1. Introduction
Respiratory metabolism constitutes a fundamental physiological mechanism in insects, functioning as the principal pathway for energy transduction. This biochemical process involves the oxidative breakdown of intracellular energy substrates, primarily carbohydrates and lipids, through oxygen-mediated catabolic reactions to generate adenosine triphosphate (ATP) and concomitantly release carbon dioxide as a metabolic byproduct [
1,
2]. The regulation of insect respiratory metabolism operates through complex interplay among endogenous physiological determinants and exogenous environmental parameters. The key modulatory factors include organismal characteristics (body mass, developmental phase, and species-specific behavioral patterns) and ambient conditions (thermal regime, humidity gradients, atmospheric oxygen tension, and photoperiodic cycles) [
3,
4,
5,
6,
7,
8]. Notably, under nutritional deprivation or environmental stressors, various insect species demonstrate adaptive metabolic depression characterized by significant downregulation of respiratory rates. This hypometabolic state facilitates conservation of energy reserves, thereby enhancing survival capacity during prolonged adverse conditions [
4,
7,
9,
10].
Schlechtendalia chinensis (Bell) is the gall-forming aphid. Its horned galls induced on
Rhus chinensis constitute approximately 70–75% of the total national gallnut yield, representing the principal determinant of commercial gallnut yield [
11,
12]. The life cycle of
S. chinensis is complex. Each year in late March, newly emerged sexuparae (spring migrants) migrate from their winter host (mosses) to their summer host,
R. chinensis. They reproduce, forming sexuales (females and males) in cracks of the host tree bark. These sexuales undergo three to four molts to reach maturity, after which mating occurs. Within one week post-mating, the males die, while the females continue development for approximately 20 days before producing the fundatrices. Each fundatrix relocates to the leaf tissues of
R. chinensis, where its feeding activity induces the formation of a gall. Inside the gall, it reproduces parthenogenetically, yielding three successive generations of fundatrigeniae. The first two generations consist of apterous (wingless) fundatrigeniae, while the third generation are alate (winged) fundatrigeniae. Between late September and early October, the mature galls dehisce, releasing the alate fundatrigeniae, which then return to mosses—the winter host. There, they deposit overwintering nymphs. These overwintering nymphs undergo development and emerge the following spring (late March) as sexuparae, thereby completing the annual cycle. Thus,
S. chinensis exhibits a heteroecious holocyclic life history, characterized by six successive morphotypes: sexuales, fundatrices, apterous fundatrigeniae, alate fundatrigeniae (autumn migrants), overwintering nymphs, and sexuparae (spring migrants) [
13,
14,
15]. Notably, the sexuales present unique biological constraints: the complete absence of functional mouthparts, rendering them incapable of nutrient acquisition by means of feeding [
16]. Furthermore, sexuales constitute the only stage in which sexual reproduction occurs, facilitating genetic recombination through mating. This process enhances genetic diversity within the population, thereby increasing evolutionary adaptability and enabling the offspring—specifically, the fundatrices—to better respond to heterogeneous and dynamic environmental conditions. Moreover, their reproductive strategy is restricted to single-ovum sexual reproduction, with each gravid female producing merely one offspring—a fundatrix. Given that individual fundatrices typically initiate solitary gall formation, the reproductive success of sexuales fundamentally governs gallnut productivity through strict 1:1 female-to-gall numerical correspondence.
The reproductive cycle of S. chinensis sexuales necessitates sequential mate localization and copulatory behavior, followed by embryonic development and subsequent oviposition by gravid females. These energetically demanding processes—including mate-seeking locomotion, gametogenesis, and parturition—require substantial nutrient allocation. Paradoxically, sexual morphs exhibit complete mouthpart degeneration, eliminating feeding capacity throughout their whole life. This physiological constraint raises critical questions regarding their energy homeostasis: Could metabolic rate modulation, particularly respiratory downregulation, serve as an evolutionary adaptation to offset nutritional deficits during sexual reproduction? Current entomological research lacks empirical data on respiratory metabolism dynamics in S. chinensis, with no published studies quantifying its catabolic processes or energy budgeting strategies during critical life stages.
