Laboratory and Semi-Field Cage Demography Studies of Diachasmimorpha longicaudata Mass-Reared on Two Ceratitis capitata Strains
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect-Rearing Procedures
2.2. Experimental Setup
2.2.1. Laboratory Trials
2.2.2. Semi-Field Cage Trials
2.3. Data Analysis
3. Results
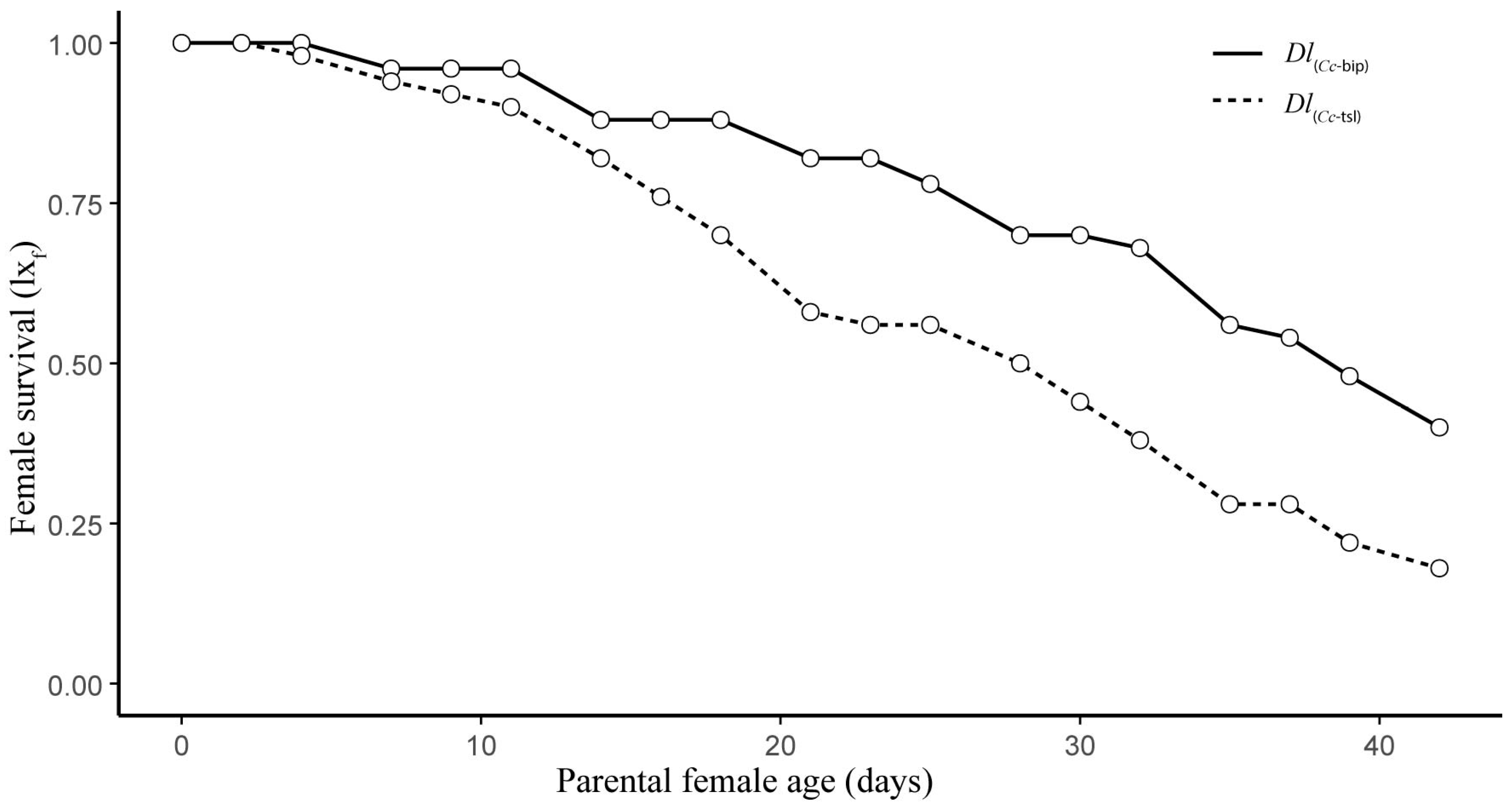
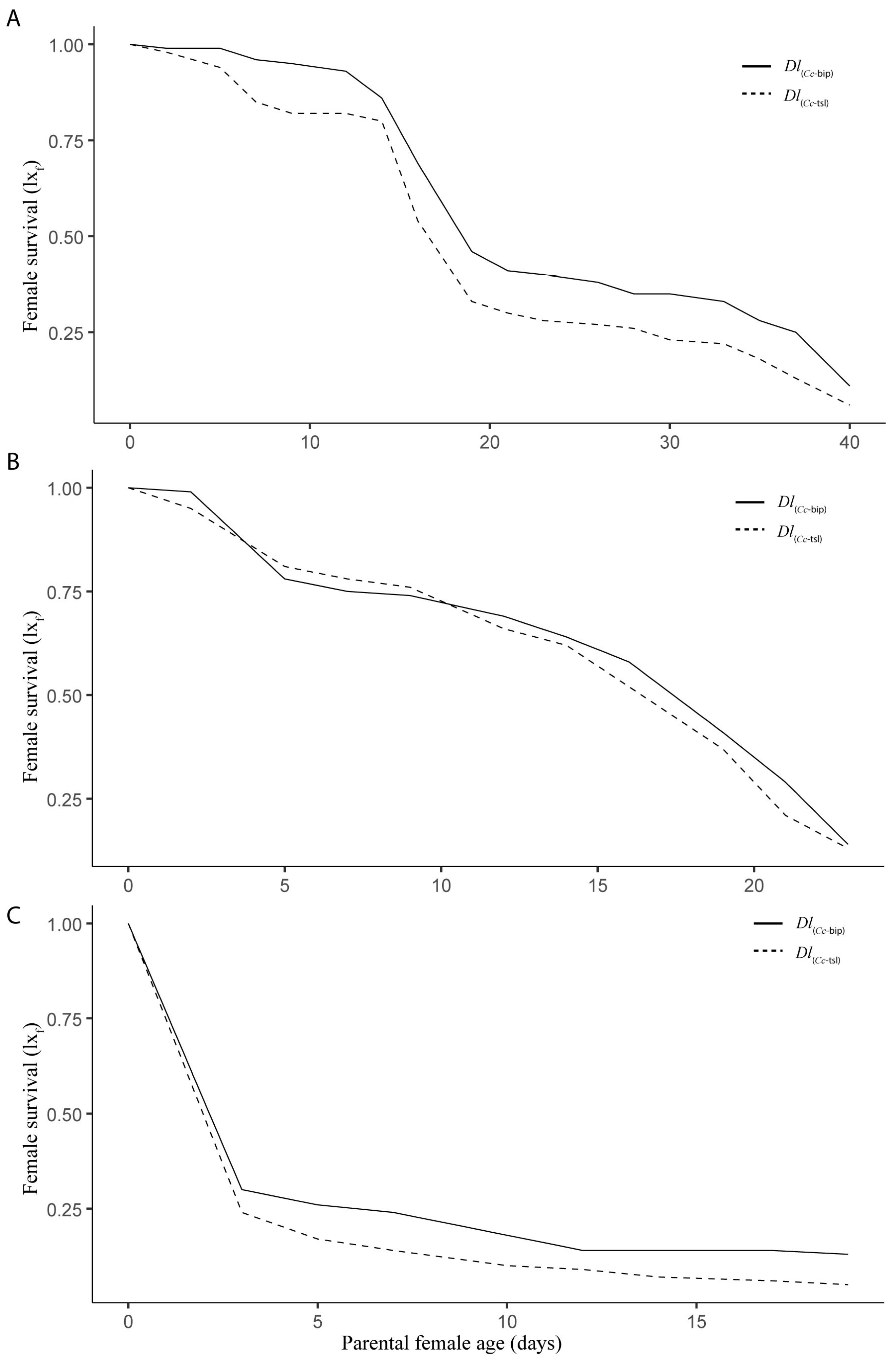
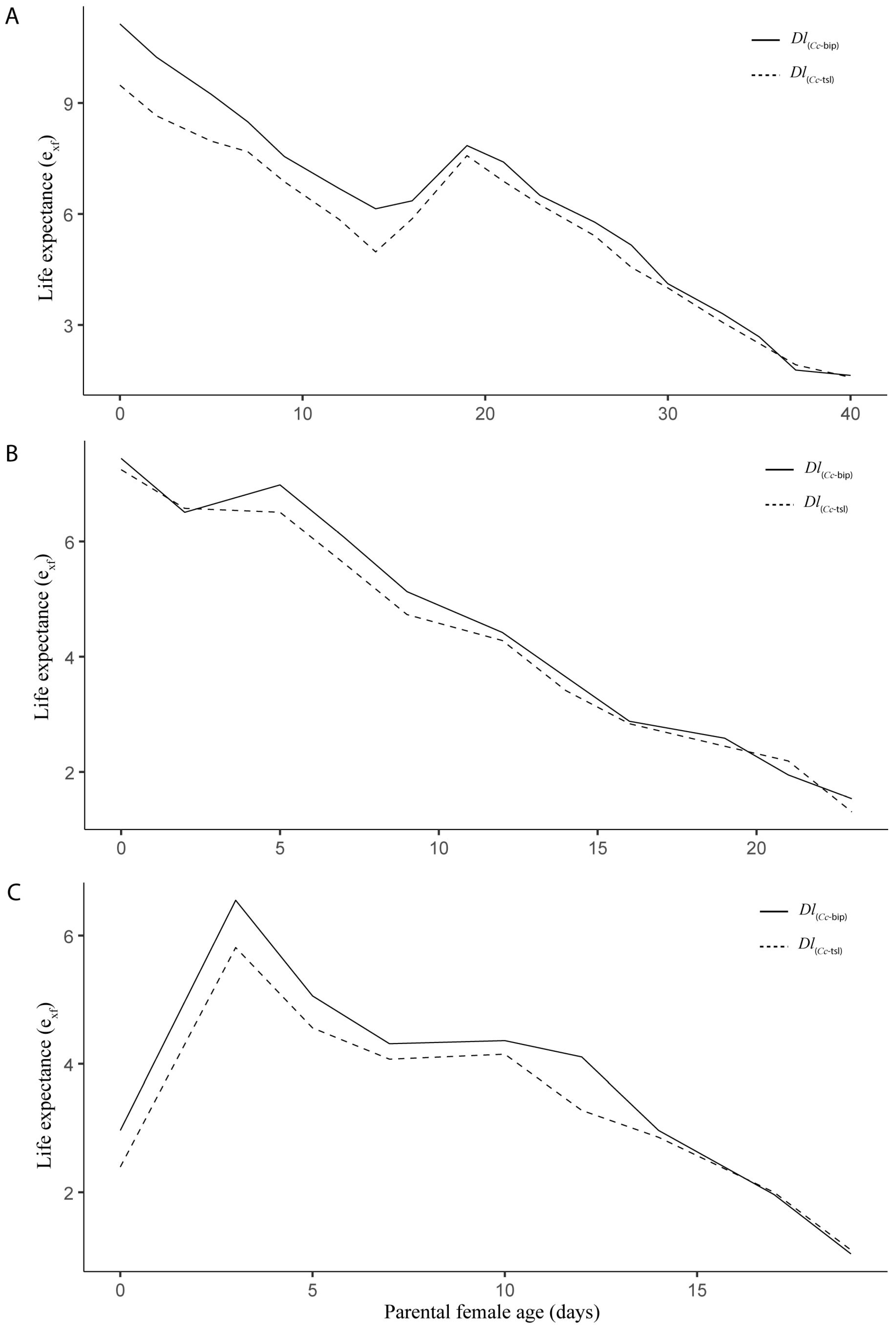
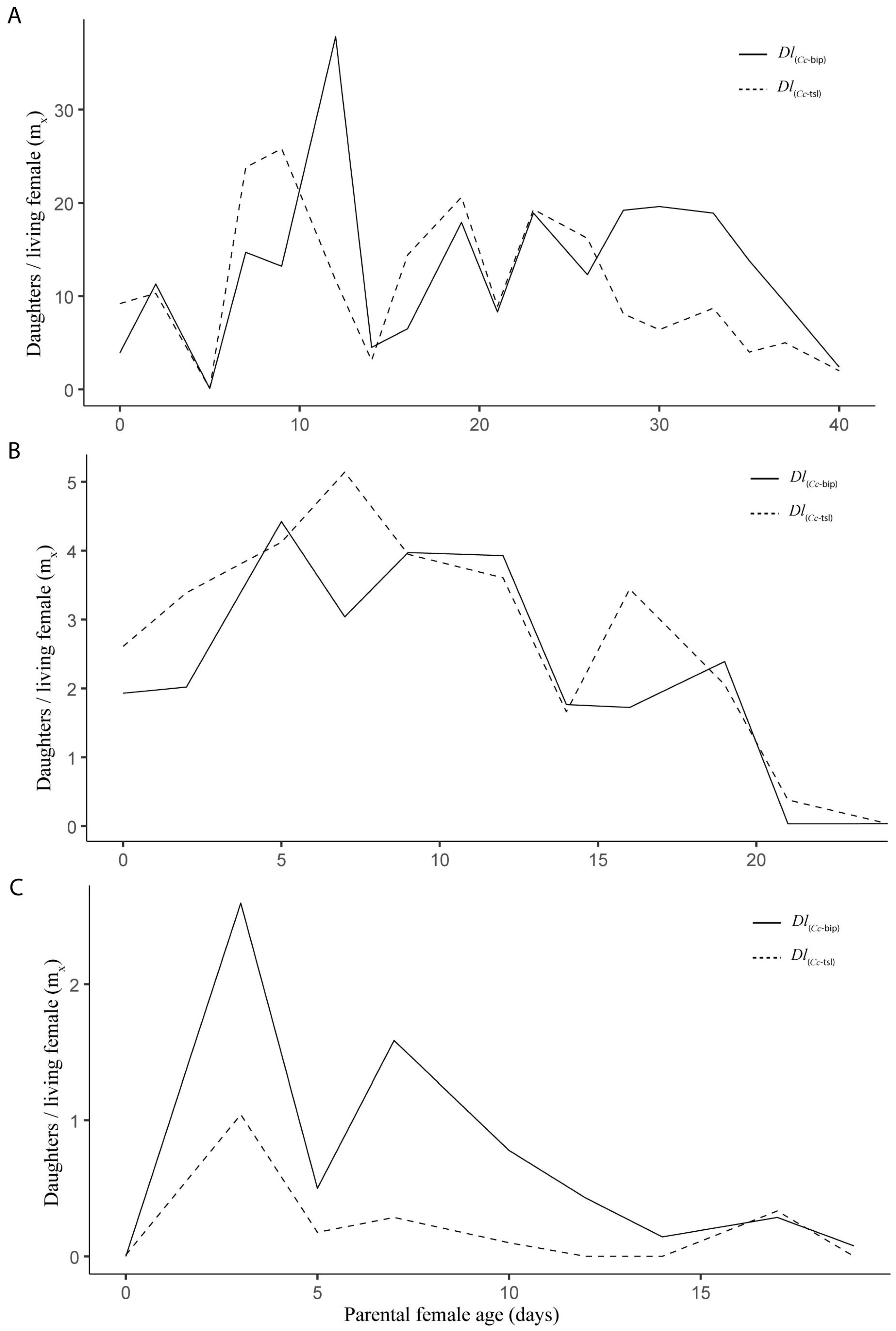
3.1. Life Table and Population Increase Parameters Under Laboratory Conditions
3.2. Life Table and Population Increase Parameters Under Semi-Field Cage Conditions
4. Discussions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Montoya, P.; López, P.; Cruz, J.; López, F.; Cadena, C.; Cancino, J.; Liedo, P. Effect of Diachasmimorpha longicaudata releases on the native parasitoid guild attacking Anastrepha spp. larvae in disturbed zones of Chiapas, Mexico. BioControl 2017, 62, 581–593. [Google Scholar] [CrossRef]
- Dias, N.P.; Zotti, M.J.; Montoya, P.; Carvalho, I.R.; Nava, D.E. Fruit fly management research: A systematic review of monitoring and control tactics in the world. J. Crop Prot. 2018, 112, 187–200. [Google Scholar] [CrossRef]
- Garcia, F.R.M.; Ovruski, S.M.; Suárez, L.; Cancino, J.; Liburd, O.E. Biological control of tephritid fruit flies in the Americas and Hawaii: A review of the use of parasitoids and predators. Insects 2020, 11, 662. [Google Scholar] [CrossRef]
- Aluja, M.; Ovruski, S.M.; Garcia, F.R.M.; Hurtado, M.; Enkerlin, W. Fruit fly (Tephritidae) management in the Neotropical Region: History, state of the art, and perspectives. In Management of Fruit Flies in the Americas; Garcia, F.R.M., Ed.; Springer Nature: Cham, Switzerland, 2024; pp. 11–66. [Google Scholar] [CrossRef]
- Lima de Brida, A.; Jean-Baptiste, M.-C.; Suárez, L.; Ovruski, S.M.; Cancino, J.; Liburd, O.E.; Garcia, F.R.M. Biological control of fruit flies with emphasis on microbial control. In Management of Fruit Flies in the Americas; Garcia, F.R.M., Ed.; Springer Nature: Cham, Switzerland, 2024; pp. 127–142. [Google Scholar] [CrossRef]
- Dias, N.P.; Montoya, P.; Nava, D.E. A 30-Year Systematic Review Reveals Success in Tephritid Fruit Fly Biological Control Research. Entomol. Exp. Appl. 2022, 170, 370–384. [Google Scholar] [CrossRef]
- Wang, X.G.; Ramadan, M.M.; Hoelmer, K.A. Biological control introductions against invasive tephritid fruit flies (Diptera: Tephritidae) in the US: Achievements, opportunities, and challenges. In Management of Fruit Flies in the Americas; Garcia, F.R.M., Ed.; Springer Nature: Cham, Switzerland, 2024; pp. 461–500. [Google Scholar] [CrossRef]
