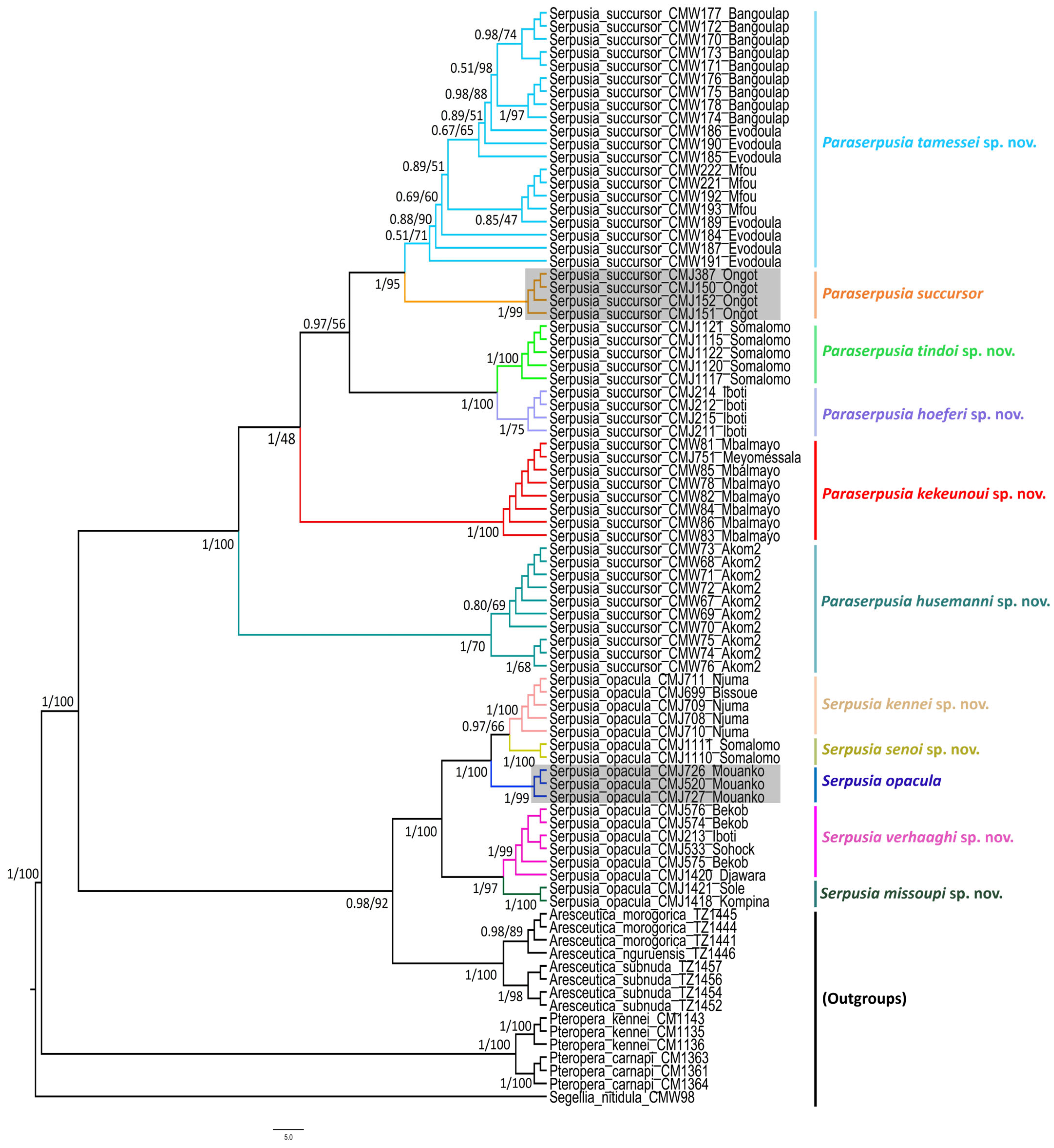
3.2.2. The Genus Serpusia Karsch, 1891
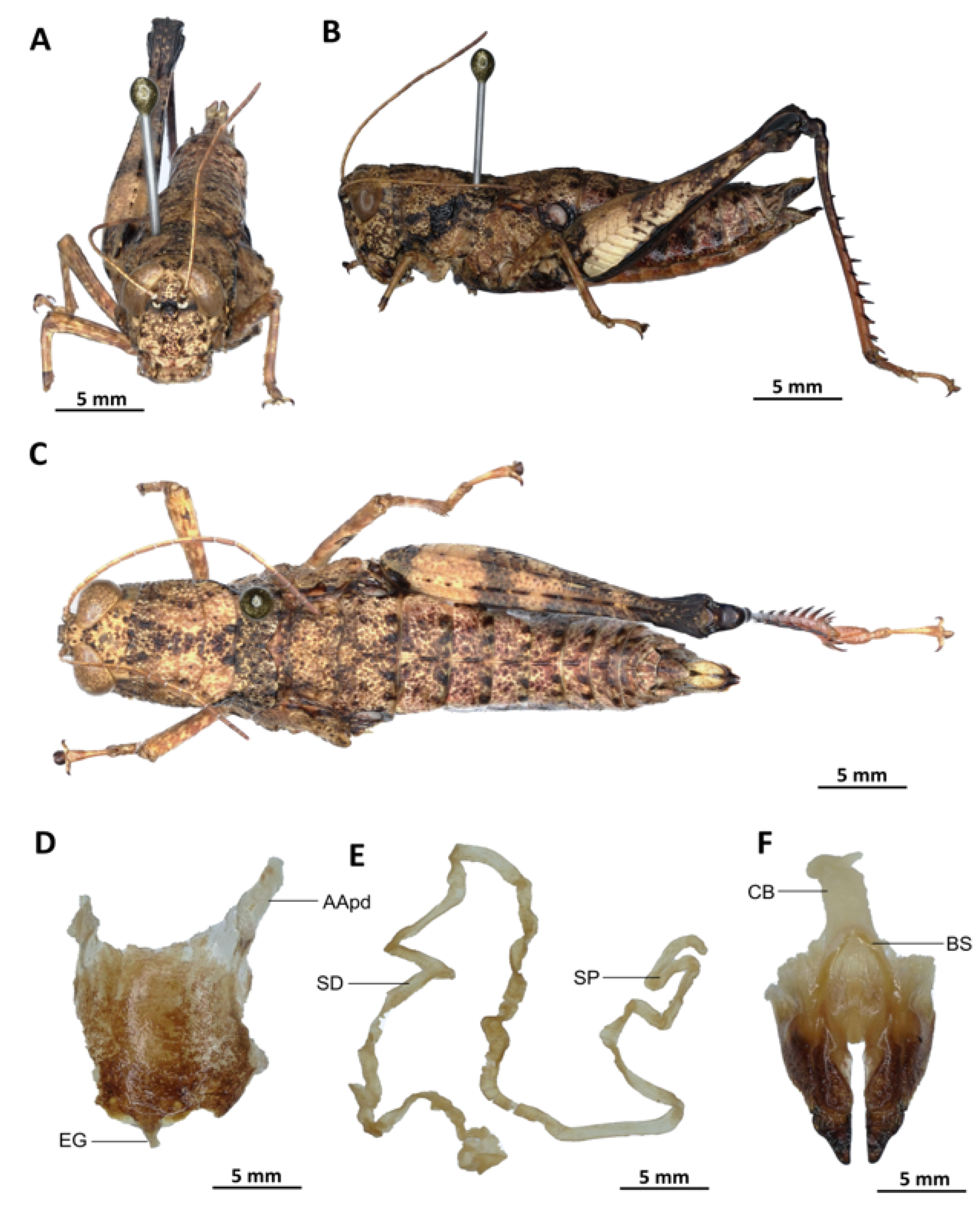
With S. opacula as the type, species of the genus Serpusia are morphologically very similar.
Material examined. Cameroon • 1 ♂, 1 ♀; Mouanko, in more or less stable forest; 3°38′0” N, 9°47′0” E, 89 m a.s.l.; 3 May. 2020; J.A. Yetchom Fondjo leg.; SMNK. Cameroon • 1 ♀, Mouanko, in the forest habitat; 3°38′0″ N, 9°47′0″ E, 89 m a.s.l.; 11 October 2020; J.A. Yetchom Fondjo leg.; SMNK.
Diagnosis. Serpusia opacula strongly resembles to Paraserpusia succursor in coloration, but is clearly distinct in the following characters: the elytra are rudimentary, vestigial and narrow, covering only a small portion of the tympanum and not reaching the posterior part of the metanotum (vs. elytra lobiform, with subparallel margins, slightly exceeding the posterior part of the metanotum in P. succursor); the hind femora are black in the basal part (vs. dark red in P. succursor); the posterior margin of the pronotum is strongly emarginate and acute (vs. rounded or only slightly emarginate in P. succursor); the male genitalia differ by their apodemes of cingulum with almost straight apex, and strongly exceeding the endophallic apodemes’ tip (vs. the apodemes of cingulum exhibit a slight curvature ventrally at the apex, with the tip of the endophallic apodemes being reached in P. succursor); the rami is of a length that is equal to or slightly exceeds its width in lateral view (vs. in P. succursor, the rami is longer than its width in lateral view); the ectophallic sheath is long (vs. in P. succursor, the ectophallic sheath is short); the aedeagus is long, only slightly curved upwards, and has almost straight margins and parallel tips (vs. the aedeagus is of medium size, with roughly divergent margins and broad tip in P. succursor); the gonopore sac is small in size and subtriangular in shape, covering approximately one-half of the endophallus (vs. the gonopore sac of P. succursor is of a larger size and covers approximately two-thirds of the endophallus).
Serpusia opacula is very similar to S. missoupi sp. nov. from which it can easily be distinguished by the presence of a large shiny black spot at the apex of the elytra, a large spot resulting from the fusion between the two apical spots (vs. two shiny black spots well separated in S. missoupi sp. nov.); furthermore, the pale spot located in the inferior-external region of the lateral lobes of the pronotum is evident and discernible (vs. pale spot inconspicuous or absent in S. missoupi sp. nov.); the female genitalia are distinguished by the presence of a subgenital plate with long anterior apodemes (vs. broad and short in S. missoupi sp. nov.), and a short spermatheca duct (vs. very long in S. missoupi sp. nov.).
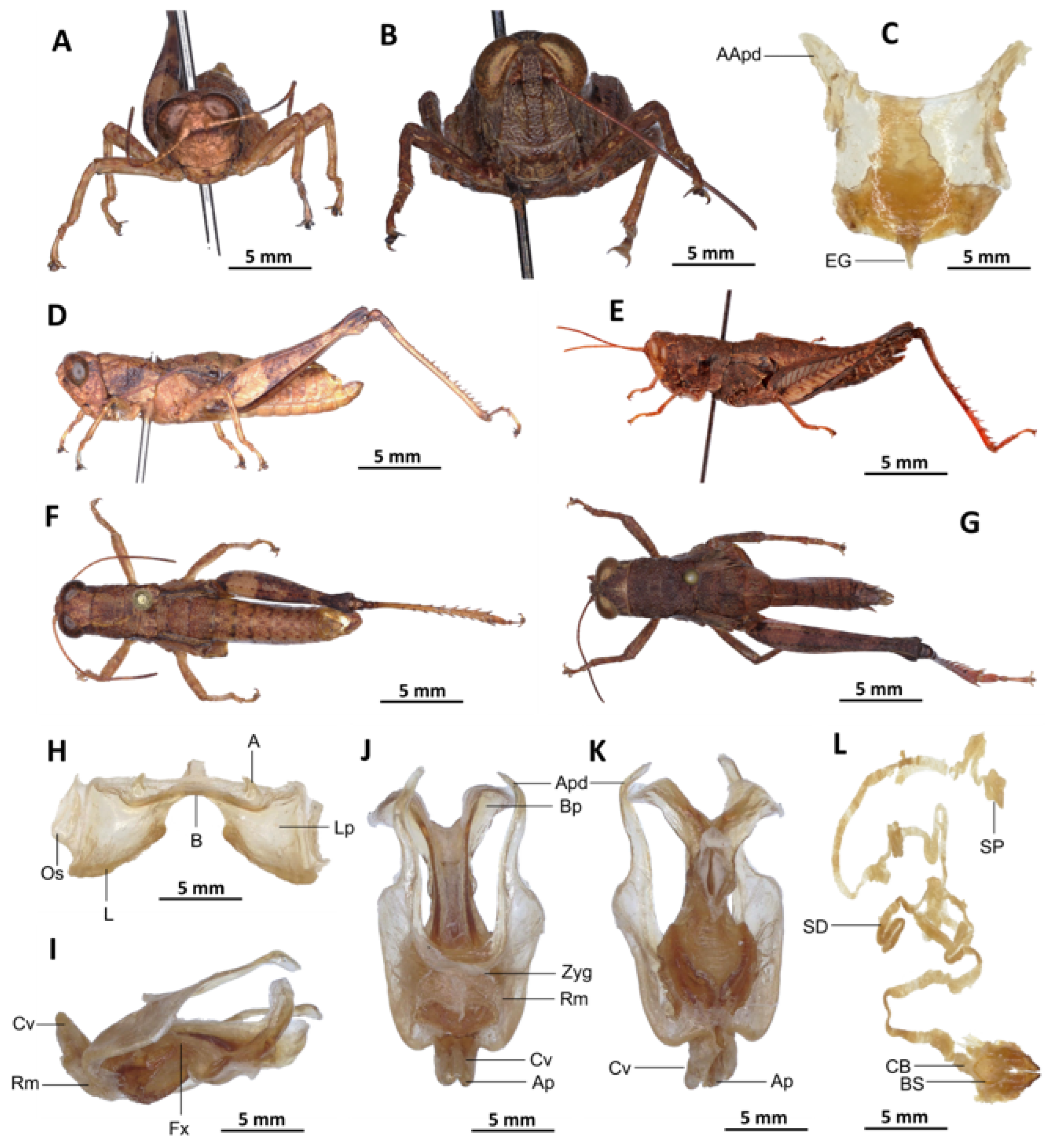
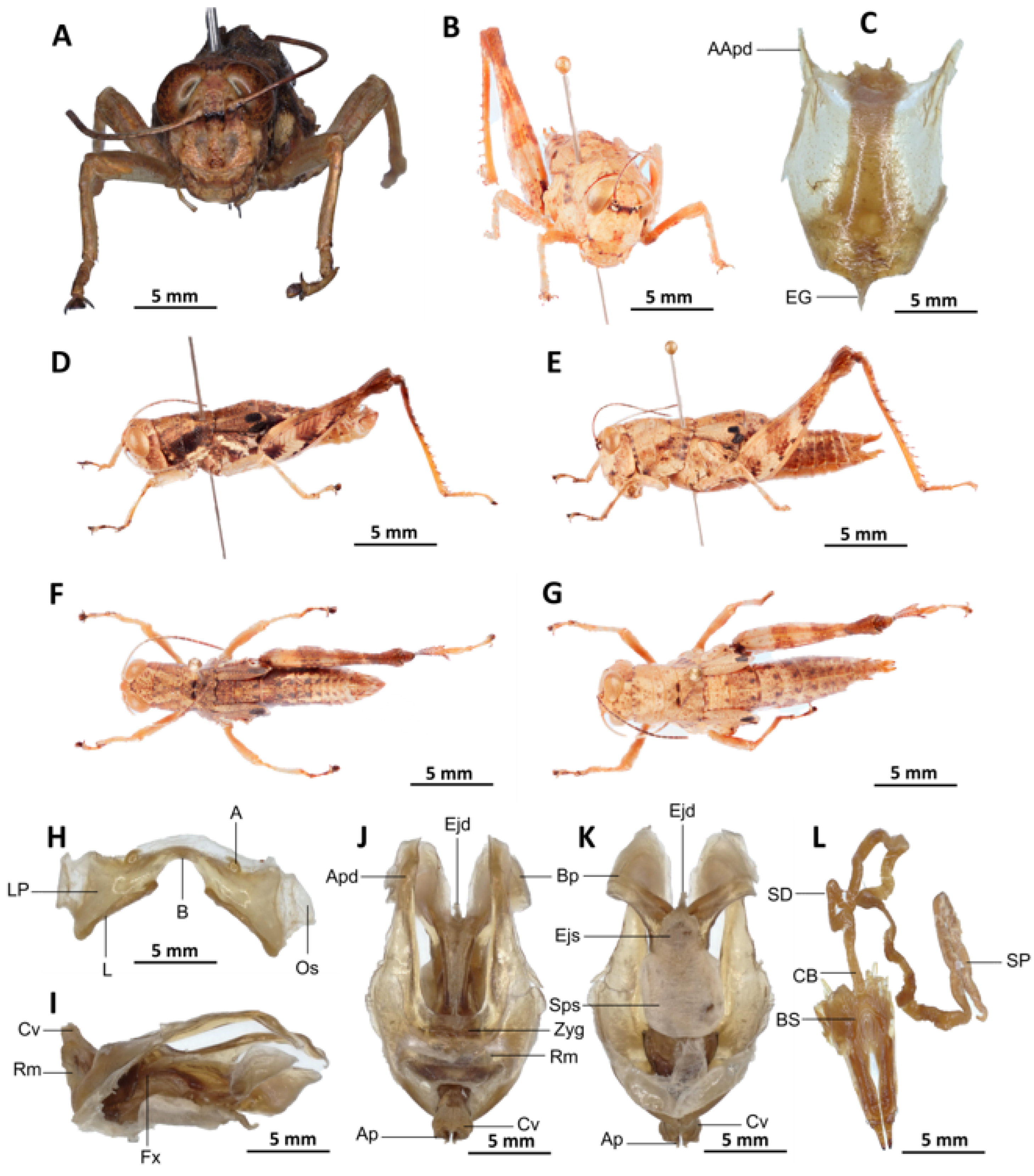
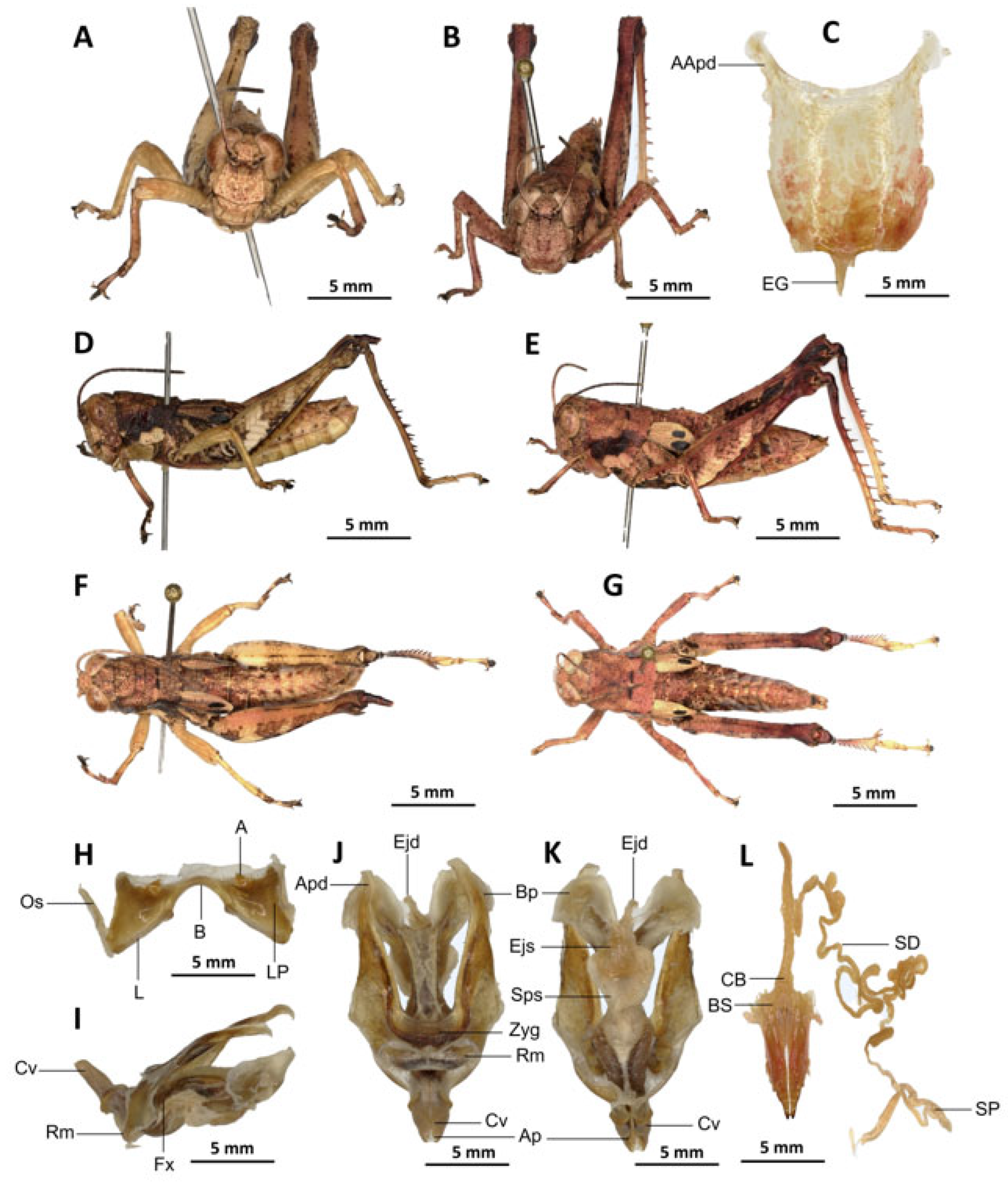
Redescription. Male. The abdomen is characterised by a shiny, sub-unicoloured appearance and a subtle olivaceous tint. The head is erect, and the fastigium of the vertex is flattened. The lateral lobes of the pronotum exhibit an irregular black spot that is more or less clearly defined. The median carina of the pronotum is visible and is crossed by three transverse grooves. The prozona is approximately three times longer than the metazona. The pronotum, mesonotum, metanotum, and dorsal surface of the abdomen exhibit pronounced wrinkling and granularity. The elytra are rudimentary and vestigial, exhibiting a very narrow configuration.