This investigation employed an LI-6400XT portable photosynthesis system with a customized insect respiration chamber (6400-89) to quantify respiratory metabolic rates (RMRs) at different developmental stages of S. chinensis sexual morphs, including their parental sexuparae and progeny fundatrices. Utilizing high-resolution respirometry, we systematically evaluated the hypothesis that sexual aphids mitigate endogenous nutrient depletion through respiratory depression, while elucidating their evolutionary metabolic trade-offs. These findings establish mechanistic links between hypometabolic adaptation and reproductive success, providing actionable insights for optimizing gallnut production in controlled cultivation systems.
2. Materials and Methods
2.1. Insect Materials
Field-collected specimens of S. chinensis were obtained from the designated area for gallnut cultivation (30°10′12″ N, 110°52′36″ E; elevation 960 ± 15 m) in Bainianguan, Wufeng Tujia Autonomous County, Hubei Province. Newly emerged sexuparae were collected from the cultivated moss nursery and subsequently transferred to 90 mm Petri dishes lined with moistened filter paper under controlled laboratory conditions (25 ± 1 °C, 80% RH, 16:8 L:D). Continuous monitoring commenced upon initiation of parturition, with daily collection of neonate progeny (males and females) and the newborn fundatrices produced by females.
2.2. RMR Measurements
We employed an LI-6400XT portable photosynthesis system (LI-COR Biosciences, Lincoln, NE, USA)) equipped with a customized insect respiration chamber (6400-89) to quantify respiratory metabolic rates across three distinct daily phases: 9:00–12:00, 14:00–17:00, and 19:00–22:00. The experimental design incorporated four aphid morphotypes (sexuparae, females, males, and fundatrices), with all specimens selected for uniform size and confirmed vitality through pre-experimental screening. Prior to measurements, 100 sexuparae or 400 individuals for other morphotypes underwent 30 min of environmental acclimation within the respiration chamber under standardized conditions (24 ± 0.5 °C, 70 ± 5% RH). Continuous CO2 flux monitoring commenced following stabilization of the emission curves (defined as <5% variation over 5-min intervals), with high-frequency data acquisition at 2-s resolution. Each group of samples was measured for 15 min, and the respiratory metabolic rate (μg·g−1·min−1) was automatically recorded by the instrument recorder. The experimental design implemented five independent biological replicates per morphotype–temporal combination, with strict avoidance of individual reuse across the experimental groups.
2.3. Statistics and Analysis
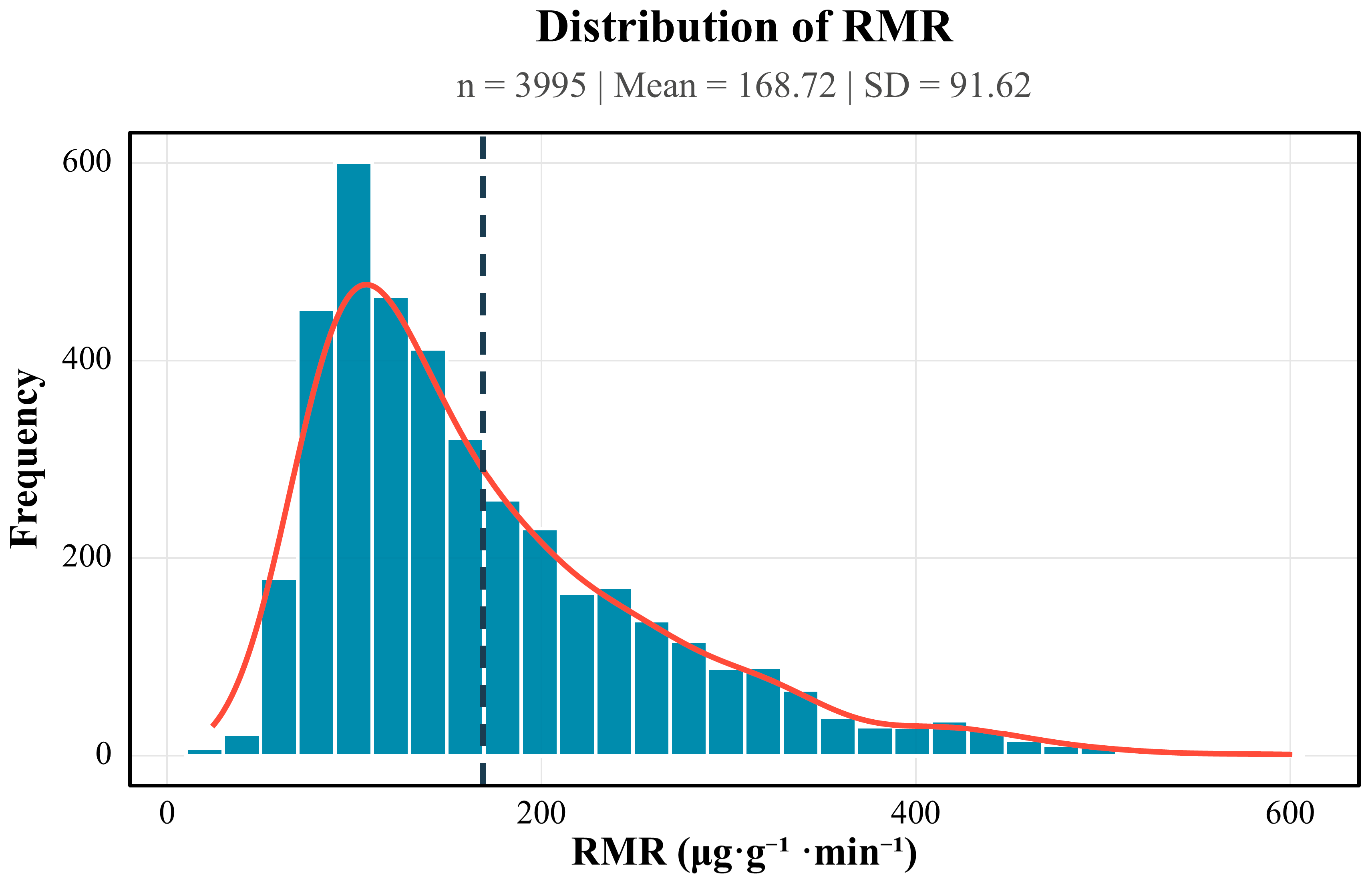