- Cancino, J.; García-Coapio, G.; López, P.; Juárez, G.; Albores, F.S.; Juárez, M.; Montoya, P. Biological control of Ceratitis capitata in the Mexico-Guatemala border region and its advantages in opening areas with social conflict. In Management of Fruit Flies in the Americas; Garcia, F.R.M., Ed.; Springer Nature: Cham, Switzerland, 2024; pp. 461–500. [Google Scholar] [CrossRef]
- Gómez-Llano, J.H.; Galvão-Silva, F.L.; Acevedo, F.E.; Castro-Llanos, F.; Gottschalk, M.S.; Nava, D.E. Biological control under climate change: Distribution patterns of the South American fruit fly Anastrepha fraterculus and two of its parasitoids in the Americas. PLoS ONE 2025, 20, e0325761. [Google Scholar] [CrossRef]
- van Lenteren, J.C.; Bueno, V.H. Augmentative biological control of arthropods in Latin America. BioControl 2003, 48, 123–139. [Google Scholar] [CrossRef]
- Montoya, P.; Cancino, J.; Zenil, M.; Santiago, G.; Gutierrez, J.M. The augmentative biological control component in the Mexican national campaign against Anastrepha spp. fruit flies. In Area-Wide Control of Insect Pests: From Research to Field Implementation; Vreysen, M.J.B., Robinson, A.S., Hendrichs, J., Eds.; Springer: Dordrecht, The Netherlands, 2007; pp. 661–670. [Google Scholar]
- Aluja, M.; Sivinski, J.; Ovruski, S.M.; Guillén, L.; López, M.; Cancino, J.; Torres-Anaya, A.; Gallegos-Chan, G.; Ruiz, L.C. Colonization and domestication of seven species of native new world hymenopterous larval-prepupal and pupal fruit fly (Diptera: Tephritidae) parasitoids. Biocontrol Sci. Technol. 2009, 19, 49–79. [Google Scholar] [CrossRef]
- Cancino, J.; Pérez, B.; Johnson, A.C.; Reynolds, O.L. Parasitoids are choosy: Increase in the capacity to discriminate parasitised tephritid pupae by Coptera haywardi. BioControl 2019, 64, 357–366. [Google Scholar] [CrossRef]
- Núñez-Campero, S.R.; Suárez, L.; Buonocore Biancheri, M.J.; Cancino, J.; Murúa, F.; Molina, D.; Laría, O.; Aluja, M.; Ovruski, S.M. Host suitability and fitness-related parameters in Coptera haywardi (Hymenoptera: Diapriidae) reared on irradiated Ceratitis capitata (Diptera: Tephritidae) pupae stemming from the tsl Vienna-8 genetic sexing strain. J. Econ. Entomol. 2020, 113, 1666–1674. [Google Scholar] [CrossRef]
- Estrada-Marroquín, M.D.; Cancino, J.; Sánchez, D.; Montoya, P.; Liedo, P. Host-specific demography of Utetes anastrephae (Hymenoptera, Braconidae), a native parasitoid of Anastrepha spp. fruit flies (Diptera, Tephritidae). J. Hymenopt. Res. 2022, 93, 53–69. [Google Scholar] [CrossRef]