They are adorned with two shiny black subapical spots, which are sometimes confluent, and extend to the posterior edge of the metanotum in some individuals. The inner surface of the hind femur is red, the black plume is located in the apical half, the basal third is black, and the dorsal carina is serrated. The hind tibiae and tarsi are characterised by the presence of white hairs and setae. The supra-anal plate of the male exhibits a broad basal half, longitudinal grooves in the middle, and furrowed edges. The cerci of the male are short and conical in shape.
Epiphallus (
Figure 6H). The bridge is narrow, arched, and forms an obtuse angle; the ancorae are small, pointed inwards; the lophi are broad, wide, lobiform; the oval sclerites are small, roughly elongate, and strap-like; and the anterior projections are strongly reduced.
Phallic complex (
Figure 6I–K). It is characterised by an elongated, less robust form. The dorsal arch of the cingulum is U-shaped and open, with the anterior margin situated beneath the zygoma that is broadly emarginate in the midline. The apodemes of cingulum are more or less straight, running approximately parallel to each other distally and strongly exceeding the distal tip of the endophallic apodemes. The endophallus is characterised by its short, thread-like flexure. The endophallic valves are distinguished by their length and slenderness, exhibiting a wide and dorso-ventrally flattened base that extends almost to the tip of the ectophallic aedeagal sheath. The endophallic valves are deeply cupped and laterally directed, while the zygoma is notable for its pronounced sclerotisation. The rami are well-developed and sclerotised, as long as or slightly longer than their width in lateral view, extending to the ventral midline of the phallus. The ventral extremities are not fused nor overlapping. The ectophallic sheath is long. The aedeagus is projecting, large in size, long, only slightly curved upwards, and with almost straight margins and parallel tips. The aedeagal valves are slender and pointed, and are enveloped or covered by a thick, semitransparent sheath that is closely appressed to the endophallic valves. The gonopore sac is small, subtriangular, and approximately half of the endophallus is covered by it, as well as the endophallic sclerites ventrally.
Female (
Figure 6B,C,E,G,L). As the male, but larger, the valves of the ovipositor are small and short. The subgenital plate (
Figure 6C) is pentagonal in shape, diminutive in size, with truncated posterior margins; anterior apodemes of considerable length; egg-guide narrow and short; ventral pockets of the vaginal floor of significant size; copulatory bursa large in size, almost straight, gradually narrowing towards the front; each basivalvar sclerite exhibits minimal curvature, forming an obtuse angle. The spermatheca duct (
Figure 6L) is of a reduced length, and the base of the spermathecal duct opens directly at the apex of the copulatory bursa. The recurrent distal trunk of the lateral spermathecal diverticulum is five times longer than that of the proximal trunk.
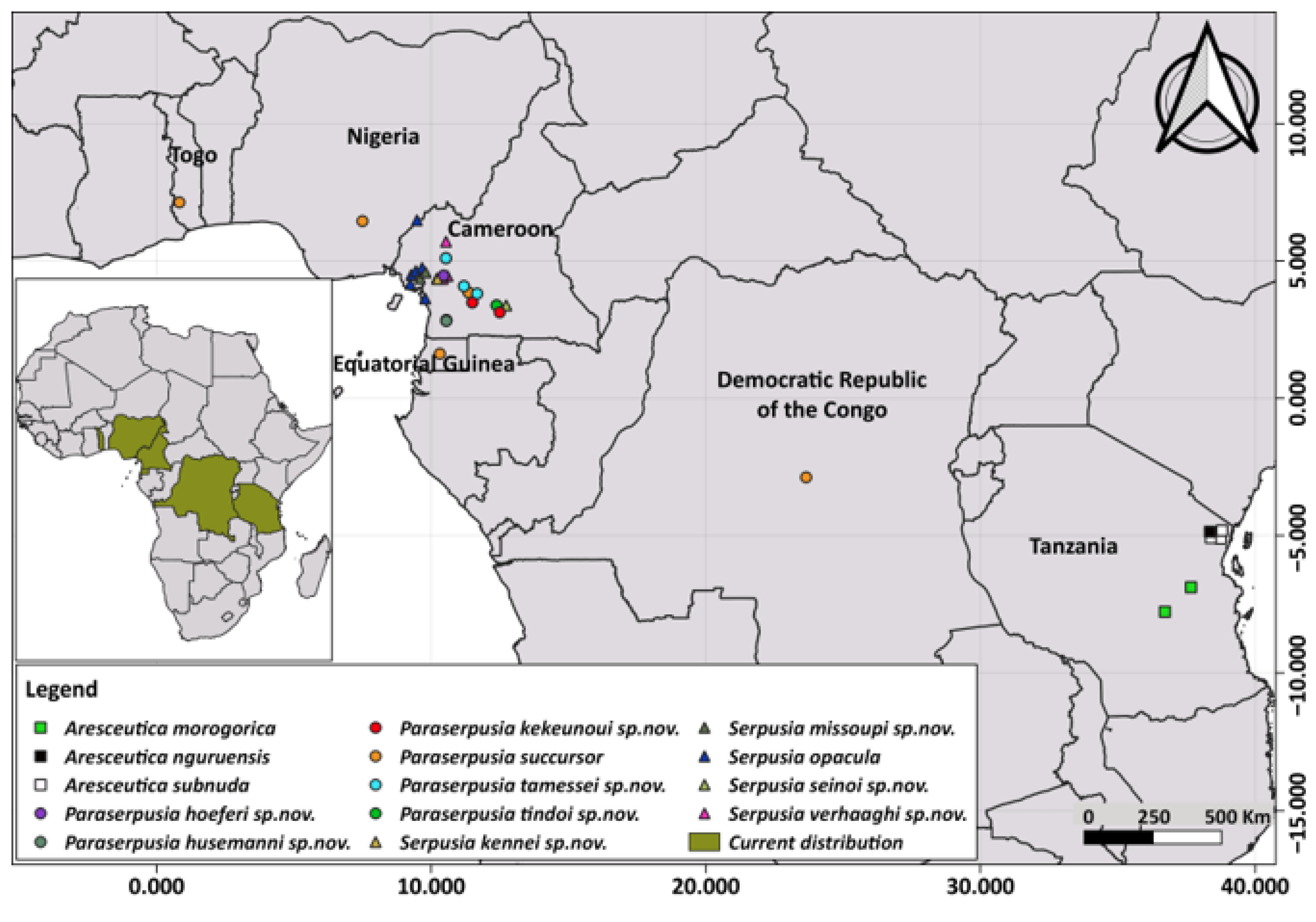
Distribution. Barombi Station, Buea, Kumba, Mouanko, Tombel, Cameroon; Nigeria.
Biology and Ecology. Serpusia opacula is present throughout the year in Cameroon’s humid forests. The species is presently confined to the regions of Cameroon that experience an equatorial climate, exhibiting a unimodal distribution of rainfall.
Material examined. Holotype. Cameroon • ♂; Bissoue, Ebo Forest, more or less stable forest habitat; 4°21′42″ N, 10°12′31″ E; 17 April 2022; J.A. Yetchom Fondjo leg.; SMNK, SMNK-ORTH-0000007. Paratypes. Cameroon • 1 ♀; Bissoue, Ebo Forest, more or less stable forest habitat; 4°21′42″ N, 10°12′31″ E; 17 April 2022; J.A. Yetchom Fondjo leg.; SMNK. Cameroon • 1 ♂, 3 ♀♀; Njuma, Ebo Forest, more or less stable forest habitat; 4°20′53″ N, 10°13′56″ E; 18 April 2022; J.A. Yetchom Fondjo leg.; SMNK.
Diagnosis.
Serpusia kennei sp. nov. is similar to
S. opacula (
Figure 6), but can be distinguished by the shorter elytra, which do not fully cover the tympanum and do not reach the posterior edge of the metanotum (vs. longer elytra, extending beyond the posterior edge of the metanotum and fully covering the tympanum in
S. opacula), and by the medium-sized hind legs (vs. large in
S. opacula). The following characteristics differentiate male genitalia: the apodemes of cingulum exhibit a slight curvature inwards at the apex, not reaching the tip of the endophallic apodemes (vs. having an almost straight apex and extending well beyond the tip of the endophallic apodemes in
S. opacula). The valves of cingulum are short (vs. long in
S. opacula), and the aedeagus is short (vs. large or elongate in
S. opacula), while the gonopore sac is large (vs. small in
S. opacula). The female genitalia are distinguished by the presence of a subgenital plate, which is characterised by broader anterior apodemes (vs. long in
S. opacula). Additionally, the spermatheca duct is notably elongated (vs. short in
S. opacula).
This species can be distinguished from S. seinoi sp. nov. by the shorter elytra, which do not reach the posterior margin of the tympanum (vs. longer, extending beyond the posterior margin of the tympanum in S. seinoi), and by the shiny black spots at the apex of the elytra, which are well separated and sometimes confluent in females (vs. fused into a single large shiny black spot in S. seinoi). The female genitalia are distinguished by their pentagonal subgenital plate, with truncate posterior margins (vs. hexagonal with rounded posterior margins in S. seinoi); each basivalvar sclerite exhibits minimal curvature, forming an obtuse angle (vs. forming an acute angle in S. seinoi).