Initial data handling was performed in Microsoft Excel 365. Statistical analyses were performed in R (v4.5.0; R Development Core Team, Vienna, Austria) using the “stats” package, including outlier detection (Tukey fence) and calculations of descriptive statistics (mean ± SD, median [IQR]). Visualizations were completed in R using the ggplot2 package(v4.0.0). The normality of the respiratory metabolic rate (RMR) variable was formally evaluated with the Shapiro–Wilk test (
n = 3995). The statistic was significant (W = 0.912,
p < 0.001), indicating departure from normality; visual inspection revealed a pronounced right-tail skew (
Figure 1). Consequently, inter-group comparisons were conducted using non-parametric tests. The Kruskal–Wallis rank-sum test was chosen as the primary inferential procedure because it accommodates multiple independent samples without assuming normality and is robust to outliers. When the Kruskal–Wallis test indicated a significant difference among groups (
p < 0.05), post hoc pairwise comparisons were performed with Dunn’s test, applying Bonferroni correction to control the family-wise Type I error rate and to pinpoint the specific pairs driving the overall effect. This analytical approach enabled comprehensive comparisons across two dimensions: inter-morphotype differences among the 4 aphid morphotypes and intra-morphotype variations across the 3 temporal intervals. The experimental design specifically addressed both interspecific metabolic divergence and temporal metabolic fluctuations within identical morphotypes, ensuring a rigorous evaluation of statistical significance across all comparative groups.
4. Discussion
Insect respiratory intensity serves as a direct proxy for quantifying energy metabolism [
17]. Crucially, insects modulate their respiratory intensity as a physiological strategy to enhance environmental adaptability under adverse conditions [
18,
19]. Consequently, analyses of respiratory metabolism patterns provide dual insights: they elucidate internal nutrient utilization dynamics and reveal evolutionary trade-offs between environmental adaptation and life-history strategies in insects. This study constitutes the first systematic investigation of respiratory metabolism across three distinct generations of
S. chinensis—the sexual generation (males and females), their maternal generation (sexuparae), and their offspring generation (fundatrices)—while simultaneously examining sexual dimorphism within males and females. Our findings demonstrate significant intergenerational and intersexual differentiation in respiratory metabolic rates (RMRs). These variations exhibit precise functional alignment with species-specific ecological roles and environmental adaptation mechanisms.