- Aluja, M.; Rull, J. Managing pestiferous fruit flies (Diptera: Tephritidae) through environmental manipulation. In Biorational Tree Fruit Pest Management; Aluja, M., Leskey, T.C., Vincent, C., Eds.; CABI: Oxfordshire, UK, 2009; pp. 171–213. [Google Scholar] [CrossRef]
- Aluja, M.; Sivinski, J.; Van Driesche, R.; Anzures-Dadda, A.; Guillén, L. Pest management through tropical tree conservation. Biodivers. Conserv. 2014, 23, 831–853. [Google Scholar] [CrossRef]
- Sivinski, J. Augmentative biological control: Research and methods to help make it work. CABI Rev. 2013, 8, 1–11. [Google Scholar] [CrossRef]
- Messing, R.H.; Klungness, L.M.; Purcell, M.F.; Wong, T.T. Quality control parameters reared opiinae parasitoids used in augmentative biological control of tephritid fruit flies in Hawaii. Biol. Control 1993, 3, 140–147. [Google Scholar] [CrossRef]
- van Lenteren, J.C. Need for quality control of mass-produced biological control agents. In Quality Control and Production of Biological Control Agents: Theory and Testing Procedures; van Lenteren, J.C., Ed.; CABI Publishing: Oxfordshire, UK, 2003; pp. 1–18. [Google Scholar]
- Cancino, J.; Montoya, P. Advances and perspectives in the mass rearing of fruit fly parasitoids in Mexico. In Fruit Flies of Economic Importance: From Basic to Applied Knowledge; Sugayama, R.L., Zucchi, R.A., Ovruski, S.M., Sivinski, J., Eds.; Biofabrica Moscamed: Salvador, Brazil, 2006; pp. 133–142. [Google Scholar]
- Montoya, P.; Pérez-Lachaud, G.; Liedo, P. Superparasitism in the fruit fly parasitoid Diachasmimorpha longicaudata (Hymenoptera: Braconidae) and the implications for mass rearing and augmentative release. Insects 2012, 3, 900–911. [Google Scholar] [CrossRef] [PubMed]
- Carey, J.R.; Wong, T.T.Y.; Ramadan, M.M. Demographic framework for parasitoid mass rearing: Case study of Biosteres tryoni, a larval parasitoid of tephritid fruit flies. Theor. Popul. Biol. 1988, 34, 279–296. [Google Scholar] [CrossRef]
- Vargas, R.I.; Ramadan, M.; Hussain, T.; Mochizuki, N.; Bautista, R.C.; Stark, J.D. Comparative demographic of six fruit fly (Diptera: Thephritidae) parasitoids (Hymenoptera: Braconidae). Biol. Control 2002, 25, 30–40. [Google Scholar] [CrossRef]
- Bellows, T.S.; Van Driesche, R.G.; Elkinton, J.S. Life-table construction and analysis in the evaluation of natural enemies. Annu. Rev. Entomol. 1992, 37, 587–614. [Google Scholar] [CrossRef]
- Carey, J.R.; Roach, D.A. Biodemography: An Introduction to Concepts and Methods; Princeton University Press: Princeton, NJ, USA, 2020; 480p. [Google Scholar] [CrossRef]
- Ghaemmaghami, E.; Fathipour, Y.; Bagheri, A.; Talebi, A.A.; Reddy, G.V.P. Continuous rearing on Ephestia kuehniella reshaped quality of the parasitoid wasp Trichogramma brassicae (Hymenoptera: Trichogrammatidae). J. Asia Pac. Entomol. 2021, 24, 166–174. [Google Scholar] [CrossRef]