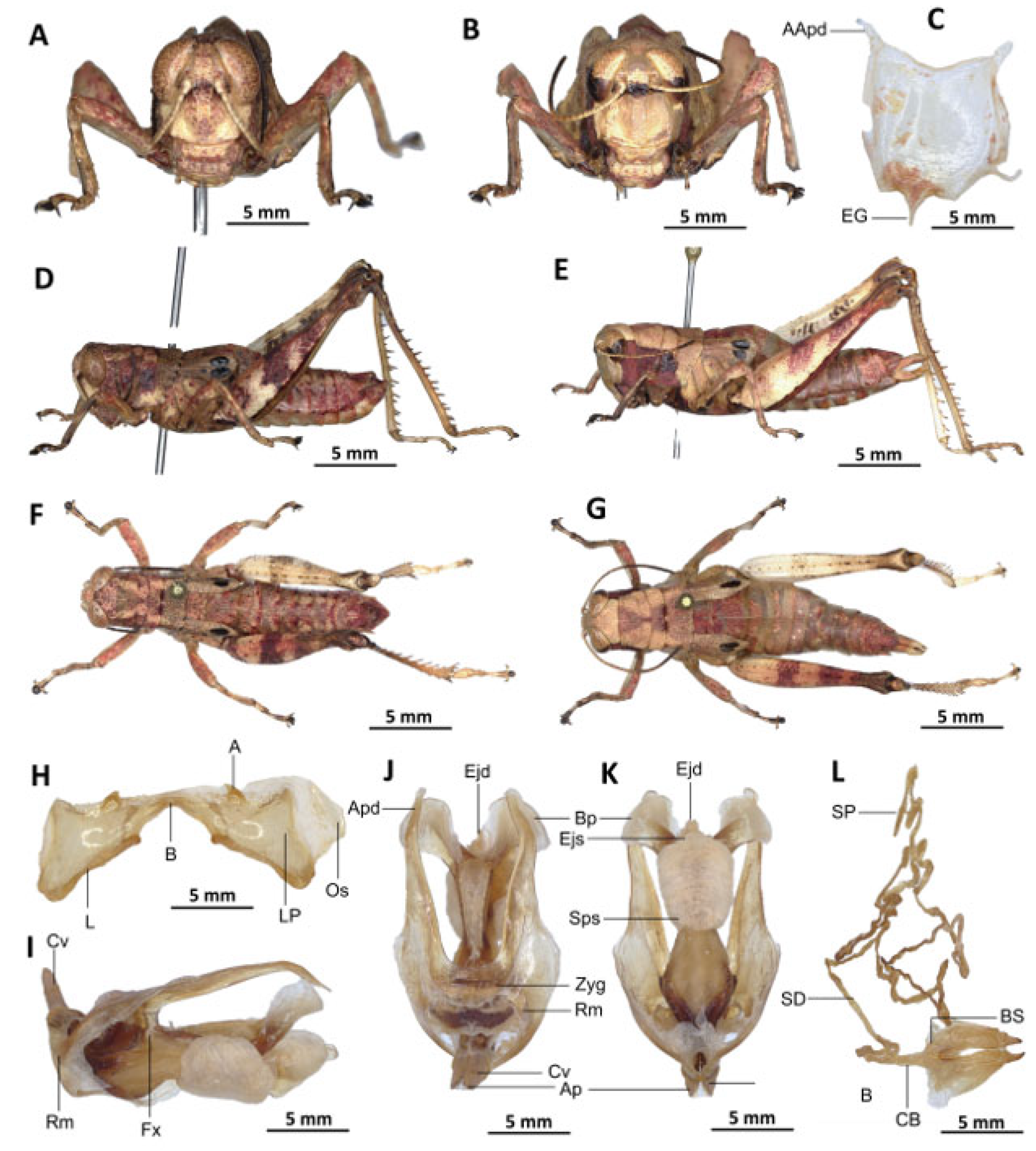
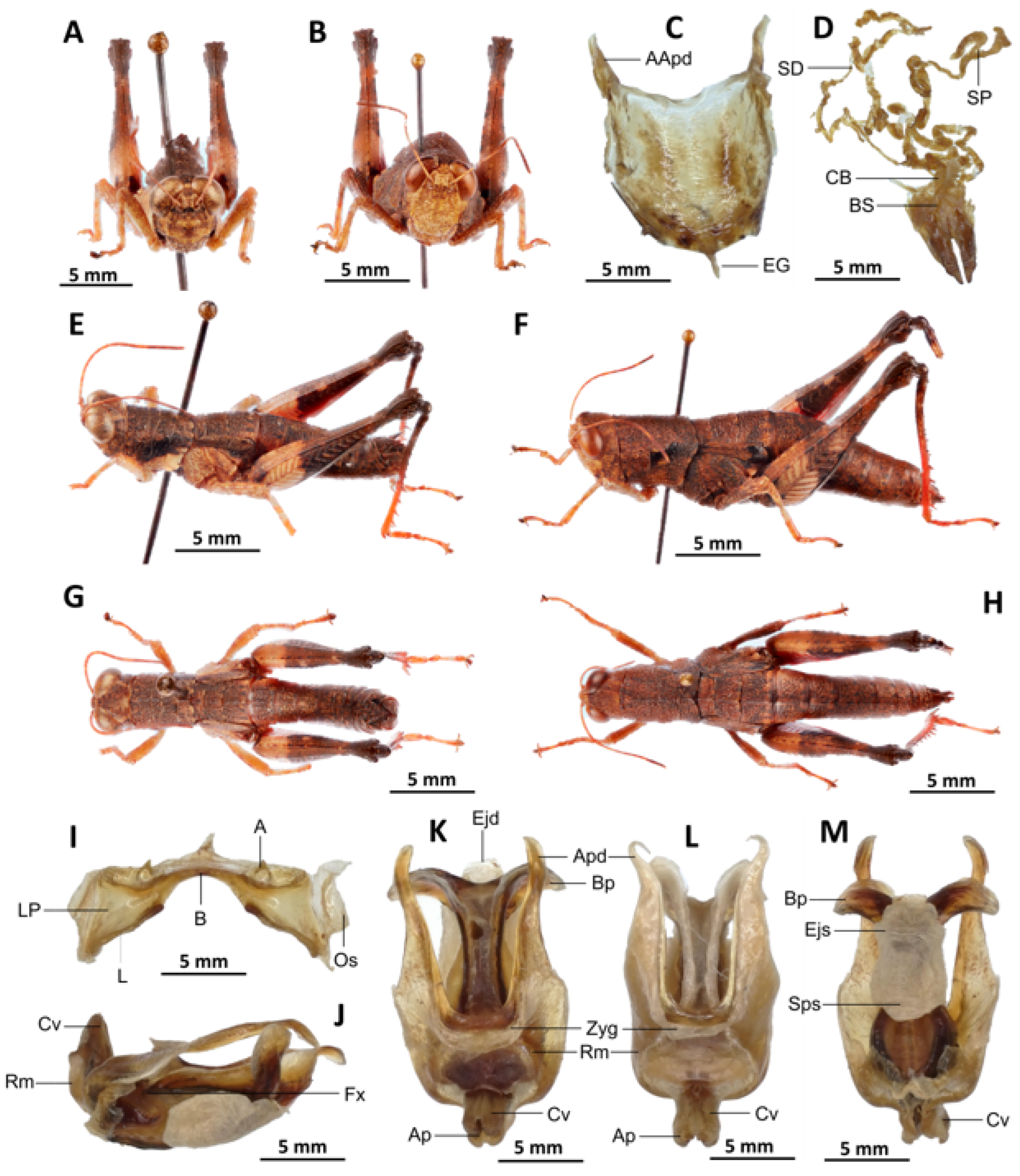
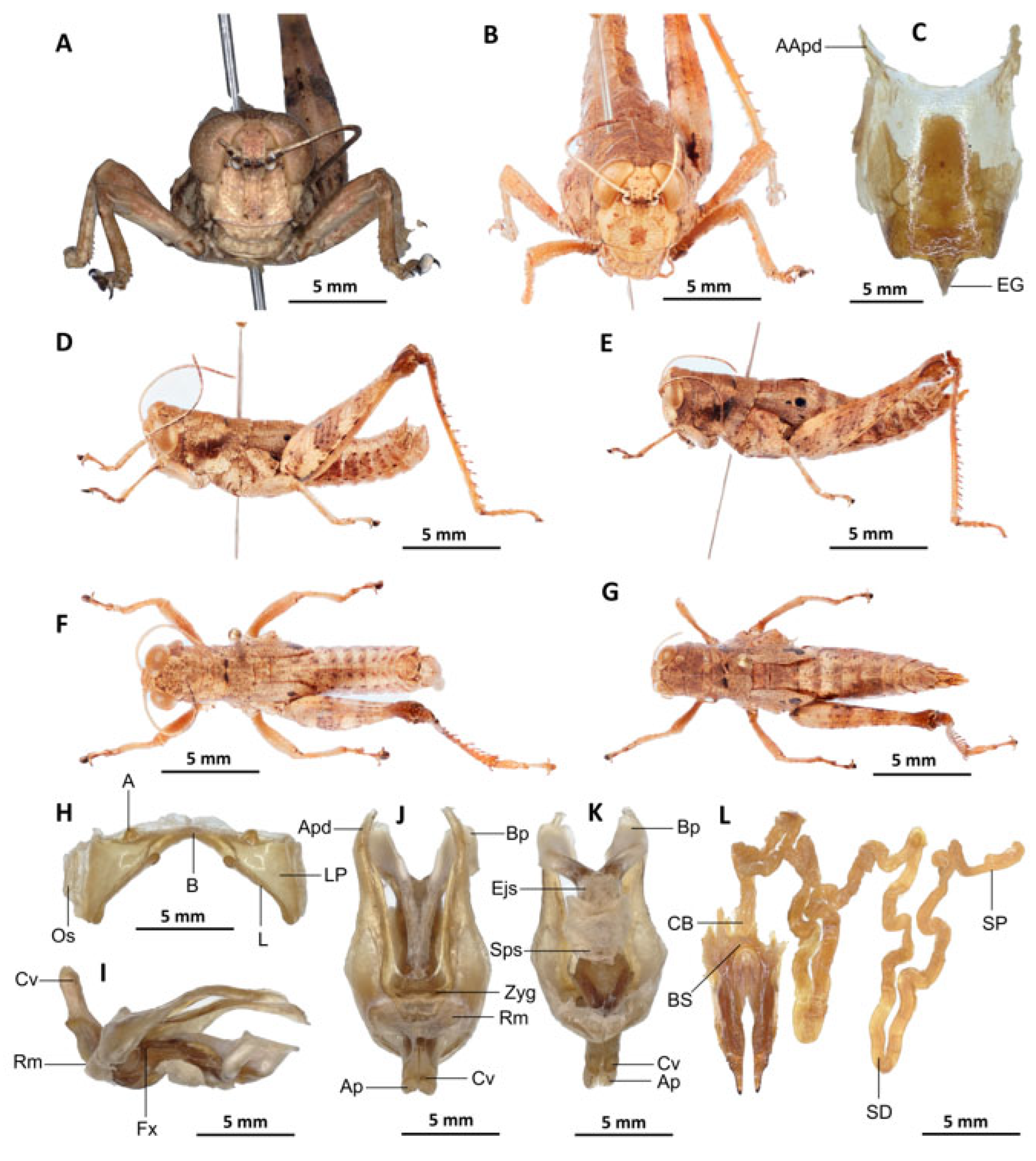
Description. Male. The general colouration is dark and opaque. The head is straight, and the fastigium of the vertex is flattened. The lateral lobes of the pronotum have a more or less clearly defined, irregular black spot. The median carina of the pronotum is perceptible and is crossed by three transverse grooves. The prozone is about three times longer than the metazona. The pronotum, mesonotum, metanotum and dorsal surface of the abdomen are strongly wrinkled and granular. The elytra are rudimentary and vestigial, and are extremely narrow and short, not reaching the posterior edge of the tympanum or the posterior edge of the metanotum. They possess two shiny black subapical spots, which are sometimes confluent in females. The hind femora are characterised by an olivaceous outer surface, a red inner surface, and a black plume in the apical half, with the basal third being black and the dorsal carina serrated. The apical half of the hind tibiae and tarsi are red, while the basal half exhibits a black plume. The hind tibiae and tarsi are adorned with white hairs and bristles.
Epiphallus (
Figure 7H). Bridge short, narrow, strongly arched, and forming an obtuse angle; ancorae small, pointed inwards towards the mid-line; lophi broad, wide, lobiform; oval sclerites small, roughly elongate, and strap-like; anterior projections strongly reduced.
Phallic complex (
Figure 7I–K). It is characterised by an elongated and slightly robust form. The dorsal arc of the cingular is U-shaped and closed, with the anterior margin situated beneath the zygoma and exhibiting a broad emargination along the midline. The apodemes of cingulum are notably long and predominantly straight, extending approximately parallel to each other in the distal region. A slight curvature is observed at the apex, and the apodemes do not extend to the distal tip of the endophallic apodemes. The endophallic apodemes are deeply cupped, laterally directed, and possess two channels with a U-shaped cross-section that extend ventrally and posteriorly to form the gonopore. The endophallus exhibits a thread-like flexure that is visible in the lateral view. The endophallic valves are characterised by their length, slenderness, width, dorso-ventral flattening at the base, lateral compression, and narrowing at the apex.
These valves extend almost to the tip of the ectophallic aedeagal sheath. The zygoma is sclerotised; the rami are well-developed and sclerotised. In the lateral view, the rami are longer than they are wide and do not extend to the ventral midline of the phallus. They are not fused nor overlapping, and their ventral extremities are linked by a transparent membrane. The ectophallic sheath is short, and the cingular valves are short and pointed. The aedeagus is characterised by its short, curved shape, with margins converging towards an obtuse tip. It is enveloped by a thick, semitransparent sheath, closely appressed to the endophallic valves. The gonopore sac is large, approximately covering two-thirds of the endophallus, and well covering the endophallic sclerites ventrally.
Female. Similar to the male, but larger; the valves of the ovipositor are short; the subgenital plate (
Figure 7C) is pentagonal in shape, small in size, with truncated posterior margins; anterior apodemes broad; egg-guide narrow, short; ventral pockets of the vaginal floor large in size; copulatory bursa almost straight, gradually narrowing towards the front; bottom of the copulatory bursa close to the arc of the basivalvar sclerites. The basivalvar sclerite exhibits minimal curvature, forming an obtuse angle. The spermatheca duct (
Figure 7L) is characterised by its considerable length, and the base of the spermathecal duct opens directly at the apex of the copulatory bursa. Notably, the recurrent distal trunk of the lateral spermathecal diverticulum is 15× longer than the proximal trunk.
Etymology. The species was named after Professor Martin Kenne, an important and recognized entomologist in Cameroon, for his dedication and scientific contribution to insect biodiversity.
Distribution. Bissoue, Njuma (Littoral region of Cameroon).
Biology and Ecology. Adults of this species can be observed throughout the year in more or less stable forests with dark undergrowth.
Material examined. Holotype. Cameroon • ♂; Kompina, less disturbed forests; 4°21′56″ N, 9°35′58″ E; 28 November 2018; J.A. Yetchom Fondjo leg.; SMNK, SMNK-ORTH-0000008. Paratypes. Cameroon • 1 ♀; Kompina, disturbed forests; 4°21′56″ N, 9°35′58″ E; 28 November 2018; J.A. Yetchom Fondjo leg.; SMNK. Cameroon • 1 ♀; Kompina, disturbed forests; 4°21′56″ N, 9°35′58″ E; 16 September 2018; J.A. Yetchom Fondjo leg.; SMNK. Cameroon • 1 ♀; Sole, disturbed forests; 4°35′0″ N, 9°48′0″ E; 28 February 2017; J.A. Yetchom Fondjo leg.; SMNK. Cameroon • 1 ♀; Sohock, disturbed forests; 5°42′11″ N, 10°32′34″ E; 3 April 2017; J.A. Yetchom Fondjo leg.; SMNK.
Diagnosis. Serpusia missoupi sp. nov. is similar to S. verhaaghi sp. nov. from which it differs by the following characters: pale spot on the lower outer edge of the lateral lobes of the pronotum barely visible to absent (vs. present and visible in S. verhaaghi); elytra with rounded apex (vs. truncate in S. verhaaghi). The male genitalia differ by their apodemes of cingulum, which are not parallel to each other, are strongly curved, sickle-like in over two-thirds of its apical part (vs. predominantly straight, extending approximately parallel to each other in the distal part, with the apical part sometimes strongly incurved, hook-like in S. verhaaghi sp. nov.); the aedeagus is only slightly curved upwards, and with almost straight margins and obtuse tips (vs. strongly curved upwards, with roughly flexuous margins, and broad and divergent tips in S. verhaaghi sp. nov.). The female genitalia differ only by their broad anterior apodemes (vs. narrow in S. verhaaghi).
The new species is also somewhat similar to
S. opacula (
Figure 6) in general colouration, from which it differs in the following characters: a medium body size (vs. slender, larger in
S. opacula); the presence of a pale spot on the lower lateral lobes of the pronotum is evident (vs. present but difficult to distinguish in
S. opacula); the elytra do not extend to the posterior edge of the metanotum, instead partially enshrouding the tympanum (vs. in
S. opacula, the elytra extend beyond the posterior edge of the metanotum, fully enclosing the tympanum). The male phalli are distinguished by their apodemes of cingulum which are not parallel to each other, are strongly curved, sickle-like in over two-thirds of its apical part (vs. they are more or less straight, running approximately parallel to each other distally in
S. opacula); the aedeagus is short, with obtuse tips (vs. the aedeagus is large, long, with parallel tips in
S. opacula). The female genitalia are distinguished by their broad and short anterior apodemes (vs. which are very long in