- Stark, J.D.; Banks, J.E.; Acheampong, S. Estimating susceptibility of biological control agents to pesticides: Influence of life history strategies and population structure. Biol. Control 2004, 29, 392–398. [Google Scholar] [CrossRef]
- Ehteshami, F.; Aleosfoor, M.; Allahyari, H.; Kavousi, A.; Fekrat, L. Comparative demography, population projection, functional response and host age preference behavior of the parasitoid Goniozus legneri on two lepidopterous insect hosts. Egypt. J. Biol. Pest Control 2023, 33, 2. [Google Scholar] [CrossRef]
- Iranipour, S.; Farazmand, A.; Saber, M.; Mashhadi, J.M. Demography and life history of the egg parasitoid Trichogramma brasicae on two moths Anagasta kuehniella and Plodia interpunctella in the laboratory. J. Insect Sci. 2009, 9, 51. [Google Scholar] [CrossRef]
- Núñez-Campero, S.R.; Aluja, M.; Rull, J.; Ovruski, S.M. Comparative demography of three Neotropical larval-prepupal parasitoid species associated with Anastrepha fraterculus (Diptera: Tephritidae). Biol. Control 2014, 69, 8–17. [Google Scholar] [CrossRef]
- Gonçalves, S.R.; Nunes, A.M.; Poncio, S.; Manica-Berto, R.; Nörnberg, S.D.; Grützmacher, A.D.; Nava, D.E. Bionomics, thermal requirements and life table of the fruit fly parasitoid Doryctobracon areolatus (Hymenoptera: Braconidae) under various thermal regimes. Biol. Control 2018, 127, 101–108. [Google Scholar] [CrossRef]
- Rashki, M.; Shirvani, A.; Hajrahmatollahi, F. Estimating some demographic parametres of Aphidius matricariae Haliday (Hymenoptera: Braconidae), the parasitoid of the greenbug aphid Schizaphis graminum (Rondani) (Hemiptera: Aphididae), at different temperatures. Egypt. J. Biol. Pest Control 2020, 30, 45. [Google Scholar] [CrossRef]
- Fernandes, E.C.; Souza, M.M.; Nava, D.E.; Silva, J.G.; Araujo, E.L. Fertility life table and biology of Tetrastichus giffardianus (Hymenoptera: Eulophidae) in the larvae of Ceratitis capitata (Diptera: Tephritidae). Bull. Entomol. Res. 2021, 111, 182–189. [Google Scholar] [CrossRef]
- Carey, J.R. Insect biodemography. Annu. Rev. Entomol. 2001, 46, 79–110. [Google Scholar] [CrossRef]
- Nyamukondiwa, C.; Weldon, C.W.; Chown, S.L.; le Roux, P.C.; Terblanche, J.S. Thermal biology, population fluctuations and implications of temperature extremes for the management of two globally significant insect pests. J. Insect Physiol. 2013, 59, 1199–1211. [Google Scholar] [CrossRef]
- Terblanche, J.S.; Nyamukondiwa, C.; Kleynhans, E. Thermal variability alters climatic stress resistance and plastic responses in a globally invasive pest, the Mediterranean fruit fly (Ceratitis capitata). Entomol. Exp. Appl. 2010, 137, 304–315. [Google Scholar] [CrossRef]
- Krechemer, F.S.; Foerster, L.A. Temperature effects on the development and reproduction of Three Trichogramma (Hymenoptera: Trichogrammatidae) species reared on Trichoplusia ni (Lepidoptera: Noctuidae) eggs. J. Insect Sci. 2015, 15, 90. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.Y.; Chen, K.W.; Zeng, L. Effects of temperature on the development and fecundity of Diachasmimorpha longicaudata (Ashmead). Chin. J. Appl. Ecol. 2012, 23, 3051–3056. [Google Scholar]
- Poncio, S.; Nunes, A.M.; Gonçalves, R.S.; Lisboa, H.; Manica-Berto, R.; Garcia, M.S.; Nava, D.E. Biology of Doryctobracon brasiliensis at different temperatures: Development of life table and determining thermal requirements. J. Appl. Entomol. 2016, 140, 775–785. [Google Scholar] [CrossRef]
- Groth, M.Z.; Loeck, A.E.; Nornberg, S.D.; Bernardi, D.; Nava, D.E. Biology and thermal requirements of Fopius arisanus (Sonan, 1932) (Hymenoptera: Braconidae) reared on Ceratitis capitata eggs (Wiedemann) (Diptera: Tephritidae). Neotrop. Entomol. 2017, 46, 554–560. [Google Scholar] [CrossRef] [PubMed]
- Harbi, A.; Beitia, F.; Chermiti, B.; de Pedro, L.; Ferrarra, F.; Asís, J.D.; Polidori, J.C.; Tormos, J.; Sabater-Muñoz, T. Abiotic factors affecting Diachasmimorpha longicaudata (Hymenoptera: Braconidae) activity as a natural enemy of Ceratitis capitata (Diptera: Tephritidae) under semi-natural conditions in the Mediterranean region. J. Appl. Entomol. 2018, 142, 755–764. [Google Scholar] [CrossRef]
- Harbi, A.; Beitia, F.; Ferrara, F.; Chermiti, B.; Sabater-Muñoz, T. Functional response of Diachasmimorpha longicaudata (Ashmead) over Ceratitis capitata (Wiedemann): Influence of temperature, fruit location and host density. Crop Prot. 2018, 109, 115–122. [Google Scholar] [CrossRef]
- Ndlela, S.; Azrag, A.G.A.; Mohamed, S.A. Determination of temperature thresholds for the parasitoid Diachasmimorpha longicaudata (Hymenoptera: Braconidae), using life cycle simulation modeling: Implications for effective field releases in classical biological control of fruit flies. PLoS ONE 2021, 16, e0255582. [Google Scholar] [CrossRef]
- Spahn, R.; Lill, J.T. Higher temperatures reduce the efficacy of a key biocontrol parasitoid. Biol. Control 2022, 176, 105079. [Google Scholar] [CrossRef]
- Servicio Nacional de Sanidad y Calidad Agroalimentaria (SENASA). Resolución SENASA 0218/2024; SENASA-Biblioteca: Buenos Aires, Argentina, 2025; Available online: https://biblioteca.senasa.gob.ar/items/show/4277 (accessed on 15 July 2025).