S. opacula), and the spermatheca duct is notably long (vs. short in
S. opacula).
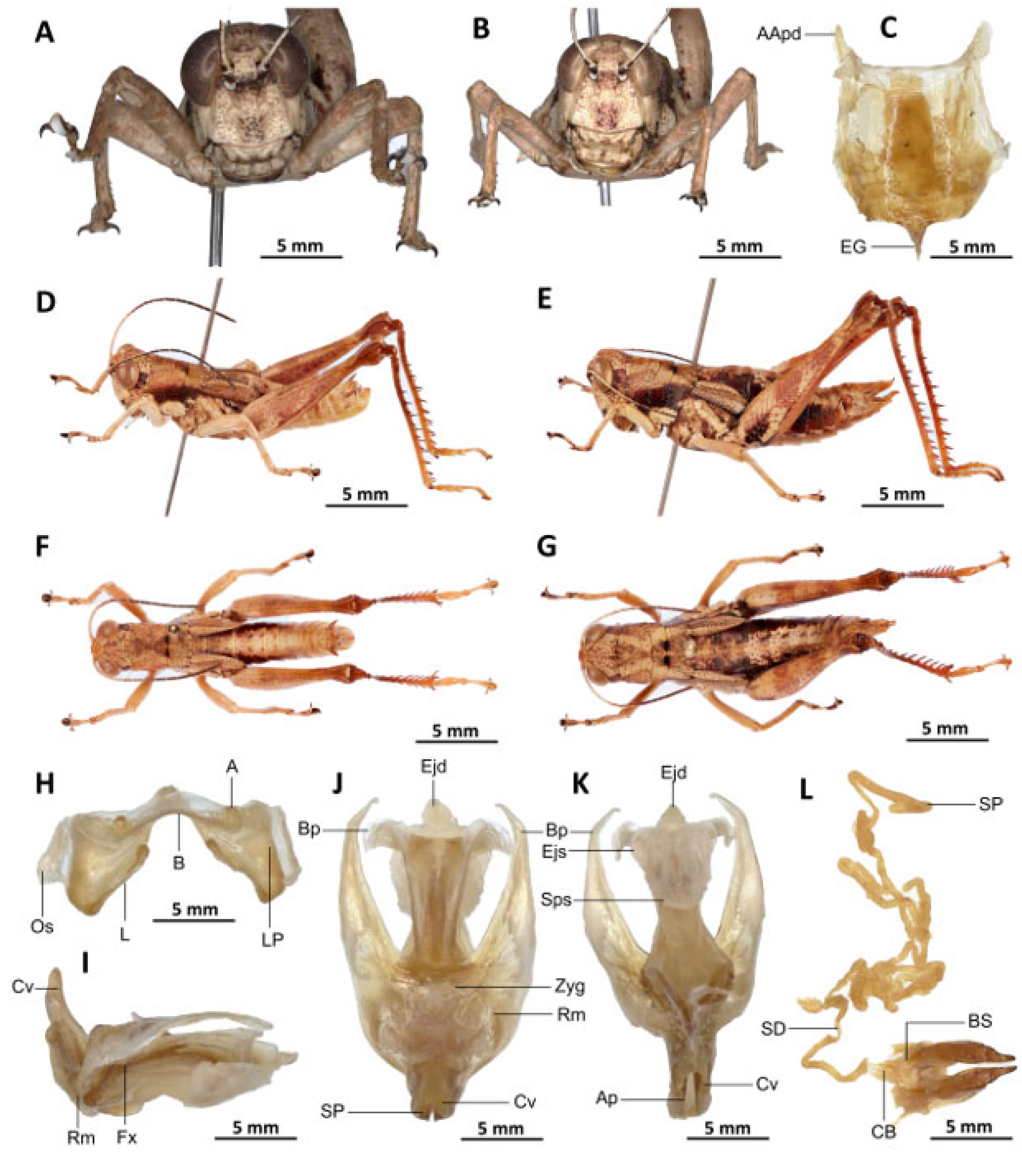
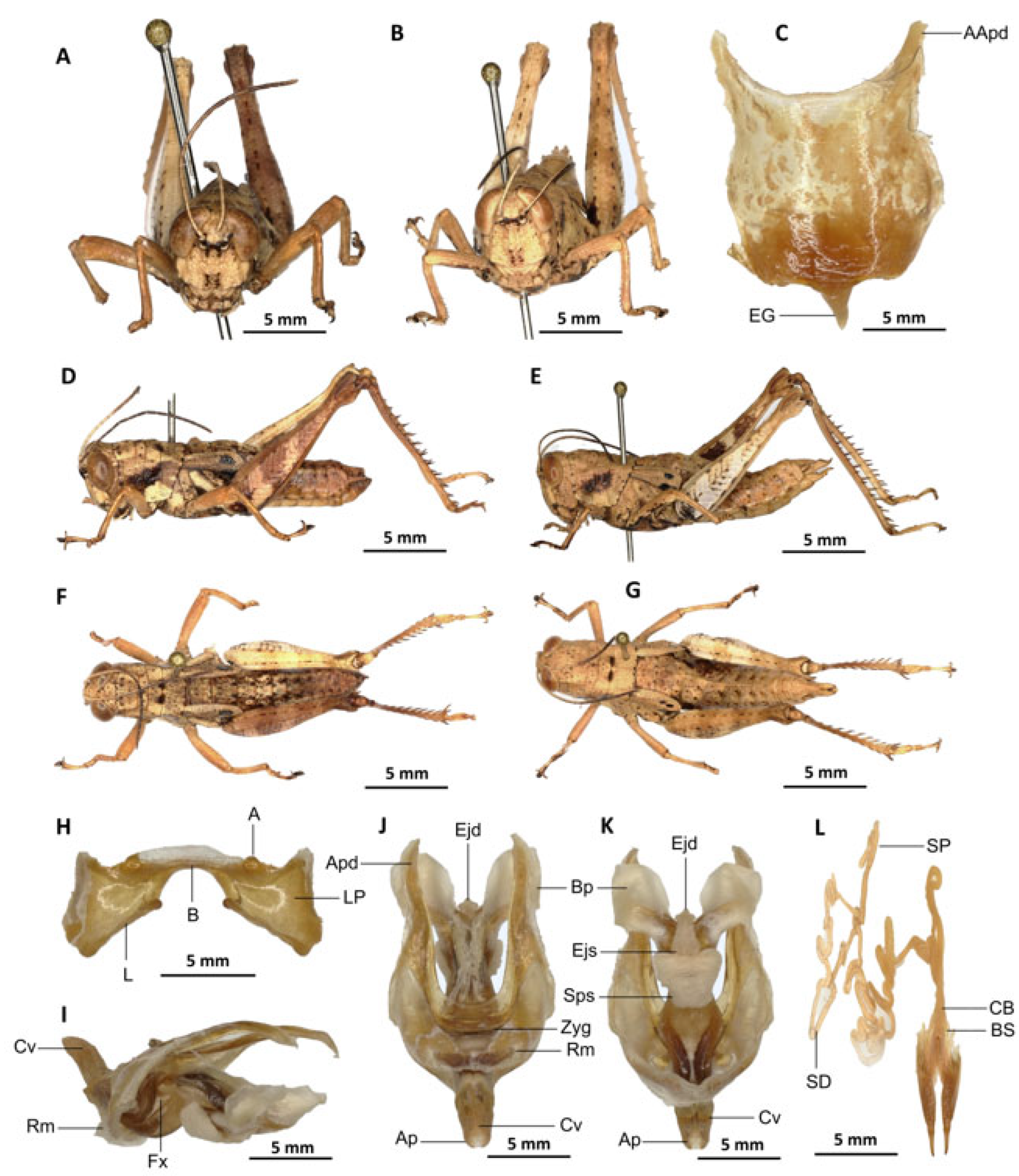
Description. Male. Of medium size. The head is straight in lateral view, the front is flattened, and the filiform antennae are longer than the head and pronotum combined. The general colouration is uniformly dark brown, the integument is opaque, and the pronotum, mesonotum, metanotum, and dorsal surface of the abdomen are strongly wrinkled and granular. The lateral lobes of the pronotum are adorned with a large, irregular, shiny black spot, and the pale spot on the lower outer edge of the lateral lobes of the pronotum is inconspicuous to absent. The elytra are rudimentary, vestigial, and extremely narrow with a rounded apex. They are adorned with two well-separated subapical shiny black spots, extending to the posterior edge of the metanotum. The elytra partially cover the tympanum and have a rounded apex. The outer surface of the hind femur is predominantly black, particularly dark on the lower outer zone, while the inner surface of the same structure exhibits a red hue, accompanied by a prominent dark black spot in the apical half. The basal third of the hind femur is dark black, and numerous small teeth mark its dorsal carina. Finally, the basal half of the hind tibia is black-purple.
Epiphallus (
Figure 8H). Bridge narrow, arched; ancorae small, slanted inwards; lophi broad, wide, lobiform; oval sclerites small, roughly elongated, and subtriangular; anterior projections strongly reduced.
Phallic complex (
Figure 8I–K). The phallic complex is characterised by an elongated structure. The dorsal arc of the cingulum is U-shaped, closed, and its anterior margin is situated beneath the zygoma, exhibiting a broad emargination along the midline. The apodemes of cingulum are of considerable length, not parallel to each other, strongly curved, sickle-like in over two-thirds of its apical part (
Figure 8I–K), and strongly exceeding the tip of the endophallic apodemes. The endophallic apodemes are characterised by their deeply cupped structure, and from their ventral margins, two channels with a U-shaped cross-section extend ventrally and posteriorly, thereby forming the gonopore processes. The endophallus exhibits a short, thread-like flexure. The endophallic valves are long and slender, wide and dorsoventrally flattened basally, but laterally compressed and narrow apically, and extend almost to the tip of the ectophallic aedeagal sheath. The zygoma is sclerotised, and the rami are well developed and sclerotised. In the lateral view, the rami are longer than they are wide, and they extend to the ventral midline of the phallus. The rami are not fused, nor overlapping, and their ventral extremities are linked by a transparent membrane. The ectophallic sheath is short and sometimes of medium size (
Figure 8J). The valves of cingulum are short and pointed. The aedeagus is short and dorsally directed, only slightly curved upwards, and with almost straight margins and obtuse tips. The aedeagal valves are enveloped or covered by a thick, semitransparent sheath, closely appressed to the endophallic valves. The gonopore sac is small.
Female. Similar to male but larger; cerci short, conical; supra-anal plate subtriangular, divided at the base and with a transverse rectilinear groove; valves of ovipositor short, 2.5× longer than wide in coalescence position.
The subgenital plate (
Figure 8C) is pentagonal, with truncated posterior margins; anterior apodemes broad and short; egg-guide thin and short; ventral pockets of the vaginal chamber large; copulatory bursa almost straight, gradually narrowing towards the front. The copulatory bursa is located at the base, near the arc of the basivalvar sclerites. Each basivalvar sclerite exhibits a slight curvature, forming an obtuse angle. The spermatheca duct (
Figure 8L) is characterised as simple and notably elongated, with the base of the spermathecal duct opening directly at the apex of the bursa.
Etymology. The species is named after Prof. Alain Didier Missoup in recognition of his work and achievements in the systematic and evolutionary biology of small mammals in Cameroon.
Distribution. Kompina, Sole (Littoral region of Cameroon) (
Figure 17).
Biology and Ecology. The species is present throughout the year in disturbed and undisturbed forests in the Littoral evergreen forests of Cameroon.
Material examined. Holotype. Cameroon • ♀; Somalomo, Dja Biosphere Reserve, forest habitat; 3°22′55″ N, 12°44′30″ E; 10 April 2022; J.A. Yetchom Fondjo leg.; SMNK-ORTH-0000009; SMNK. Paratypes. Cameroon • 1 ♀; Somalomo, Dja Biosphere Reserve, forest habitats; 3°22′55″ N, 12°44′30″ E; 10 April 2022; J.A. Yetchom Fondjo leg.; SMNK.
Diagnosis.
Serpusia seinoi sp. nov. is similar to
S. opacula (
Figure 6) in its general colouration. Still, it can be distinguished from
S. opacula specimens by its shorter tegmina, which do not reach the posterior margin of the metanotum (vs. extending well beyond in
S. opacula). Female genitalia differ by the following characteristics: subgenital plate hexagonal, with rounded posterior margins (vs. pentagonal, with truncated posterior margins in
S. opacula); anterior apodemes broad (vs. long in
S. opacula); each basivalvar sclerite barely curved, forming an acute angle (vs. forming an obtuse angle in
S. opacula); spermatheca duct long (vs. short in
S. opacula)
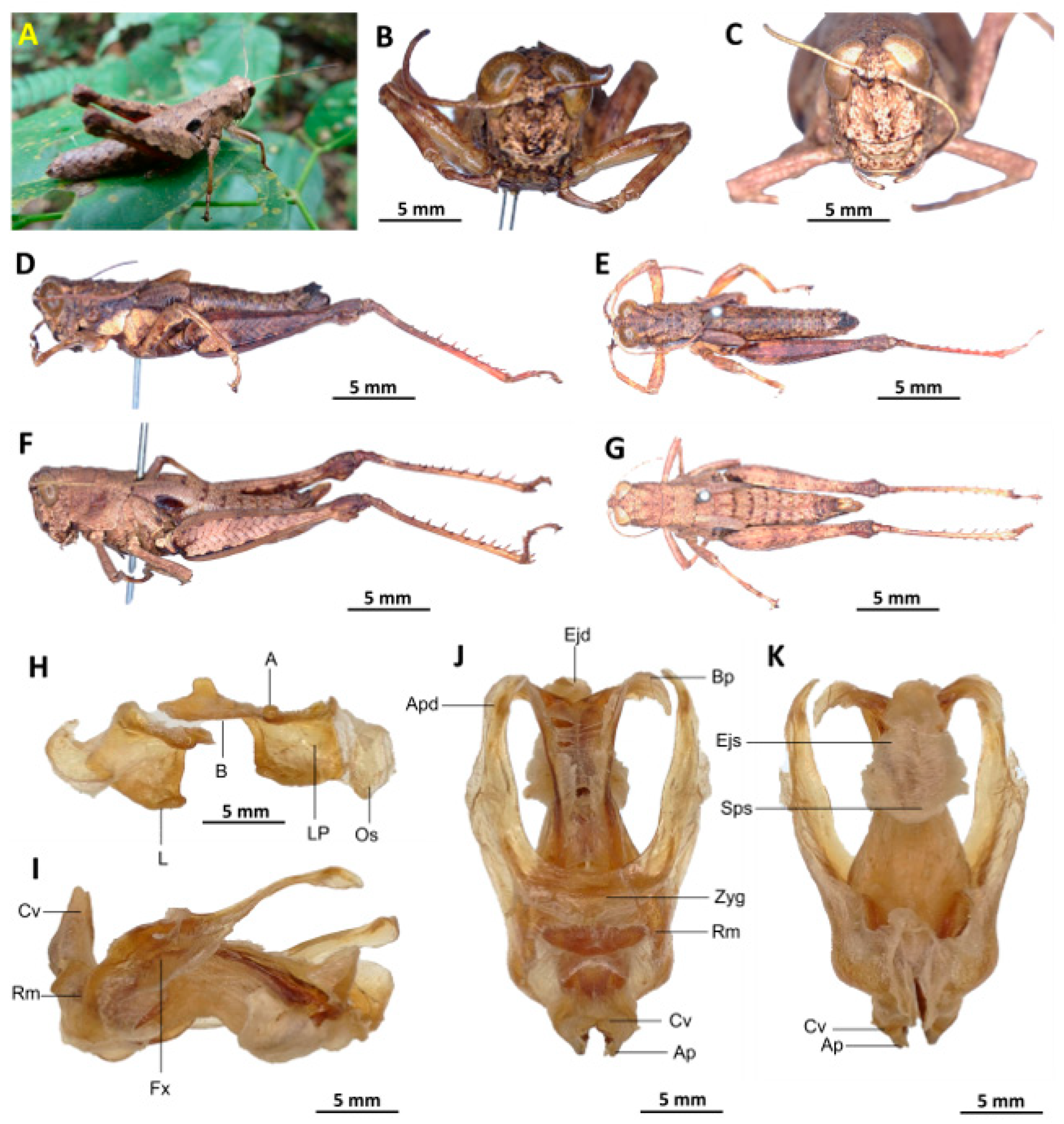
Description. Female. The general colouration is dark and opaque. The head is erect, and the fastigium of the vertex is flattened. The lateral lobes of the pronotum have a more or less clearly defined irregular black spot. The median carina of the pronotum is perceptible and crossed by three transverse grooves. The prozona is about three times longer than the metazona. The pronotum, mesonotum, metanotum, and dorsal surface of the abdomen are strongly wrinkled and granular. The elytra are rudimentary and vestigial, and are very narrow, with two shiny black subapical spots, which are sometimes confluent and do not reach the posterior margin of the metanotum. The hind femora have an olivaceous outer surface, a red inner surface, a black plume in the apical half, a black basal third, and a serrated dorsal carina. The apical half of the hind tibiae and tarsi are red, while the basal half has a black plume. The hind tibiae and tarsi are hairy and bristly.
Female genitalia. The valves of the ovipositor are short, and the subgenital plate (
Figure 9D) is hexagonal, small, and possesses rounded posterior margins. The anterior apodemes are broad, the egg-guide narrow and acute, the ventral pockets of the vaginal chamber are large, the copulatory bursa is straight and short, and the bottom of the copulatory bursa is close to the arc of the basivalvar sclerites. The basivalvar sclerite exhibits minimal curvature, forming an acute angle. The spermatheca duct (
Figure 9E) is characterised by its considerable length, and the base of the spermathecal duct opens directly at the apex of the copulatory bursa. Notably, the recurrent distal trunk of the lateral spermathecal diverticulum is 17× longer than the proximal trunk.
Figure 9.
Serpusia seinoi sp. nov. (A) female frontal view; (B) female lateral view; (C) female dorsal view; (D) female subgenital plate; (E) female spermatheca; (F) female ovipositor. BS: basivalvare sclerites; CB: corpulatory bursa; EG: egg-guide; SD: spermathecal duct; SP: spermathecal.
Figure 9.
Serpusia seinoi sp. nov. (A) female frontal view; (B) female lateral view; (C) female dorsal view; (D) female subgenital plate; (E) female spermatheca; (F) female ovipositor. BS: basivalvare sclerites; CB: corpulatory bursa; EG: egg-guide; SD: spermathecal duct; SP: spermathecal.
Etymology. The species was dedicated to Professor Richard Seino Akwa-Njoh in recognition of his valuable contribution to the advancement of research in Cytogenetics, Biodiversity, and Evolutionary Biology of Orthoptera grasshoppers in Cameroon.
Distribution. Somalomo, Dja Biosphere Reserve (Eastern Cameroon) (
Figure 17).
Biology and Ecology. Adults of this species occur throughout the year in the disturbed forests of Eastern Cameroon.
Material examined. Holotype. Cameroon • ♂; Iboti, Ebo Forest, forest habitats; 04°27,999′ N, 010°27,357′ E, 742 m a.s.l.; 7 January 2022; J.A. Yetchom Fondjo leg.; SMNK, SMNK-ORTH-0000010. Paratypes. Cameroon • 3 ♂♂, 1 ♀; Iboti, Ebo Forest, forest habitats; 04°27,999′ N, 010°27,357′ E, 742 m a.s.l.; 7 January 2022; J.A. Yetchom Fondjo leg.; SMNK. Cameroon • 1 ♂, 1 ♀; Bekob, Ebo Forest, forest habitats; 4°21′9″ N, 10°25′16″ E; 02 Mar. 2021; J.A. Yetchom Fondjo leg.; SMNK. Cameroon • 1 ♀; Djawara, more or less stable forest; 4°27′15″ N, 10°35′58″ E; 27 March 2017; J.A. Yetchom Fondjo leg.; SMNK.
Diagnosis. The new species exhibits external morphological characteristics similar to
S. opacula (
Figure 6). The distinction between the two species is determined by the presence of shiny black spots on the elytra, which are invariably fused (vs. though sometimes confluent in
S. opacula), the vestigial nature of the elytra with truncate apices (vs. rounded in
S. opacula), and the elytra not reaching the posterior margin of the metanotum (vs. extending beyond the posterior margin of the metanotum in
S. opacula). The male genitalia are characterised by the presence of a dorsal arch of cingulum closed (vs. open in
S. opacula). In addition, the apodemes of the cingulum are occasionally observed to possess strongly curved hook-like apices (vs. with almost straight apices in
S. opacula). The ectophallic sheath is characterised by its short or medium size, which is long in
S. opacula. The aedeagus is short, with roughly flexuous margins and broadened and divergent tips (vs. in
S. opacula, the aedeagus is long, more or less straight, with roughly parallel margins and tips). The female genitalia are distinguished by the presence of a subgenital plate, characterised by narrow and short anterior apodemes (vs. long in
S. opacula). The spermatheca duct is notably elongated, a feature that is absent in
S. opacula.
Description. Male. The species is characterised by a slight olivaceous, subunicoloured, opaque colouration and a shiny abdomen. The head is erect, and the fastigium of the vertex is flattened. The lateral lobes of the pronotum are ornamented with a more or less clearly defined irregular black spot, and the median carina of the pronotum is perceptible and crossed by three transverse furrows. The prozona is approximately three times as long as the metazona. The pronotum, mesonotum, metanotum, and dorsal surface of the abdomen are strongly wrinkled and granular. The elytra are rudimentary, vestigial, and very narrow, clearly reaching the posterior edge of the metanotum. The two shiny black spots are always fused. The inner face of the posterior femora is red, black in its apical half, and black in the basal third. The dorsal carina has teeth. The apical half of the hind tibiae and tarsi are red, while the basal half is plumed black. The hind tibiae and tarsi are characterised by the presence of white hairs and bristles. The male supra-anal plate is distinguished by a broad basal half, longitudinally grooved in the middle, and furrowed at the edges. The cerci of the male is short and conical.
Epiphallus (
Figure 10I). Bridge narrow, strongly arched; ancorae small, slanted inwards; lophi broad, wide, lobiform; oval sclerites small, roughly elongated, and strap-like; anterior projections strongly reduced.
Phallic complex (
Figure 10J–M). The phallic complex is characterised by an elongated and slightly robust structure. The dorsal arc of the cingulum is U-shaped, more or less closed, and its anterior margin is situated beneath the zygoma, exhibiting a broad emargination along the midline. The apodemes of cingulum are of considerable length and predominantly straight, extending approximately parallel to each other in the distal part, with the apical part sometimes strongly incurved, hook-like (
Figure 10K,L), and strongly exceeding the tip of the endophallic apodemes.
The endophallic apodemes are characterised by their deeply cupped structure, and from their ventral margins, two channels with a U-shaped cross-section extend ventrally and posteriorly, thereby forming the gonopore processes. The endophallus exhibits a short, thread-like flexure that is visible in the lateral view. The endophallic valves are long and slender, wide, and dorsoventrally flattened at the base, but laterally compressed and narrow at the apex, and extend almost to the tip of the ectophallic aedeagal sheath. The zygoma is sclerotised, and the rami are well developed and sclerotised. In the lateral view, the rami are longer than they are wide, and they extend to the ventral midline of the phallus. The rami are not fused, nor overlapping, and their ventral extremities are linked by a transparent membrane. The ectophallic sheath is short and sometimes of medium size (
Figure 10L). The valves of cingulum are short and pointed, and sometimes they are of medium size and broad. The aedeagus is characterised by its short and dorsally directed projection, its roughly flexuous margins, and its broad and divergent tips. The aedeagal valves are enveloped or covered by a thick, semitransparent sheath, closely appressed to the endophallic valves. The gonopore sac is large, roughly covering two-thirds of the endophallus and completely covering the endophallic sclerites ventrally, and situated more anteriorly.
Female. Similar to male, but larger; the valves of the ovipositor are short; the subgenital plate (
Figure 10C) pentagonal, small, with truncated posterior margins; anterior apodemes narrow and reduced in length; egg-guide filiform and short; ventral pockets of the vaginal floor reduced; copulatory bursa almost straight, gradually narrowing towards the anterior end; bottom of the copulatory bursa close to the arc of the basivalvar sclerites. The basivalvar sclerite exhibits minimal curvature, forming an obtuse angle. The spermatheca duct (
Figure 10D) is characterised by its considerable length, and the base of the spermathecal duct opens directly at the apex of the copulatory bursa. Notably, the recurrent distal trunk of the lateral spermathecal diverticulum is 15× longer than the proximal trunk.
Etymology. The species is named after Dr. Manfred Verhaagh, in recognition of his contribution to the biodiversity and species discovery of Tropical ant fauna.
Distribution. Iboti, Bekob, Sohock.
Biology and Ecology. This species occurs throughout the year in more or less disturbed forests in the coastal zones of Cameroon.
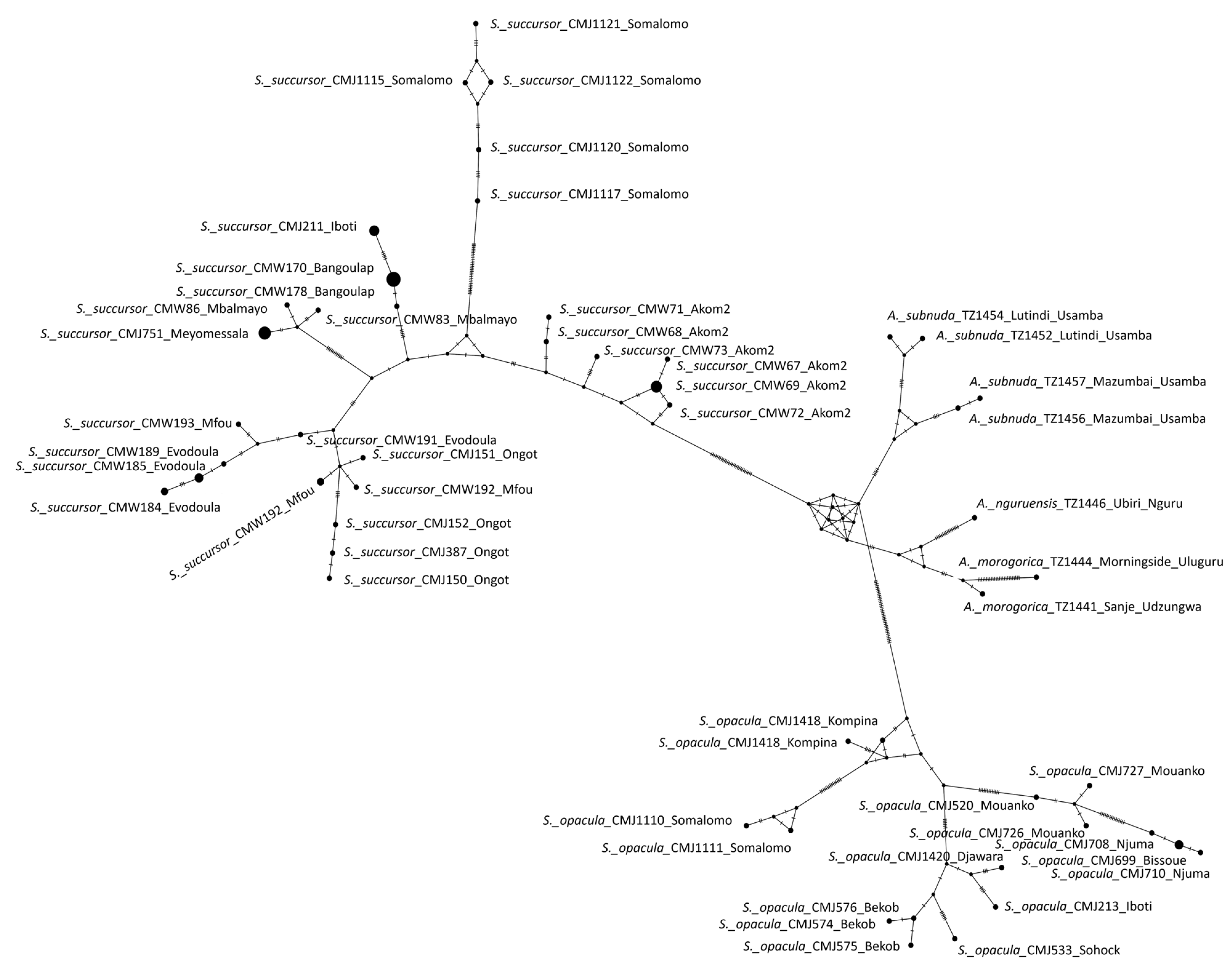
One of the five species of the genus Serpusia was described based on the female morphology. Here, we present two keys, one based on external morphology (including all five species) and one on the male internal phallic structures (including four species).
Elytra rudimentary, vestigial, narrow, with truncate apices ( Figure 7D,E)
|
| Serpusia kennei Yetchom & Wandji sp. nov. |
- -
| 2 |
- 2.
Elytra extending beyond the posterior margin of the metanotum ( Figure 6D,E)
|
| Serpusia opacula Karsch, 1891 |
- -
Elytra not reaching the posterior margin of the metanotum
| 3 |
- 3.
The two apical shiny black spots at the apex of the elytra are always separated, female subgenital plate hexagonal ( Figure 9D)
|
| Serpusia seinoi Yetchom & Wandji sp. nov. |
- -
The two apical shiny black spots at the apex of the elytra are confluent or fused
| 4 |
- 4.
The two apical shiny black spots at the apex of the elytra are about the same size ( Figure 10E,F)
|
| Serpusia verhaaghi Yetchom & Wandji sp. nov. |
- -
The internal spot is smaller ( Figure 8D,E)
|
| Serpusia missoupi Yetchom & Wandji sp. nov. |
- B.
Based on the anatomy of the phallus
| 2 |
- -
Dorsal arch of cingulum open, apodemes of cingular long, with more or less straight apices, and strongly exceeding the tip of the endophallic apodemes, ectophallic sheath and valves of cingulum always long, aedeagus long and slender, with almost straight and parallel margins and tip ( Figure 6I,J,K)
|
| Serpusia opacula Karsch, 1891 |
- 2.
Apodemes of cingulum slightly curved inwards at its apex, and not reaching the tip of the endophallic apodemes, ectophallic sheath and valves of cingulum of medium size, aedeagus short with oblique margins and converging tip ( Figure 7I–K)
|
| Serpusia kennei Yetchom & Wandji sp. nov. |
- -
Apodemes of cingular sometimes strongly curved, hook-like, or sickle-like, and strongly exceeding the tip of the endophallic apodemes
| 3 |
- 3.
Aedeagus with almost straight margins and obtuse tips ( Figure 8J–K)
|
| Serpusia missoupi Yetchom & Wandji sp. nov. |
- -
Aedeagus with roughly flexuous margins, broad and divergent tips ( Figure 10J–M)
|
| Serpusia verhaaghi Yetchom & Wandji sp. nov. |
3.2.3. Other Species
Here, we redescribe
S. catamita based on examined collection materials, whereas the original description of
S. blanchardi and
S. inflata by [
29,
30], respectively, is repeated and transcribed, as we lack specimens of these species.
Serpusia blanchardi,
S. catamita, and
S. inflata should be removed from
Serpusia and included in a separate genus. Molecular data are needed to provide insight into the systematic position of these three species.
Material examined. Togo • 1 ♂, 4 ♀♀; Bismarckburg; 20 September–31 October 1990; R. Büttner S. leg.; SMNK Stuttgart; Togo • 1 ♀; West Africa; 1984; Akem Mohr leg.; SMNK Stuttgart; Togo • 1 ♀; West Africa; 1980; Akem Mohr leg.; SMNK Stuttgart.
Diagnosis. Similar to S. opacula in shape and colouration. The elytra are broad, with an anterior or outer margin that is initially straight and then rounded (vs. in S. opacula, they are narrower and truncate at the anterior margin). The supra-anal plate has a longitudinal groove at the front with raised edges, in the middle of a strong and massive transverse carina, flat behind and, after a slight inflection of the lateral edge, on each side, quite flat in the middle and pointed towards the end (vs. in S. opacula, behind the strong transverse carina that closes the basal half, two strong longitudinal carinae converge towards the rear, giving the impression that the posterior half is suddenly very narrow, when in fact it is not).
Serpusia catamita is very similar and close to
S. inflata (
Figure 12A,B,D–G), but with slightly protruding fastigium, distinctly wavy and generally finely mottled with light brown and black posterior margin of pronotum (vs.
S. inflata has a flatter and wider fastigium, a more swollen prozona of pronotum, a more or less smooth and flat posterior edge of the pronotum); in
S. catamita, the most pronounced bulge on the outer margin of the elytra, where the black spot is also located, is more central than apical (vs. in
S. inflata, the elytra are rounded and broader).
Redescription. Male. The fastigium is slightly protruding forward; the antennae are one-fourth longer than the head and pronotum combined. The prozona of the pronotum is rather strongly convex, giving the metazona a relatively sunken appearance. The posterior margin of the pronotum is distinctly wavy and generally finely mottled with light brown and black. The prosternal tubercle is conical and pointed; the elytra are broad, with an anterior or outer margin that is initially straight and then rounded, protruding beyond the posterior margin of metanotum; hind tibiae light red; the supra-anal plate is broadly cordate, with acute apex; the cerci are slightly longer than the supra-anal plate, pointed; the supra-anal plate has a longitudinal groove at the front with raised edges, in the middle of a strong and massive transverse carina, flat behind and, after a slight inflection of the lateral edge, on each side, quite flat in the middle and pointed towards the end.
Male genitalia (
Figure 11H–K). The description of the phallus of
S. catamita is presented for the first time in this work. Its internal genitalia are similar to those of
Serpusia and
Aresceutica, but differing in some details: (i) the ancorae are slanted forwards, rather than inwards as in
Serpusia; (ii) the lophi are curved upwards, with angular apices; (iii) the phallic complex is strongly sclerotized compared to that of
Serpusia; (iv) the endophallic apodemes are more deeply cupped as in
Serpusia, but the ejaculatory sac, which is bounded by the gonopore processes, is strongly reduced and is situated more posteriorly in position under the basal endophallus as in
Aresceutica, rather than more anterioly as in
Serpusia; (v) the flexure of the endophallus is short, thread-like, as in
Serpusia; (vi) the apodemes of cingulum of
S. catamita are long and more or less straight, running roughly parallel to each other distally as in
Serpusia. The dorsal arc of the cingulum is U-shaped, as in
Serpusia; (vii) the anterior margin of the cingulum arch under the zygoma is broadly emarginate in the midline as in
Serpusia and
Paraserpusia gen. nov.; (viii) the valves of cingulum are slender and pointed as in
Serpusia and
Paraserpusia; (ix) the endophallic valves are long and slender, dorsoventrally flattened basally as in
Serpusia, but they are narrow basally than in the latter; (x) a complex ectophallic aedeagal sheath envelops the aedeagal valves and is closely appressed to the endophallic valves, to which it is attached.
Distribution. Centre, Bismarcksburg, Togo; Ghana.
Biology. Unknown.
Description [
30]. Male. The fastigium is flat, with a steep slope, transversal to the anterior, without a transversal stripe, and merging directly into the frontal stripe. The frontal bar is not deepened above the bases of the antennae, roughly punctate, then slightly deepened, with gradually converging carinae, not widened at the level of the lateral eye, but slightly widened below the lateral eye, irregular in structure, with very irregular lateral carinae, descending towards the clypeus. The antennae are one-fourth longer than the head and pronotum combined. The prozona of the pronotum is rather strongly convex, giving the metazona a relatively sunken appearance. The posterior margin of the pronotum is protruding in the shape of a blunt wing, with an almost smooth, barely wavy ridge. The prosternal tubercle is conical and pointed. The elytra are round-oval, of unequal length, from the left to the middle, from the right to the end of the first abdominal segment. The supra-anal plate is broadly cordate (heart-shaped), with a small, almost lobed bar at the base of each side; the cerci are slightly longer than the supra-anal plate, pointed in the shape of a strap; the subgenital plate is blunt, conical.
Colouration. Medium brown, head finely mottled with light spots, and the dorsal surface of prozona of the pronotum is slightly lighter in places; the lateral lobes near the upper margin (below the protuberance of the prozona) are washed, shiny, blackish, the posterior angles slightly lighter; the elytra are with a shiny black spot on the lower margin (seen at rest), about half the length of the elytra; the inner margin of hind legs is dirty red, the hind tibiae and basal tarsi are light red.
Female. Head similar to that of the male but larger; the antennae are slightly longer than head and pronotum combined; the protuberance of pronotum is swollen, especially on the lateral margins; the posterior margin is somewhat less distinct than in male, but still clearly broad, with a blunt, protruding angle and a slight depression in the middle; the elytra are broadly oval, with a slightly pointed apex at an obtuse angle, protruding somewhat beyond the first abdominal segment.
Colouration. Uniformly dark brown; elytra with a correspondingly much smaller variegated/shiny black spot.
Distribution. Kayima, Sierra Leone
Redescription. The elytra are very reduced, barely exceeding the posterior margin of the metanotum, not spotted; the hind femora with longer base, are less enlarged than in S. opacula and longer than in S. opacula; the antennae are very short.