- Guillén, D.; Sánchez, R. Expansion of the national fruit fly control programme in Argentina. In Area-Wide Control of Insects Pests: From Research to Field Implementation; Vreysen, M.J.B., Robinson, A.S., Hendrichs, J., Eds.; Springer: Dordrecht, The Netherlands, 2007; pp. 653–660. [Google Scholar]
- Suárez, L.; Buonocore-Biancheri, M.J.; Murúa, A.F.; Beltrachini, S.; Kulichevsky, L.E.; Ovruski, S.M. Management of tephritid fruit flies in Argentina. In Management of Fruit Flies in the Americas; Garcia, F.R.M., Ed.; Springer Nature: Cham, Switzerland, 2024; pp. 461–500. [Google Scholar] [CrossRef]
- Quiroga, D.; Ramirez, W.; Ruiz, C. National fruit fly control and eradication program (PROCEM) Argentina. In Proceedings of the 8th International Symposium on Fruit Flies of Economic Importance, Valencia, Spain, 26 September–1 October 2010; Sabater-Muñóz, B., Navarro-Llopis, V., Urbaneja, A., Eds.; Editorial Universitat Politécnica de Valéncia: Valencia, Spain, 2010; p. 100. Available online: http://hdl.handle.net/10251/11200 (accessed on 10 July 2025).
- Sánchez, G.; Murúa, F.; Suárez, L.; Van Nieuwenhove, G.; Taret, G.; Pantano, V.; Ovruski, S.M. Augmentative releases of Diachasmimorpha longicaudata (Hymenoptera: Braconidae) for Ceratitis capitata (Diptera: Tephritidae) control in a fruit-growing region of Argentina. Biol. Control 2016, 103, 101–107. [Google Scholar] [CrossRef]
- Camargos, M.G.; Alvarenga, C.D.; Júnior, R.R.; Walder, J.M.M.; Castro Novais, J. Spatial and temporal dispersal patterns of Diachasmimorpha longicaudata (Hymenoptera: Braconidae) reared on Ceratitis capitata and Anastrepha fraterculus (Diptera: Tephritidae). Biol. Control 2018, 122, 84–92. [Google Scholar] [CrossRef]
- de Pedro, L.; Tormos, J.; Harbi, A.; Ferrara, F.; Sabater-Muñoz, B.; Asís, J.D.; Beitia, F. Combined use of the larvo-pupal parasitoids Diachasmimorpha longicaudata and Aganaspis daci for biological control of the medfly. Ann. Appl. Biol. 2019, 174, 40–50. [Google Scholar] [CrossRef]
- Cancino, J.; Ruiz, L.; López, E.; Aguilar, E.; Galvez, C.; Montoya, P.; Liedo, P. Suppression of Ceratitis capitata (Wied.) (Diptera: Tephritidae) populations in coffee in the Mexico-Guatemala border region through the augmentative releases of Diachasmimorpha longicaudata (Ashmead) (Hymenoptera: Braconidae). Biocontrol Sci. Technol. 2019, 29, 822–826. [Google Scholar] [CrossRef]
- Vargas, R.I.; Leblanc, L.; Harris, E.J.; Manoukis, N.C. Regional suppression of Bactrocera fruit flies (Diptera: Tephritidae) in the Pacific through biological control and prospects for future introductions into other areas of the world. Insects 2012, 3, 727–742. [Google Scholar] [CrossRef]
- Ibrahim, A.G.; Palacio, I.P.; Rohani, I. Biology of Diachasmimorpha longicaudata, a parasitoid of carambola fruit fly. Pertanika J. Trop. Agric. Sci. 1995, 17, 139–143. [Google Scholar]
- Sivinski, J.; Aluja, M. The evolution of ovipositor length in the parasitic Hymenoptera and the search for predictability in biological control. Fla. Entomol. 2003, 86, 143–150. [Google Scholar] [CrossRef]
- Alós, M.; Llera, H.; Taret, G. Resultados del cambio de estrategia adoptada desde 2011. In Programa de Control y Erradicación de Mosca de los Frutos; Gobierno de San Juan Ediciones: San Juan, Argentina, 2014; 14p. [Google Scholar]
- Suárez, L.; Biancheri, M.J.B.; Murúa, F.; Ordano, M.; Wang, X.G.; Cancino, J.; Garcia, F.R.M.; Sánchez, G.; Beltrachini, S.; Kulichevsky, L.E.; et al. Medfly population suppression through augmentative release of an introduced parasitoid in an irrigated multi-fruit orchard of central–western Argentina. Insects 2023, 14, 387. [Google Scholar] [CrossRef] [PubMed]
- Díaz, L.M.; Murua, A.F.; Acosta, J.C.; Escobar, J.M. Dispersal behavior of Ceratitis capitata (Diptera: Tephritidae) among agricultural valleys in San Juan, Argentina. Rev. Soc. Entomol. Argent. 2008, 67, 155–161. [Google Scholar]
- Suárez, L.; Buonocore Biancheri, M.J.; Murúa, F.; Bilbao, M.; García, M.; Cancino, J.; Martín, O.; Molina, D.; Laría, O.; Ovruski, S.M. Effects of host age and radiation dose in Diachasmimorpha longicaudata (Hymenoptera: Braconidae) mass reared on Medfly larvae of the tsl Vienna-8 genetic sexing strain. Biol. Control 2019, 130, 51–59. [Google Scholar] [CrossRef]
- Carey, J.R. Applied Demography for Biologists: With Special Emphasis on Insects; Oxford University Press: New York, NY, USA, 1993; 206p. [Google Scholar]
- Campos, V.E.; Gatica, G.; Andino, N.; Maldonado, V.N.F.; Cardús, A. Land Surface Temperature in an Arid City: Assessing Spatio-temporal Changes. Remote Sens. Earth Syst. Sci. 2023, 6, 90–104. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2024; Available online: https://www.R-project.org/ (accessed on 28 July 2025).
- Wickham, H.; Averick, M.; Bryan, J.; Chang, W.; McGowan, L.D.; François, R.; Grolemund, G.; Hayes, A.; Henry, L.; Hester, J.; et al. Welcome to the tidyverse. J. Open Source Softw. 2019, 4, 1686. [Google Scholar] [CrossRef]
- Davison, A.C.; Hinkley, D.V. Bootstrap Methods and Their Applications; Cambridge University Press: Cambridge, UK, 1997; 95p, ISBN 0-521-57391-2. [Google Scholar]
- Canty, A.; Ripley, B. Boot: Bootstrap R (S-Plus) Functions, R package version 1.3-31; R Foundation for Statistical Computing: Vienna, Austria, 2024.
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016; 260p. [Google Scholar]
- Therneau, T.M.; Grambsch, P.M. Modeling Survival Data: Extending the Cox Model; Springer: NY, USA, 2000; 350p, ISBN 0-387-98784-3. [Google Scholar]
- Therneau, T. A Package for Survival Analysis in R, R Package Version 3.8-3; 2024. Available online: https://CRAN.R-project.org/package=survival (accessed on 28 July 2025).