Distribution. Liberia
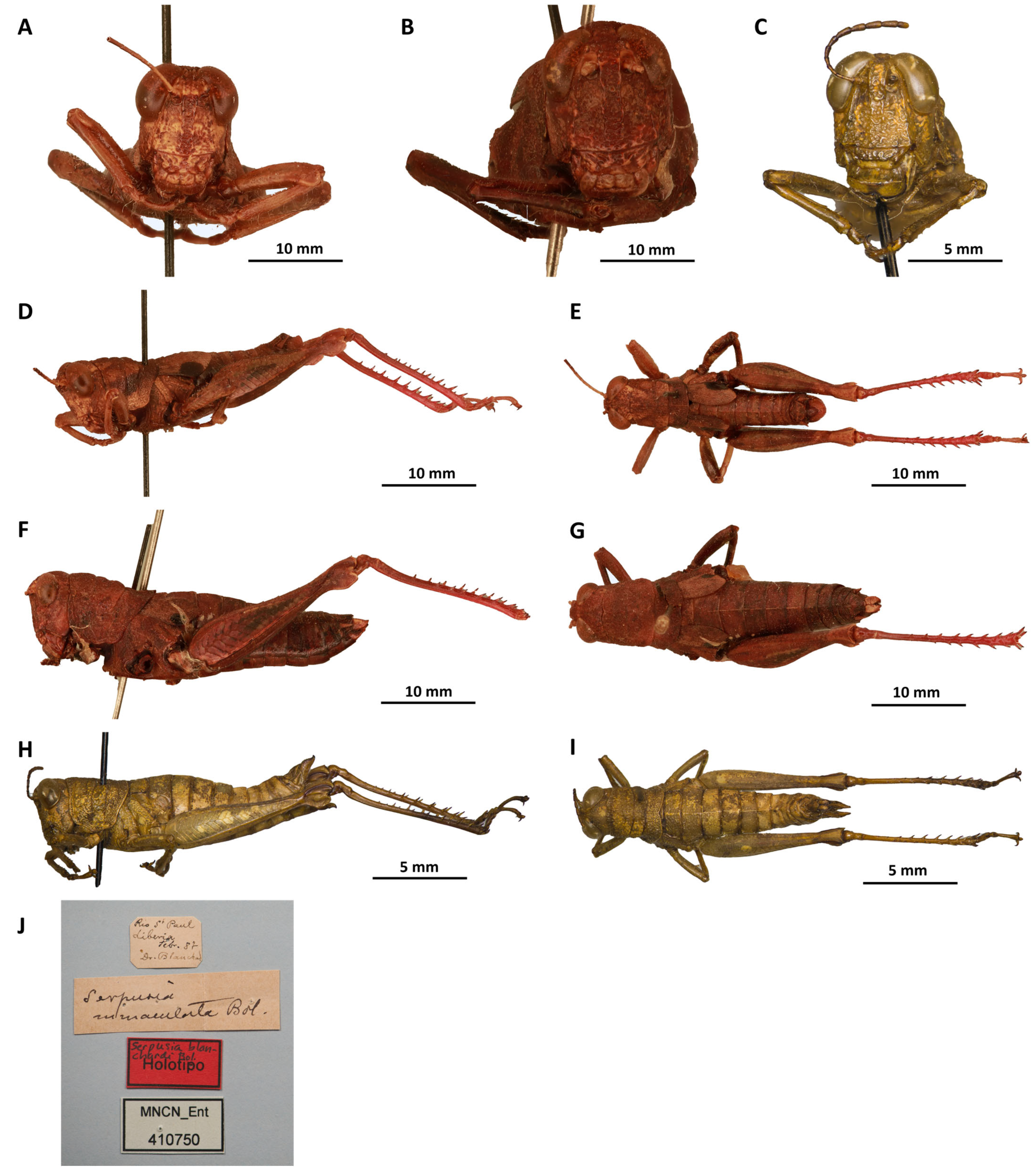
Remarks. This species is known only from its type, a single female, deposited in the Entomology Collection of the National Museum of Natural Sciences, Madrid, Spain (MNCN).
Remarks.
Serpusia catamita,
S. blanchardi, and
S. inflata are quite different from the true
Serpusia externally. For instance, (i)
S. catamita and
S. inflata have large lobiform tegmina as
Paraserpusia species, whereas the true
Serpusia have vestigial, rudimentary tegmina; (ii) the forewings in the true
Serpusia have two shiny black spots, whereas there is always only one in
S. catamita and
S. inflata; (iii) the posterior margin of the pronotum is distinctly incised and strongly emarginated in the middle, forming an acute angle in
Serpusia. In contrast, it is rounded and distinctly wavy, with a light brown mottling in the middle in
S. catamita, and more or less smooth and flat in
S. inflata. After examining the male internal genitalia of
S. catamita in search of additional differential characteristics, we found that the phalli of
S. catamita as a whole differ from those of
Serpusia in five details: (i) the ancorae of the epiphallus point directly inwards in
Serpusia, whereas they are slanted forwards in
S. catamita; (ii) the lophi of the epiphallus are large, lobiform, and rounded in
Serpusia, whereas they are curved upwards, with angular apices in
S. catamita; (iii) the phallic complex is robust and more strongly sclerotised in
S. catamita than in
Serpusia; (iv) the ejaculatory sac is large, roughly covering two-thirds of the endophallus and completely covering the endophallic sclerites ventrally in
Serpusia, whereas it is strongly reduced in
S. catamita; and (v) the endophallic valves are flattened basally in
Serpusia, whereas they are narrow basally in
S. catamita. In addition, the tegmina in
S. blanchardi are much reduced and not spotted as in true
Serpusia; the hind femora are longer basally and less enlarged than in
Serpusia; the antennae are shorter than the head and pronotum together in
S. blanchardi, whereas they are longer than the head and pronotum together in
Serpusia. Based on these consistent morphological differences, we therefore suggest removing
S. catamita and
S. inflata from
Serpusia and including them in a separate genus, as previously proposed by [
4].
3.2.4. The Genus Paraserpusia gen. nov.
Type species: Paraserpusia succursor (Karsch, 1896), by present designation.
Ptemoblax succursor Karsch, 1896
Serpusia succursor (Karsch, 1896)
Material examined. Cameroon • 17 ♂♂, 11 ♀♀; Ongot, disturbed forests and forest edges; 3°51′0″ N, 11°22′1″ E; 15 June 2020; J.A. Yetchom Fondjo leg.; SMNK. Cameroon • 16 ♂♂, 13 ♀♀; Ongot, disturbed forests and forest edges; 3°51′0″ N, 11°22′1″ E; 5 December 2021; J.A. Yetchom Fondjo leg.; SMNK. Cameroon • 7 ♂♂, 3 ♀♀; Ongot, in disturbed forests and forest edges; 3°51′0″ N, 11°22′1″ E; 20 March 2022; J.A. Yetchom Fondjo leg.; SMNK.
Diagnosis. Paraserpusia succursor is similar to P. hoeferi sp. nov. in general coloration, but can be distinguished by the following characteristics: tegmina less broad, only reaching the anterior margin of the metanotum (vs. very broad, strongly widened towards the apex and fairly reaching the posterior margin of the metanotum in P. hoeferi sp. nov.; elytra with a rounded apex (vs. with a pointed or acute apex in P. hoeferi sp. nov.). Male genitalia differ by its aedeagus, which is of medium size with roughly divergent margins and divergent (vs. long with almost straight margins and parallel tip in P. hoeferi sp. nov.); female genitalia differ by its narrow egg-guide (vs. broad in P. hoeferi).
Paraserpusia succursor is identical to P. kekeunoui sp. nov. in general coloration, but can be distinguished by the following features: elytra subpointed more enlarged with a rounded apex (vs. slightly enlarged towards the apex in P. kekeunoui sp. nov.); inner subapical spot small or not reduced, almost the same size as the outer apical spot (vs. much reduced, rounded and smaller than the outer apical spot in P. kekeunoui sp. nov.); posterior margin of pronotum incised in the middle (vs. almost straight, not incised in P. kekeunoui sp. nov.); male genitalia differ by its dorsal arc of cingulum open (vs. close in P. kekeunoui sp. nov.); apodemes of cingulum slightly curved ventrally at its apex, and only reaching the tip of the endophallic apodemes (vs. almost parallel or slightly orthogonally arranged basally, converging apically towards an obtuse apex, long fairly exceeding the tip of the endophallic apodemes in P. kekeunoui sp. nov.); endophallic apodemes small (vs. endophallic apodemes large in P. kekeunoui sp. nov.); aedeagus of medium size, and with roughly divergent margins and broad tip (vs. long, with abruptly oblique margins and acute in P. kekeunoui sp. nov.).
Paraserpusia succursor is similar to
P. tindoi sp. nov. (
Figure 18) in general coloration, but can be easily distinguished by several characteristics: the male cercus is long (vs. short in
P. tindoi); the elytra have two shiny black spots (vs. no shiny black spots in
P. tindoi sp. nov.); the apex of the elytra is slightly widened and rounded (vs. apex of the elytra is narrowed and slightly truncated in
P. tindoi sp. nov.); male genitalia differ by its elongate phallic complex (vs. less elongate and slightly robust in
P. tindoi sp. nov.); apodemes of cingulum only reaching the tip of the endophallic apodemes (vs. fairly exceeding the tip of the endophallic apodemes in
P. tindoi sp. nov.); aedeagus short or of medium size, and with roughly divergent margins and broad tip (vs. aedeagus long, with roughly convergent margins and rounded tip in
P. tindoi sp. nov.).
Redescription. Male. Antennae black, filiform, longer than the head and pronotum combined; pronotum with lateral carinae plumed black, median carina crossed by three transverse furrows, the typical furrow strongly indented in the middle, with a deep concavity on each side; lateral lobes of the pronotum with a broad, shiny black stripe not reaching the anterior margin, inferior-external half pale, anterior and posterior margins with numerous pale lines; pale metathoracic episternites; elytra lobiform, with subparallel margins, slightly enlarged towards the apex, rounded apex, slightly exceeding the posterior margin of the metanotum, and adorned with two shiny black spots on the outer apical and inner subapical sides, spots sometimes fused in males; lower inner surface, inner basal half of posterior femora dark red, apical half blackish or dark; posterior tibiae with basal half black and apical half red; male cercus short conical, with flattened base and pointed apex, not extending beyond the extremity of the abdomen; male supra-anal plate trigonal, with dorsal surface provided with longitudinal carinae and a transverse groove.
Epiphallus (
Figure 13H). Bridge narrow, arched; ancorae small, slanted inwards towards the mid-line; lophi broad, wide, lobiform; oval sclerites small, roughly elongate and strap-like; anterior projections strongly reduced.
Phallic complex (
Figure 13I–K). Elongate, small; dorsal arc of cingulum U-shaped, open, its anterior margin not emarginate; the apodemes of cingulum are long and more or less straight, running roughly parallel to each other distally, but slightly curved ventrally at its apex, and only reaching the tip of the endophallic apodemes; endophallic apodemes deeply cupped, and with two channels with a U-shaped cross section running ventrally and posteriorly from their ventral margins to form the gonopore process; endophallic apodemes laterally directed; the flexure of the endophallus is short and thread-like; zygoma only slightly sclerotised; the anterior margin of the cingular arch under the zygoma is broadly emarginate in the midlinerami well developed, sclerotised somewhat, longer than its wide in lateral view, and extending to the ventral midline of the phallus, not fused nor overlaping at their ventral extremities; ectophallic sheath short; aedeagus projecting, of medium size, slightly curved, antero-dorsally directed, and with roughly divergent margins and broad tip; aedeagal valves enveloped/covered by a thick semitransparent sheath, closely appressed to the endophallic valves; endophallic valves long and slender, wider and dorsoventrally flattened basally, but laterally compressed and narrow apically.
They extend almost to the tip of the ecotophallic aedeagal sheath. The valves of cingulum are slender and pointed; the gonopore sac is large, roughly covering the 2/3 of the endophallus and well covering the endophallic sclerites ventrally; it extends more anteriorly up to the U-shaped cross section.
Female. Similar to male, but larger; valves of ovipositor of normal shaped, long; cercus short conical; subgenital plate (
Figure 13C) hexagonal, long, with truncated posterior margins; anterior apodemes short and broad; egg-guide short, narrow; ventral pockets of the vaginal floor large; copulatory bursa long, almost straight, with parallel margins; bottom of the copulatory bursa close to the arc of the basivalvar sclerites; each basivalvar sclerite barely curved, forming a round angle; Spermatheca duct (
Figure 13L) simple, of medium size; the base of the spermathecal duct opening directly at the apex of the copulatory bursa.
Distribution. Cameroon (Ongot, Centre region); Democratic Republic of Congo; Nigeria; Togo.
Habitat. This species occurs in undisturbed and disturbed forests, and in agroforests of the humid zones of Cameroon.
Biology and Ecology. It can be observed throughout the year in its natural habitats in Cameroon’s humid forests. In Cameroon, the species has a narrow distribution range.
Material examined. Holotype. Cameroon • ♂; Iboti, in the Ebo Forest; 04°27.999′ N, 010°27.357′ E, 742 m a.s.l.; 7 January 2022; J.A. Yetchom Fondjo leg.; SMNK, SMNK-ORTH-0000011.
Paratypes. Cameroon • 4 ♂♂, 2 ♀♀; ♂; Iboti, in the Ebo Forest; 04°27.999′ N, 010°27.357′ E, 742 m a.s.l.; 7 January 2022; J.A. Yetchom Fondjo leg.; SMNK.
Diagnosis.
Paraserpusia hoeferi sp. nov. is similar to
P. succursor (
Figure 13) in general coloration, but can be distinguished by the following characteristics: tegmina very broad, strongly widened towards the apex and reaching the posterior margin of the metanotum (vs. less broad, only reaching the anterior margin of the metanotum in
P. succursor); elytra with a pointed or acute apex (vs. a rounded apex in
P. succursor). Male genitalia differ by its aedeagus, which is cylindrical, long, with almost straight margins parallel tip (vs. short or of medium size, with roughly divergent margins in
P. succursor); female genitalia differ by its broad egg-guide (vs. narrow in
P. succursor).
Description. Male. Tegument dark brown; fastigium of vertex flattened or short; frons pale; frontal sides speckled with black in front of and behind the eyes; antennae black, filiform, longer than the head and pronotum combined; pronotum with lateral carinae plumed with black, median carinae slightly tectiforme, crossed by three transverse furrows, the typical furrow strongly indented in the middle and bordered by two deep concavities, one on each side; lateral lobes of the pronotum with a broad shiny black band not reaching the anterior margin, pale infero-external half, anterior and posterior margins with numerous pale spots; pale metathoracic episternites; elytra lobiform, greatly enlarged towards the apex, with a pointed or acute apex and exceeding the posterior margin of the metanotum, and reaching the posterior margin of the first abdominal tergite, and adorned with two shining black spots on the outer apical and inner subapical sides; on the inner side, the inner basal half of the posterior femora dark red, the apical half black or dark; posterior tibiae with basal half black and apical half red; male cercus short conical, with flattened base and pointed apex, not extending beyond the extremity of the abdomen; male supra-anal plate trigonal, with dorsal surface provided with longitudinal carinae and a transverse groove.
Epiphallus (
Figure 14H). Bridge narrow, slightly more open, forming a more or less reflex angle; ancorae slanted inwards towards the mid-line; lophi broad, wide, lobiform; oval sclerites small, roughly elongate, and strap-like; anterior projections strongly reduced.
Phallic complex (
Figure 14I–K). Long; the dorsal arc of cingulum is U-shaped, open, its anterior margin under the zygoma is broadly emarginate in the midline; apodemes of cingulum are long and more or less straight, running roughly parallel to each other distally, slightly curved inwards at its apex, and only reaching the tip of the endophallic apodemes; the endophallic apodemes are more deeply cupped, and from their ventral margins two channels with a U-shaped cross section run ventrally and posteriorly to form the gonopore processes; the ejaculatory sac is situated more anteriorly; the zygoma sclerotised; the flexure of the endophallus is thread-like; the rami well developed, sclerotised, longer than its wide in lateral view, and extending to the ventral midline of the phallus, not fused nor overlaping at their ventral extremities; the valves of cingulum are slender and pointed, extending along the upper margin of the endophallic valves; ectophallic sheath of short; the endophallic valves are long and slender, wider and more dorsoventrally flattened basally; aedeagus projecting, long, antero-dorsally directed, with almost straight margins and parallel and broaded tip; aedeagal valves enveloped/covered by a thick semitransparent sheath, closely appressed to the endophallic valves; gonopore sac large, roughly covering the 1/2 of the endophallus and well covering the endophallic sclerites ventrally.
Female. Similar to male, but larger; cercus short conical; ovipositor of medium size; subgenital plate (
Figure 14C) hexagonal, small, with truncated posterior margins; anterior apodemes short and broad; egg-guide short, broad; ventral pockets of the vaginal floor large; copulatory bursa long, almost straight.
Bottom of the copulatory bursa close to the arc of the basivalvar sclerites; each basivalvar sclerite barely curved, forming around angle; spermatheca duct (
Figure 14L) simple, long; the base of the spermathecal duct opening directly at the apex of the copulatory bursa.
Etymology. The species is named after Dr. Hubert Hoefer, in recognition of his contribution to the biodiversity and species discovery of Tropical and European spider fauna.
Distribution. Iboti, Ebo Forest, Littoral region of Cameroon (
Figure 19).
Habitat. This species is associated with the bare ground in undisturbed and disturbed forests of the humid zones of Cameroon.
Biology and Ecology. It can be observed throughout the year in forest edges in the Ebo Forest zones.
Remarks. Three individuals of the juvenile stage were collected in the same habitat and locality.
Material examined. Holotype. Cameroon • ♂; Akom2, disturbed Forest; 2°49′4″ N, 10°33′9″ E, 470 m a.s.l.; 3 July 2024; A.C. Wandji leg.; SMNK, SMNK-ORTH-0000012. Paratypes. Cameroon • 9 ♂♂, 12 ♀♀; Akom2, disturbed Forest and cocoa farms; 2°49′4″ N, 10°33′9″ E, 470 m a.s.l.; 3 July 2024; A.C. Wandji leg.; SMNK.
Diagnosis. The new species P. husemanni sp. nov. is similar to P. kekeunoui sp. nov. from which it can be easily distinguished by: elytra with almost straight apex forming a right angle (vs. elytra with rounded apex in P. kekeunoui); elytra barely reaching the front edge of the first abdominal segment (vs. elytra extending well beyond the front edge of the first abdominal segment in P. kekeunoui sp. nov.); posterior margin of pronotum curved in the middle (vs. almost straight, not curved in P. kekeunoui sp. nov.); male genitalia differ by its long aedeagus, with almost straight or only slightly oblique margins and an obtuse tip (vs. long, with margins abruptly oblique and sharply narrowing towards an acute apex in P. kekeunoui sp. nov.); the gonopore sac is small, and is situated more anteriorly (vs. of medium size, is situated more posteriorly in position under the basal endophallus in P. kekeunoui sp. nov.).
The new species P. husemanni sp. nov. is also similar to P. tindoi sp. nov. in general coloration, but can be easily distinguished by a number of characteristics: the elytra are decorated with two conspicuous shiny black spots at the apical and subapical ends (vs. elytra without shiny black spots in P. tindoi sp. nov.); elytra barely reaching the front edge of the first abdominal segment (vs. elytra extending beyond the front edge of the first abdominal segment in P. tindoi sp. nov.); apex of elytra almost straight, forming a right angle with the lower margin (vs. apex of elytra truncate, obtuse to rounded in P. tindoi sp. nov.); male genitalia differ by its elongated phallic complex (vs. slightly robust in P. tindoi sp. nov.); apodemes of cingulum almost parallel or slightly orthogonally arranged, long (vs. curved, almost forming a horseshoe-like profile basally, oblique apically in P. tindoi sp. nov.); endophallic apodemes large/wide (vs. small in P. tindoi sp. nov.); aedeagus conical, long, slightly curved upwards, with almost straight or only slightly oblique margins narrowing towards the obtuse tip (vs. cylindrical, long, strongly curved, and with converging margins and rounded tip in P. tindoi sp. nov.); the gonopore sac is situated more anteriorly (vs. it is situated more posteriorly in position under the basal endophallus in P. tindoi sp. nov.).
Description. Male. Fastigium of the vertex flattened; tegument pale, plume of black; fros pale; subocular facial spot single, rounded, central; antenna filiform, longer than the head and pronotum combined in both sexes; median carina of the pronotum obtuse, tectiform, crossed by three transverse furrows, the typical furrow bordered by two hollow concavities mottled brilliant black, one on each side.
Posterior margin of the pronotum slightly curved in the middle; lateral lobes of pronotum with a shiny black band; infero-posterior half of lateral lobes of pronotum, pro-, meso- and methatoracic episternites pale; elytra lobiform, not enlarged, with subparallel margins and an almost straight/subrectilinear apex, with two well-formed and separate shiny black spots; elytra barely reaching the anterior margin of the first abdominal segment; inferoexternal surface of posterior femora dark red, internal basal half of posterior femora pale, internal apical half dark, with a black band; posterior tibiae basal half dark and apical half light red; male and female cerci conical.
Epiphallus (
Figure 15H). Bridge narrow; ancorae small, slanted inwards towards the midline; lophi wide, lobiform; oval sclerites small; anterior projections strongly reduced.
Phallic complex (
Figure 15I–K). Elongate; dorsal arch of cingulum U-shaped, closed, its anterior margin under the zygoma is broadly emarginate in the midline; ectophallic sheath long; apodemes of cingulum almost parallel or slightly orthogonally arranged, long, ritching or slightly exceeding the tip of endophallic apodemes; the endophallic apodemes are wide, more deeply cupped, and from their ventral margins two channels with a U-shaped cross section run ventrally and posteriorly to form the gonopore processes; the flexure of the endophallus is thread-like; zygoma well sclerotised; rami well developed, sclerotised, longer than its wide in lateral view, and extending to the ventral midline of the phallus, and overlaping but not fused at their ventral extremities; aedeagus projecting, long, slightly curved upwards, with almost straight or only slightly oblique margins and an obtuse apex with a ridge-like structure; aedeagal valves enveloped/covered by a thick semitransparent sheath, closely appressed to the endophallic valves; the endophallic valves are long and slender, wide and dorsoventrally flattened basally; the valves of cingulum are slender and pointed; the gonopore sac is small, progressively narrowing towards the posterior margin, and is situated more anteriorly.
Female. Similar to male, but larger; valves of ovipositor narrow, long; subgenital plate (
Figure 15C) hexagonal, short, with emaginated posterior margins; anterior apodemes short and broad; egg-guide of medium size; ventral pockets of the vaginal floor large; copulatory bursa long, almost straight, with parallel margins; bottom of the copulatory bursa close to the arc of the basivalvar sclerites; each basivalvar sclerite barely curved, forming around angle; spermatheca duct (
Figure 15L) simple, long; the base of the spermathecal duct opening directly at the apex of the copulatory bursa.
Etymology. The species is named after Prof. Dr. Martin Husemann, a distinguished taxonomist in Germany, for his dedication and significant contribution to the evolution of biodiversity, population genetics, phylogenetics, and biogeography of Orthoptera.
Distribution. Akom 2 (Southern Cameroon).
Biology and Ecology. This species occurs throughout the year in disturbed forests and cocoa farms of Southern Cameroon, where it is abundant.
Material examined. Holotype. Cameroon • ♂; Mbalmayo, disturbed forest; 3°29′17″ N, 11°30′6″ E, 679 m a.s.l.; 3 August 2024; A.C. Wandji leg.; SMNK, SMNK-ORTH-0000013. Paratypes. Cameroon • 1 ♂; Meyomessala, disturbed forest; 3°7′29″ N, 12°29′48″ E; 18 August 2021; J.A. Yetchom Fondjo leg.; SMNK. Cameroon • 7 ♂♂, 6 ♀♀; Mbalmayo, disturbed forest, forest edges, and cocoa farms; 3°29′17″ N, 11°30′6″ E, 679 m a.s.l.; 3 August 2024; A.C. Wandji leg.; SMNK.
Diagnosis.
Paraserpusia kekeunoui sp. nov. is identical to
P. succursor (
Figure 13) in general coloration, but can be distinguished by the following features: elytra slightly enlarged towards the apex, apex subpointed (vs. more enlarged with a rounded apex in
P. succursor); inner subapical shiny black spot much reduced, rounded and fairly smaller than the outer apical spot (vs. inner subapical spot little or not reduced, almost the same size as the outer apical spot in
P. succursor); posterior margin of pronotum almost straight, not incised (vs. incised in the middle in
P. succursor). Male genitalia differ by its apodemes of the cingulum that are almost parallel or slightly orthogonally arranged basally, convergent apically, long, and fairly exceeding the tip of the endophallic apodemes (vs. slightly curved ventrally at its apex, and only reaching the tip of the endophallic apodemes in
P. succursor); endophallic apodemes large (vs. endophallic apodemes small in
P. succursor); aedeagus long with abruptly oblique margins and converging, narrowing sharply towards an acute tip (vs. short or of medium size, and with roughly divergent margins and broad tip in
P. succursor); the gonopore sac is situated more posteriorly in position under the basal endophallus (vs. more anteriorly in
P. succursor).
The new species is similar to
P. hoeferi sp. nov. (
Figure 14) from which it can easily be distinguished by several characteristics: elytra strongly reduced, barely reaching the anterior margin of the metanotum (vs. elytra greatly enlarged towards the apex and reaching the posterior margin of the metanotum in
P. hoeferi sp. nov.); inner subapical glossy black spot on elytra greatly reduced to one point (vs. almost equal in size in
P. hoeferi sp. nov.); posterior margin of pronotum almost straight, not curved (vs. curved in the middle in
P. hoeferi sp. nov.). Male genitalia differ by its strongly arched epiphallus bridge (vs. slightly arched in
P. hoeferi sp. nov.), its apodemes of cingulum almost parallel or slightly orthogonally arranged basally, fairly exceeding the tip of the endophallic apodemes (vs. slightly curved inwards at its apex, and only reaching the tip of the endophallic apodemes in
P. hoeferi sp. nov.); its slightly upcurved aedeagus, and with abruptly oblique margins narrowing sharply towards the tip (vs. antero-dorsally directed, and with almost straight margins, and parallel and broaded tip in
P. hoeferi sp. nov.); the gonopore is situated more posteriorly in position under the basal endophallus (vs. more anteriorly in
P. hoeferi sp. nov.).
Description. Male. Tegument dark brown; fastigium of vertex flattened; frons pale; frontal sides mottled with black in front of and behind the eyes; filiform antenna longer than the head and pronotum combined, with a black apex; lateral carinae of pronotum mottled with black, median carina tectiform, crossed by three transverse furrows, the typical furrow strongly indented and bordered by two hollow concavities, one on each side; posterior margin of the pronotum almost straight, not incised in the middle; lateral lobes of the pronotum with a broad shiny black stripe not reaching the anterior margin, anterior and posterior margins with numerous small pale spots; the lower half of the lateral lobes of the pronotum pale; elytra lobiform, slightly enlarged, with a subpointed apex barely reaching the anterior margin of the metanotum, ornamented with two shiny black spots on the outer apical and inner subapical, the inner subapical strongly reduced to a point; neck conical; inner basal half of posterior femora dark red, apical half red with a black band; posterior tibiae basal half dark and apical half red.
Epiphallus (
Figure 16H). The bridge of the epiphallus is narrow, strongly arched; the ancorae are small, slanted inwards towards the midline; the lophi are wide, lobiform; oval sclerites are longer than wide; the anterior projections are strongly reduced.
Phallic complex (
Figure 16I–K). Elongate; dorsal arc of cingulum U-shaped, close, its anterior margin under the zygoma is broadly emarginate in the midline; ectophallic sheath short; apodemes of cingulum almost parallel or slightly orthogonally arranged basally, convergent apically, long fairly exceeding the tip of the endophallic apodemes; endophallic apodemes large, deeply cupped, and from their ventral margins two channels with a U-shaped cross section run ventrally and posteriorly to form the gonopore processes; the flexure of the endophallus is thread-like; zygoma well sclerotized.
Rami well developed, sclerotised, longer than wide in lateral view, and extending to the ventral midline of the phallus, not fused nor overlapping at their ventral extremities; aedeagus projecting, long, slightly curved upwards, with abruptly oblique margins and converging narrow acute tip, with a ridge-like structure at the apex; aedeagal valves enveloped/covered by a thick semitransparent sheath, closely appressed to the endophallic valves; the endophallic valves are long and slender, wider and more dorsoventrally flattened basally, but laterally compressed and narrow apically; the valves of cingulum are slender and pointed; the gonopore sac is of medium size, and is situated more posteriorly in position under the basal endophallus.
Female. Similar to male, but larger; cercus short conical; valves of ovipositor of medium size; subgenital plate (
Figure 14C) hexagonal, large, with slightly emaginated posterior margin; anterior apodemes broad; egg-guide short, broad; ventral pockets of the vaginal floor large; copulatory bursa long, almost straight, with parallel margings; bottom of the copulatory bursa close to the arc of the basivalvar sclerites; each basivalvar sclerite barely curved, forming around angle; spermatheca duct (
Figure 16L) simple, of medium size; the base of the spermathecal duct opening directly at the apex of the copulatory bursa.
Etymology. The species was named in honour of Professor Sévilor Kekeunou, an important Orthoptera expert in Cameroon, for his dedication and scientific contribution to the advancement of the knowledge of research in Orthoptera Ecology, Biodiversity Research, and Taxonomy.
Distribution. Meyomessala, Mbalmayo, Cameroon.
Habitat. This species is associated with the herbaceous vegetation of forest zones.
Biology and Ecology. Paraserpusia kekeunoui sp. nov. is abundant throughout the year in disturbed forests, forest edges, and cocoa farms of the Centre and Southern Cameroon.
Material examined. Holotype. Cameroon • ♂; Bangoulap, disturbed forest; 5°5′58″ N, 10°32′20″ E, 1358 m a.s.l.; 29 December 2024; A.C. Wandji & M. Mbadjoun Nzike leg.; SMNK, SMNK-ORTH-0000014. Paratypes. Cameroon • 12 ♂♂, 8 ♀♀; Bangoulap, disturbed forest; 5°5′58″ N, 10°32′20″ E, 1358 m a.s.l.; 29 December 2024; A.C. Wandji & M. Mbadjoun Nzike leg.; SMNK. Cameroon • 7 ♂♂, 5 ♀♀; Evodoula, in disturbed Forest, forest edges, and cocoa farms; 4°4′54″ N, 11°11′41″ E, 612 m a.s.l.; 10 January 2025; A.C. Wandji leg.; SMNK. Cameroon • 3 ♂♂, 1 ♀; Mfou, in disturbed Forest and cocoa farms; 3°48′32″ N, 11°40′32″ E, 697 m a.s.l.; 25 January 2025; A.C. Wandji & M. Mbadjoun Nzike leg.; SMNK.
Diagnosis.
Paraserpusia tamessei sp. nov. is similar to
P. succursor (
Figure 13) from which it can be distinguished by the following characteristics: the tegmina strongly exceed the posterior margin of the metanotum, but not reaching the posterior edges of the first abdominal tergite, with a pointed or acute apex (vs. they are broad, strongly widened towards the apex, and barely reaching the anterior margin of the metanotum, with rounded apex in
P. succursor); the aedeagus of the male phalli is cylindrical, long, with roughly convergent margins and rounded tip (vs. it is cylindrical, of medium size, with roughly divergent margins and broad tip in
P. succursor).
The new species is also similar to
P. hoeferi sp. nov. (
Figure 14) from which it can be distinguished by the following characteristics: the tegmina strongly exceed the posterior margin of the metanotum, but not reaching the posterior edges of the first abdominal tergite (vs. they strongly exceed the posterior margin of the metanotum, and fairly reaching the posterior edges of the first abdominal tergite in
P. hoeferi sp. nov.); the aedeagus of the male phalli is cylindrical, long, with roughly convergent margins and rounded tip (vs. it is cylindrical, long, with almost straight margins and parallel tip in
P. hoeferi sp. nov.).
The new species is also identical to
P. husemanni sp. nov. (
Figure 15) from which it differs by the tegmina having a pointed or acute apex (vs. they have an almost straight apex, forming a right angle in
P. husemanni sp. nov.); the aedeagus of the male phalli is cylindrical, long, with roughly convergent margins and a rounded tip (vs. it is conical, long, with almost straight margins and an obtuse tip in
P. husemanni sp. nov.).
Description. Male. Fastigium of the vertex slightly protroduing forwards; tegument pale, plume of black; frons pale; subocular facial spot single, rounded, central; antenna filiform, much longer than the head and pronotum combined in both sexes; median carina of the pronotum obtuse, tectiform, crossed by three transverse furrows, the typical furrow bordered by two hollow concavities mottled brilliant black, one on each side; the posterior margin of the pronotum posterior is more or less wavy; lateral lobes of pronotum with a shiny black band; infero-posterior half of lateral lobes of pronotum, pro-, meso- and methatoracic episternites pale; a large brown band extending from the cheeks to the apical 1/3 of the lateral lobes of pronotum in soe individuals; elytra lobiform, large/wide, with truncate, acute apex, and with two well-formed and separated shiny black spots; elytra strongly exceeding the posterior margin of the metanotum, but not reaching the posterior edges of the first abdominal tergite; inferoexternal surface of posterior femora dark red, internal basal half of posterior femora pale, internal apical half with alternated pale and black bands; posterior tibiae basal half dark and apical half light red; male cerci conical.
Epiphallus (
Figure 17H). Bridge narrow; ancorae small, slanted inwards towards the midline; lophi wide, lobiform; oval sclerites small; anterior projections strongly reduced.
Phallic complex (
Figure 17I–K). Elongate; dorsal arch of cingulum U-shaped, open, its anterior margin under the zygoma is broadly emarginate in the midline; ectophallic sheath long; apodemes of cingulum almost parallel or slightly orthogonally arranged, long, only reaching the tip of endophallic apodemes; endophallic apodemes wide, more deeply cupped, and from their ventral margins two channels with a U-shaped cross section run ventrally and posteriorly to form the gonopore processes; flexure of the endophallus thread-like; zygoma well sclerotised; rami well developed, sclerotised, longer than its wide in lateral view, and extending to the ventral midline of the phallus, and overlapping but not fused at their ventral extremities; aedeagus long, slightly curved upwards, with parallel margins and an rounded apex with a ridge-like structure; aedeagal valves covered by a thick semitransparent sheath, closely appressed to the endophallic valves; endophallic valves long and slender, wide and dorsoventrally flattened basally; valves of cingulum slender and pointed; gonopore sac large and situated more posteriorly as in
Serpusia.
Female. Similar to male, but larger; valves of ovipositor long; subgenital plate (
Figure 17C) hexagonal, short, with emaginated posterior margins; anterior apodemes short and broad; egg-guide of thin; ventral pockets of the vaginal floor large; copulatory bursa long, almost straight, with parallel margins; bottom of the copulatory bursa close to the arc of the basivalvar sclerites; each basivalvar sclerite barely curved, forming an obtuse angle; spermatheca duct (
Figure 17L) simple, long; the base of the spermathecal duct opening directly at the apex of the copulatory bursa.
Etymology. The species is named after Professor Joseph Lebel Tamesse, a distinguished entomologist and researcher in Cameroon, for his dedication and contribution to the taxonomy and ecology of various insect groups, including Psyllids.