- Pritchard, D.W.; Paterson, R.A.; Bovy, H.C.; Barrios-O’Neill, D. Frair: An R package for fitting and comparing consumer functional responses. Methods Ecol. Evol. 2017, 8, 1528–1534. [Google Scholar] [CrossRef]
- Wood, S.N.; Pya, N.; Saefken, B. Smoothing parameter and model selection for general smooth models (with discussion). J. Am. Stat. Assoc. 2016, 111, 1548–1575. [Google Scholar] [CrossRef]
- Wickham, H.; François, R.; Henry, L.; Müller, K.; Vaughan, D. dplyr: A Grammar of Data Manipulation, R package version 1.1.4; R Foundation for Statistical Computing: Vienna, Austria, 2023. Available online: https://CRAN.R-project.org/package=dplyr (accessed on 28 July 2025).
- Wickham, H.; Vaughan, D.; Girlich, M. tidyr: Tidy Messy Data, R package version 1.3.1; R Foundation for Statistical Computing: Vienna, Austria, 2024. Available online: https://CRAN.R-project.org/package=tidyr (accessed on 28 July 2025).
- Groemping, U. Relative Importance for Linear Regression in R: The Package relaimpo. J. Stat. Softw. 2006, 17, 1–27. [Google Scholar] [CrossRef]
- Cribari-Neto, F.; Zeileis, A. Beta Regression in R. J. Stat. Softw. 2010, 34, 1–24. [Google Scholar] [CrossRef]
- Fox, J.; Weisberg, S. An R Companion to Applied Regression, 3rd ed.; Sage: Thousand Oaks, CA, USA, 2019; Available online: https://www.john-fox.ca/Companion/ (accessed on 28 July 2025).
- Wood, S.N. Fast stable restricted maximum likelihood and marginal likelihood estimation of semiparametric generalized linear models. J. R. Stat. Soc. Ser. B Stat. Methodol. 2011, 73, 3–36. [Google Scholar] [CrossRef]
- Jalmar, M.F.; Carrasco, S.; Ferrari, L.P.; Arellano–Valle, R.B. Multiplicative errors-in-variables beta regression. Braz. J. Probab. Stat. 2023, 37, 249–262. [Google Scholar] [CrossRef]
- Nchu, F. Sustainable biological control of pests: The way forward. Appl. Sci. 2024, 14, 2669. [Google Scholar] [CrossRef]
- Iranipour, S.; Vaez, N.; Ghanbalani, G.N.; Zakaria, R.A.; Jafarloo, M.M. Effect of host change on demographic fitness of the parasitoid, Trichogramma brassicae. J. Insect Sci. 2010, 10, 78. [Google Scholar] [CrossRef]
- Lü, X.; Qiu, R.; He, X.; Li, J. Evaluation of key factors for mass rearing the egg parasitoid Telenomus remus Nixon (Hymenoptera: Scelionidae). CABI Agric. Biosci. 2024, 5, 55. [Google Scholar] [CrossRef]
- Meirelles, R.N.; Redaelli, L.R.; Ourique, C.B. Comparative biology of Diachasmimorpha longicaudata (Hymenoptera: Braconidae) reared on Anastrepha fraterculus and Ceratitis capitata (Diptera: Tephritidae). Fla. Entomol. 2013, 96, 412–418. [Google Scholar] [CrossRef]
- Meirelles, R.N.; Redaelli, L.R.; Ourique, C.B. Thermal requirements and annual number of generations of Diachasmimorpha longicaudata (Hymenoptera: Braconidae) reared in the South American fruit fly and the Mediterranean fruit fly (Diptera: Tephritidae). Fla. Entomol. 2015, 98, 1223–1227. [Google Scholar] [CrossRef]
- Viscarret, M.M.; La Rossa, R.; Segura, D.F.; Ovruski, S.M.; Cladera, J.L. Evaluation of the parasitoid Diachasmimorpha longicaudata (Ashmead) (Hymenoptera: Braconidae) reared on a genetic sexing strain of Ceratitis capitata (Wied.) (Diptera: Tephritidae). Biol. Control 2006, 36, 147–153. [Google Scholar] [CrossRef]
- Caswell, H.; de Vries, C.; Hartemink, N.; Roth, G.; van Daalen, S.F. Age × stage-classified demographic analysis: A comprehensive approach. Ecol. Monogr. 2018, 88, 560–584. [Google Scholar] [CrossRef]
- Mu, M.-Y.; Chen, Y.-M.; Wang, X.-G.; Desneux, N.; Zang, L.-S. Comparative demographics, population projections and egg maturation patterns of four eupelmid egg parasitoids on the factitious host Antherae pernyi. Pest Manag. Sci. 2023, 79, 3631–3641. [Google Scholar] [CrossRef]
- Beddington, J.; Free, C.; Lawton, J. Characteristics of successful natural enemies in models of biological control of insect pests. Nature 1978, 273, 513–519. [Google Scholar] [CrossRef]
- Núñez-Campero, S.R.; Ovruski, S.M.; Aluja, M. Survival analysis and demographic parameters of the pupal parasitoid Coptera haywardi (Hymenoptera: Diapriidae), reared on Anastrepha fraterculus (Diptera: Tephritidae). Biol. Control 2012, 61, 40–46. [Google Scholar] [CrossRef]
- Lane, S.D.; Mills, N.J.; Getz, W.M. The effects of parasitoid fecundity and host taxon on the biological control of insect pests: The relationship between theory and data. Ecol. Entomol. 2001, 24, 181–190. [Google Scholar] [CrossRef]