Distribution. Bangoulap, Evodoula, Mfou (West Highlands and Centre regions, Cameroon).
Figure 17.
Paraserpusia tamessei sp. nov. (A) male frontal view; (B) female frontal view; (C) female subgenital plate; (D) male lateral view; (E) female lateral view; (F) male dorsal view; (G) female dorsal view; (H) epiphallus dorsal view; (I) phallic complex lateral view; (J) phallic complex dorsal view; (K) phallic complex ventral view; (L) female spermatheca. A: ancorae; AApd: anterior apodeme; Ap: apical valves of penis; Apd: apodeme of cingulum; B: bridge of epiphallus; Bp: basal valves of penis; BS: basivalvare sclerites; CB: corpulatory bursa; Cv: valve of cingulum; EG: egg-guide; Ejd: ejaculatory duct; Ejs: ejaculatory sac; Fx: flexure of the endophallus; L: lophus of epiphallus; LP: lateral plate of epiphallus; Os: oval sclerite; Rm: ramus of cingulum; SD: spermathecal duct; SP: spermatheca; Sps: spermatophore sac; Zyg: zygoma of cingulum.
Figure 17.
Paraserpusia tamessei sp. nov. (A) male frontal view; (B) female frontal view; (C) female subgenital plate; (D) male lateral view; (E) female lateral view; (F) male dorsal view; (G) female dorsal view; (H) epiphallus dorsal view; (I) phallic complex lateral view; (J) phallic complex dorsal view; (K) phallic complex ventral view; (L) female spermatheca. A: ancorae; AApd: anterior apodeme; Ap: apical valves of penis; Apd: apodeme of cingulum; B: bridge of epiphallus; Bp: basal valves of penis; BS: basivalvare sclerites; CB: corpulatory bursa; Cv: valve of cingulum; EG: egg-guide; Ejd: ejaculatory duct; Ejs: ejaculatory sac; Fx: flexure of the endophallus; L: lophus of epiphallus; LP: lateral plate of epiphallus; Os: oval sclerite; Rm: ramus of cingulum; SD: spermathecal duct; SP: spermatheca; Sps: spermatophore sac; Zyg: zygoma of cingulum.
Biology and Ecology. This species is abundant in disturbed forests and cocoa farms in the West Highlands and Centre regions of Cameroon.
Material examined. Holotype. Cameroon • ♂; Somalomo, Dja Biosphere Reserve, forest habitat; 3°22′55″ N, 12°44′30″ E, 607 m a.s.l.; 10 April 2022; J.A. Yetchom Fondjo leg.; SMNK, SMNK-ORTH-0000015. Paratypes. Cameroon • 6 ♂♂, 4 ♀♀; Somalomo, Dja Biosphere Reserve, forest habitat; 3°22′55″ N, 12°44′30″ E, 607 m a.s.l.; 10 April 2022; J.A. Yetchom Fondjo leg.; SMNK. Cameroon • 5 ♂♂, 2 ♀♀; Somalomo, in the Dja Biosphere Reserve, forest habitat; 3°22′55″ N, 12°44′30″ E, 607 m a.s.l.; 10 April 2022; J.A. Yetchom Fondjo leg.; SMNK.
Diagnosis. This species is similar to
P. succursor (
Figure 13) in general coloration, but can be easily distinguished by several characteristics: the male cercus is short (vs. long in
P. succursor); the elytra have no shiny black spots (vs. two shiny black spots in
P. succursor); the apex of the elytra is narrowed and slightly truncated (vs. apex of the elytra is slightly widened and rounded in
P. succursor); male genitalia differ by its less elongate and slightly robust phallic complex (vs. of medium size and elongate in
P. succursor), apodemes of cingulum fairly exceeding the tip of the endophallic apodemes (vs. only reaching the tip of the endophallic apodemes in
P. succursor); aedeagus long, with converging margins and rounded tip (vs. aedeagus of medium size, with roughly divergent margins and broad tip in
P. succursor); the gonopore is situated more posteriorly in position under the basal endophallus (vs. more anteriorly in
P. succursor).
The new species is also similar to
P. hoeferi sp. nov. (
Figure 14) from which it can be easily distinguished by the following characteristics: elytra reduced, with no black spots and a narrowed and slightly truncated apex (vs. elytra large and greatly enlarged towards the apex, and a pointed apex with two shiny black spots in
P. hoeferi sp. nov.); elytra extending beyond the posterior margin of the metanotum and limited to the anterior margin of the first abdominal segment (vs. elytra not reaching the posterior margin of the metanotum in
P. hoeferi). Male genitalia differ by its apodemes of cingulum fairly exceeding the tip of the endophallic apodemes (vs. only reaching the tip of the endophallic apodemes in
P. hoeferi sp. nov.); aedeagus cylindrical, long, with converging margins and rounded tip (vs. cylindrical, long, with almost straight margins and parallel tip in
P. hoeferi sp. nov.); gonopore sac of medium size, and situated more posteriorly in position under the basal endophallus (vs. more anteriorly in
P. hoeferi sp. nov.).
This species is also similar to
P. kekeunoui sp. nov. (
Figure 16) from which it can be distinguished by the following characteristics: elytra without a shiny black spot (vs. two shiny black spots in
P. kekeunoui); inner surface of posterior femora completely red (vs. inner surface of posterior femora apically red, basally pale in
P. kekeunoui); anterior and posterior edges of pronotum curved in the middle (vs. not curved, almost straight in
P. kekeunoui); antennae longer than head and pronotum combined in both sexes (vs. antennae shorter or as long as head and pronotum combined in
P. kekeunoui); male genitalia differ by its less elongated and slightly robust phallic complex (vs. elongate in
P. kekeunoui); the apodemes of cingulum are curved, forming a horseshoe-like profile basally, oblique apically (vs. almost parallel or slightly orthogonally arranged basally, convergent apically in
P. kekeunoui); endophallic apodemes small (vs. large in
P. kekeunoui); aedeagus of medium size, strongly curved, antero-dorsally directed, and with converging margins and rounded tip (vs. conical long, slightly curved upwards, with abruptly oblique margins narrowing sharply towards an acute tip in
P. kekeunoui).
Description. Male. Head straight in lateral view, fastigium of the vertex, frons and cheeks pale; subocular facial spot rounded in the centre; antennae pale-black, filiform, longer than the head and pronotum combined; a broad shiny black ribbon running from the back of the eyes, across the superolateral face of the lateral lobes of the pronotum and extending to the lateral-superior face of the abdomen; dorsal surface of pronotum pale, with black plume and three transverse furrows; typical furrow strongly indented on either side of median carina; anterior margin of pronotum slightly curved in the middle, posterior margin of pronotum with numerous furrows dorsally; lateral lobes of pronotum with a shiny black band clearly reaching the anterior margin; infero-anterior and infero-posterior margins of lateral lobes of pronotum pale; pale pro-, meso-, and metathoracic episternites; elytra lobiform, narrowing towards the apex, with parallel margins, and a slightly truncate apex, slightly exceeding the posterior margin of the metanotum; shining black spots on the elytra complement absent; inferior margins of the elytra outlined in black; prosternal tubercle short conical with flattened base; front and middle legs pale; lower outer surface, inner surface of posterior femora dark red; outer surface of posterior femora with three broad black facis or spots, variegated with pale spots; posterior tibiae with dark basal half and light red apical half; external apical spines on posterior tibiae absent; arolium and claws well developed; ventral surface of abdomen pale; male supra-anal plate trigonal, with a groove through the basal third; male cerci short conical with slightly flattened base and acute apex; male subgenital plate conical with rounded apex.
Epiphallus (
Figure 18H). The bridge is narrow, arched; the ancorae are small and slanted inwards towards the midline; lophi wide, lobiform; oval sclerites small and roughly triangular; anterior projections strongly reduced.
Phallic complex (
Figure 18I–K). Less elongate and lightly robust; dorsal arch of cingulum is U-shaped, strongly open, and curved to form a slightly horseshoe-like profile in dorsal view, its anterior margin under the zygoma is broadly emarginate in the midline; ectophallic sheath short; apodemes of cingulum curved, forming a horseshoe-like profile basally, oblique apically, and fairly exceeding the tip of the endophallic apodemes; endophallic apodemes small, deeply cupped and ventrally directed, and from their ventral margins two channels with a U-shaped cross section run ventrally and posteriorly to form the gonopore processes; the flexure of the endophallus is very slender; zygoma only slightly sclerotised; rami well developed, sclerotised somewhat, longer than its wide in lateral view, and extending to the ventral midline of the phallus, not fused nor overlaping at their ventral extremities; the valves of cingulum are slender and pointed; the endophallic valves are long and slender, wide and dorsoventrally flattened basally, but more reduced than in
P. surccusor; aedeagus cylindrical, long, strongly curved upwards, antero-dorsally directed, and with oblique margins and rounded tip; aedeagal valves covered by a thick semitransparent sheath, closely appressed to the endophallic valves; the gonopore sac is of medium size, and is situated more posteriorly in position under the basal endophallus.
Female. Similar to male, but larger; cerci conical; supra-anal plate trigonal with obtuse apex, crossed by a groove in its basal half; valves of ovipositor narrow, long; subgenital plate (
Figure 18C) hexagonal, long, with truncated posterior margins; anterior apodemes broad; egg-guide of medium size; ventral pockets of the vaginal floor large; copulatory bursa long, almost straight, with parallel margins; bottom of the copulatory bursa close to the arc of the basivalvar sclerites; each basivalvar sclerite barely curved, forming a round angle; spermatheca duct (
Figure 18L) simple, of medium size; the base of the spermathecal duct opening directly at the apex of the copulatory bursa.
Figure 18.
Paraserpusia tindoi sp. nov. (A) male frontal view; (B) female frontal view; (C) female subgenital plate; (D) male lateral view; (E) female lateral view; (F) male dorsal view; (G) female dorsal view; (H) epiphallus dorsal view; (I) phallic complex lateral view; (J) phallic complex dorsal view; (K) phallic complex ventral view; (L) female spermatheca. A: ancorae; AApd: anterior apodeme; Ap: apical valves of penis; Apd: apodeme of cingulum; B: bridge of epiphallus; Bp: basal valves of penis; BS: basivalvare sclerites; CB: corpulatory bursa; Cv: valve of cingulum; EG: egg-guide; Ejd: ejaculatory duct; Ejs: ejaculatory sac; Fx: flexure of the endophallus; L: lophus of epiphallus; LP: lateral plate of epiphallus; Os: oval sclerite; Rm: ramus of cingulum; SD: spermathecal duct; SP: spermatheca; Sps: spermatophore sac; Zyg: zygoma of cingulum.
Figure 18.
Paraserpusia tindoi sp. nov. (A) male frontal view; (B) female frontal view; (C) female subgenital plate; (D) male lateral view; (E) female lateral view; (F) male dorsal view; (G) female dorsal view; (H) epiphallus dorsal view; (I) phallic complex lateral view; (J) phallic complex dorsal view; (K) phallic complex ventral view; (L) female spermatheca. A: ancorae; AApd: anterior apodeme; Ap: apical valves of penis; Apd: apodeme of cingulum; B: bridge of epiphallus; Bp: basal valves of penis; BS: basivalvare sclerites; CB: corpulatory bursa; Cv: valve of cingulum; EG: egg-guide; Ejd: ejaculatory duct; Ejs: ejaculatory sac; Fx: flexure of the endophallus; L: lophus of epiphallus; LP: lateral plate of epiphallus; Os: oval sclerite; Rm: ramus of cingulum; SD: spermathecal duct; SP: spermatheca; Sps: spermatophore sac; Zyg: zygoma of cingulum.
Etymology. The species was named in honour of Professor Maurice Tindo, a distinguished entomologist and researcher in Cameroon, for his significant contribution and achievement in the field of insect ecology, biodiversity, species interactions, and conservation biology.
Distribution. Somalomo, Dja Biosphere Reserve (Cameroon) (
Figure 19).
Biology and Ecology. This species is associated with disturbed forests in southern Cameroon. Adults are present throughout the year in the natural environment. Mating is observed between April and June.
Except for
P. tindoi sp. nov., whose phallus is slightly robust as in
Aresceutica, all
Paraserpusia species have a very similar phallic complex, but differ from each other by the shape of the aedeagus. As such, five types of aedeagus can be found in
Paraserpusia (
Figure 11,
Figure 12,
Figure 13,
Figure 14,
Figure 15 and
Figure 16): (1) aedeagus cylindrical, short or of medium size, with divergent margins and broad tip, (2) aedeagus cylindrical, long with almost straight margins and parallel tip, (3) aedeagus cylindrical, long, with convergent margins and rounded tip; (4) aedeagus conical, long, with abruptly oblique margins narrowing towards an obtuse tip, and (5) aedeagus conical, long, with almost straight or only slightly oblique margins and obtuse apex having a ridge-like structure.
- A.
Based on external morphology
Tegmina lobiform, without shiny black spots ( Figure 18D–G)
|
| Paraserpusia tindoi Yetchom & Wandji sp.nov. |
- -
Tegmina lobiform, with two shiny black spots
| 2 |
- 2.
Tegmina broad, strongly widened towards the apex, and reaching the posterior margin of the metanotum
| 4 |
- -
Tegmina broad, strongly widened towards the apex, and not reaching the anterior margin of the metanotum
| 3 |
- 3.
|
| Paraserpusia succursor (Karsch, 1896) |
- -
Tegmina with a subpointed apex ( Figure 16D–G)
|
| Paraserpusia kekeunoui Yetchom & Wandji sp. nov. |
- 4.
Tegmina with almost straight apex forming a right angle ( Figure 15D–G)
|
| Paraserpusia husemanni Yetchom & Wandji sp. nov. |
- -
Tegmina with a pointed or acute apex
| 5 |
- 5.
Tegmina strongly exceeding the posterior margin of the metanotum, but not reaching the posterior edges of the first abdominal tergite ( Figure 17D–G)
|
Paraserpusia tamessei Yetchom & Wandji sp. nov.
|
- -
Tegmina strongly exceeding the posterior margin of the metanotum, and fairly reaching the posterior edges of the first abdominal tergite ( Figure 14D–G)
|
| Paraserpusia hoeferi Yetchom & Wandji sp. nov. |
| The distribution range of all species treated in this study is shown in Figure 19 below. |
Figure 19.
Distribution of Aresceutica, Paraserpusia, and Serpusia species in African forests.
Figure 19.
Distribution of Aresceutica, Paraserpusia, and Serpusia species in African forests.
- B.
Based on the structures of the phallus
| 2 |
- -
Phallic complex less elongate and slightly robust, apodemes of cingulum curved, forming a horseshoe-like profile basally, oblique apically ( Figure 18I–K)
|
| Paraserpusia tindoi Yetchom & Wandji sp. nov. |
- 2.
Apodemes of cingulum slightly curved ventrally at its apex, and only reaching the tip of the endophallic apodemes
| 3 |
- -
Apodemes of cingulum almost parallel or slightly orthogonally arranged, long exceeding the tip of the endophallic apodemes
| 4 |
- 3.
Aedeagus cylindrical, of medium size, with roughly divergent margins and broad tip ( Figure 13I–K)
|
| Paraserpusia succursor (Karsch, 1896) |
- -
Aedeagus cylindrical, long, with almost straight margins and parallel tip ( Figure 14J)
|
| Paraserpusia hoeferi Yetchom & Wandji sp. nov. |
- 4.
Aedeagus cylindrical, long, with roughly convergent margins and a rounded tip ( Figure 17J)
|
| Paraserpusia tamessei Yetchom & Wandji sp. nov. |
| Aedeagus conical | 5 |
- 5.
Aedeagus conical, long, with abruptly oblique margins and acute tip; gonopore sac situated more posteriorly under the basal endophallus ( Figure 16J)
|
| Paraserpusia kekeunoui Yetchom & Wandji sp. nov. |
- -
Aedeagus conical, long, with almost straight or only slightly oblique margins and an obtuse tip; gonopore sac is small, and is situated more anteriorly ( Figure 15J)
|
| Paraserpusia husemanni Yetchom & Wandji sp. nov. |
Although P. succursor and P. tamessei overlap geographically, they form distinct COI-16S clades, and they also exhibit discrete forewing and genitalia differences—thus supporting their recognition as separate species. Paraserpusia tindoi and P. hoeferi form distinct COI-16S clades, and do not overlap geographically as P. tindoi occurs exclusively in the Eastern region of Cameroon (Somalomo) and P. hoeferi in the Littoral region of Cameroon (Iboti). Both also exhibit discrete forewing and genitalia differences—thus supporting their recognition as separate species. Paraserpusia kekeunoui occurs exclusively in Mbalmayo (centre region of Cameroon), whereas P. husemanni is restricted to the southern region of Cameroon (Akom 2). Both species form distinct COI-16S clades and exhibit discrete forewing and genitalia differences—thus supporting their recognition as separate species. Additionally, although S. missoupi and S. verhaaghi overlap geographically (both species being found in the Littoral region of Cameroon), they exhibit discrete forewing and genitalia differences, and form well-supported clades in the phylogenetic tree inferred from a combined COI and 16S dataset—thus supporting their recognition as separate species. Serpusia kennei is geographically distributed in the Littoral region of Cameroon, whereas S. seinoi occurs exclusively in the Eastern region of Cameroon. Both species exhibit discrete forewing morphology and form well-supported clades in the phylogenetic tree inferred from a combined COI and 16S dataset—thus supporting their recognition as separate species.