- Jervis, M.A.; Moe, A.; Heimpel, G.E. The evolution of parasitoid fecundity: A paradigm under scrutiny. Ecol. Lett. 2012, 15, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Segoli, M.; Abram, P.K.; Ellers, J.; Greenbaum, G.; Hardy, I.C.W.; Heimpel, G.E.; Keasar, T.; Ode, P.J.; Sadeh, A.; Wajnberg, E. Trait-based approaches to predicting biological control success: Challenges and prospects. Trends Ecol. Evol. 2023, 38, 802–811. [Google Scholar] [CrossRef] [PubMed]
- Appiah, E.F.; Ekesi, S.; Salifu, D.; Afreh-Nuamah, K.; Obeng-Ofori, D.; Khamis, F.; Mohamed, S.A. Effect of temperature on immature development and longevity of two introduced opine parasitoids on Bactrocera invadens. J. Appl. Entomol. 2013, 137, 571–579. [Google Scholar] [CrossRef]


| Temperature (°C) | Relative Humidity (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| Seasons | Tmean | Tmax | Tmin | Trange | RHmean | RHmax | RHmin | RHrange |
| Spring | 19.6 ± 0.6 | 26.2 ± 0.8 | 13.7 ± 0.6 | 17.3 ± 0.9 | 48.3 ± 2.2 | 69.4 ± 2.8 | 27.4 ± 2.4 | 42.4 ± 3.0 |
| Summer | 22.0 ± 0.5 | 30.3 ± 0.7 | 13.9 ± 0.6 | 22.7 ± 0.8 | 51.2 ± 1.0 | 78.1 ± 1.5 | 23.6 ± 1.1 | 54.6 ± 1.5 |
| Autumn | 12.7 ± 0.3 | 20.0 ± 0.6 | 5.3 ± 0.5 | 13.6 ± 0.9 | 62.8 ± 1.1 | 83.5 ± 2.1 | 42.0 ± 0.9 | 41.6 ± 2.4 |
| Population Increase Parameters | ||||
|---|---|---|---|---|
| Parasitoid Population Lines | R0 (Females/Female Per Generation) | r (Per Day) | T (Days) | λ (Per Day) |
| Dl(Cc-bip) | 109.80 ± 3.46 | 0.32 ± 0.00 | 14.63 ± 0.30 | 1.37 ± 0.00 |
| [108.8–122.4] a | [0.29–0.32] a | [14.53–15.82] a | [1.34–1.38] a | |
| Dl(Cc-tsl) | 63.94 ± 3.35 | 0.29 ± 0.00 | 14.22 ± 0.58 | 1.33 ± 0.01 |
| [68.3–81.4] b | [0.24–0.28] b | [15.12–17.50] a | [1.28–1.32] b | |
| Population Increase Parameters | |||||
|---|---|---|---|---|---|
| Parasitoid Population Lines | Seasons | R0 (Females/Female Per Generation) | r (Per Day) | T (Days) | λ (Per Day) |
| Dl(Cc-bip) | Spring | 23.26 ± 3.45 [16.93–30.17] a | 0.15 ± 0.02 [0.12–0.20] b | 20.67 ± 2.75 [15.74–26.16] a | 1.16 ± 0.03 [1.12–1.23] b |
| Summer | 19.80 ± 3.37 [11.29–25.50] a | 0.35 ± 0.09 [0.21–0.56] a | 8.42 ± 1.73 [5.45–12.21] b | 1.42 ± 0.14 [1.24–1.75] a | |
| Autumn | 1.56 ± 0.72 [0.40–3.09] b | 0.08 ± 0.10 [−0.09–0.27] c | 5.72 ± 1.94 [3.78–10.82] b | 1.08 ± 0.11 [0.92–1.31] c | |
| Dl(Cc-tsl) | Spring | 19.76 ± 3.17 [13.61–26.05] a | 0.17 ± 0.03 [0.12–0.25] b | 17.15 ± 2.52 [12.65–22.15] a | 1.19 ± 0.04 [1.13–1.28] b |
| Summer | 22.48 ± 4.21 [14.41–30.05] a | 0.41 ± 0.11 [0.03–0.12] a | 7.65 ± 1.69 [4.82–11.77] b | 1.50 ± 0.18 [1.27–1.97] a | |
| Autumn | 0.36 ± 0.23 [0.06–0.85] c | −0.23 ± 0.10 [−0.44–−0.04] d | 4.50 ± 2.28 [3.19–11.07] b | 0.80 ± 0.08 [0.64–0.96] d | |
| Host Species | Intrinsic Rate of Natural Increment (r) | Laboratory-Tested Temperatures (°C) | References |
|---|---|---|---|
| Anastrepha fraterculus (Wiedemann) | 0.17 ± 0.03 | 25.0 ± 2.0 | [81] |
| Ceratitis capitata (Wiedemann) | 0.14 ± 0.02 | 25.0 ± 2.0 | [81] |
| C. capitata (wild strain) | 0.098 ± 0.005 | 22.9 ± 2.9 | [82] |
| C. capitata (genetic sexing strain Cast-191) | 0.094 ± 0.004 | 22.9 ± 2.9 | [82] |
| Bactrocera dorsalis Hendel | 0.003 ± 0.001–0.145 ± 0.001 | 15–30 | [44] |
| B. dorsalis | −0.0240–0.1318 | 15–30 | [39] |
| B. dorsalis | 0.12 | 26.0 ± 2.0 | [24] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suárez, L.; Núñez-Campero, S.R.; Carta Gadea, S.L.; Murúa, F.; Garcia, F.R.M.; Ovruski, S.M. Laboratory and Semi-Field Cage Demography Studies of Diachasmimorpha longicaudata Mass-Reared on Two Ceratitis capitata Strains. Insects 2025, 16, 1031. https://doi.org/10.3390/insects16101031
Suárez L, Núñez-Campero SR, Carta Gadea SL, Murúa F, Garcia FRM, Ovruski SM. Laboratory and Semi-Field Cage Demography Studies of Diachasmimorpha longicaudata Mass-Reared on Two Ceratitis capitata Strains. Insects. 2025; 16(10):1031. https://doi.org/10.3390/insects16101031
Chicago/Turabian StyleSuárez, Lorena, Segundo Ricardo Núñez-Campero, Silvia Lorena Carta Gadea, Fernando Murúa, Flávio Roberto Mello Garcia, and Sergio Marcelo Ovruski. 2025. "Laboratory and Semi-Field Cage Demography Studies of Diachasmimorpha longicaudata Mass-Reared on Two Ceratitis capitata Strains" Insects 16, no. 10: 1031. https://doi.org/10.3390/insects16101031
APA StyleSuárez, L., Núñez-Campero, S. R., Carta Gadea, S. L., Murúa, F., Garcia, F. R. M., & Ovruski, S. M. (2025). Laboratory and Semi-Field Cage Demography Studies of Diachasmimorpha longicaudata Mass-Reared on Two Ceratitis capitata Strains. Insects, 16(10), 1031. https://doi.org/10.3390/insects16101031