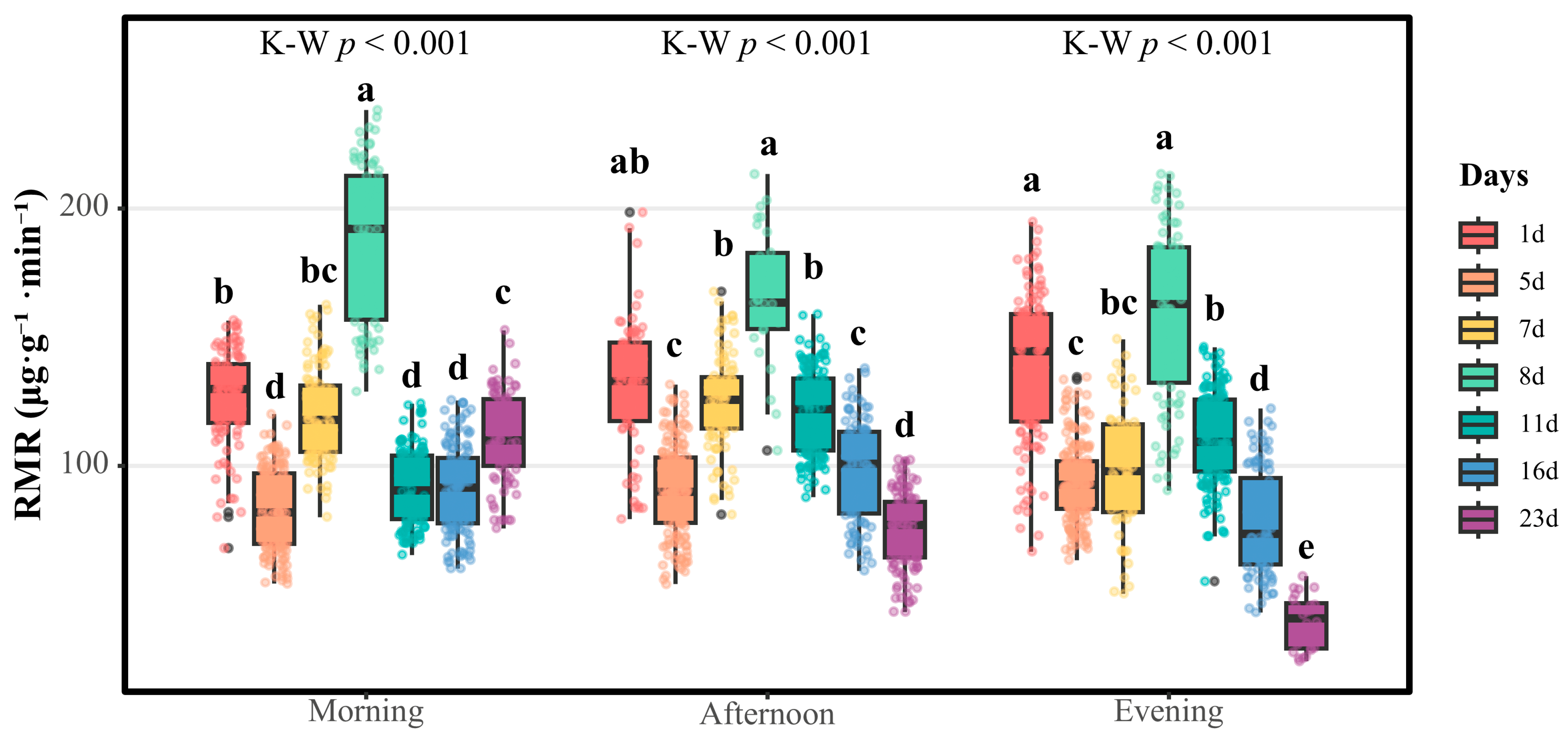
This investigation quantitatively tracked RMR dynamics across developmental stages in both sexes, revealing similar temporal RMR patterns coupled with significant sexual RMR dimorphism. Females demonstrated stage-specific RMR regulation characterized by three distinct phases: an initial neonatal downregulation, reproductive-phase modulation, and post-copulatory depression. Neonatal females exhibited elevated RMRs, followed by 34.2–40.7% suppression within postnatal days 1–5 (
p < 0.05), indicating strategic energy reallocation toward reproductive system maturation and long-term embryogenesis requirements. Subsequent mating phase activation (days 5–8) manifested progressive RMR elevation, peaking at 192.065 μg·g
−1·min
−1 on day 8—a metabolic signature associated with oocyte activation, spermathecal function, and vitellogenesis-related hormonal shifts [
4]. A post-mating RMR collapse to 40.773 μg·g
−1·min
−1 (day 23) suggests evolutionary adaptation through extreme RMR throttling to optimize maternal longevity and fundatrix production success.
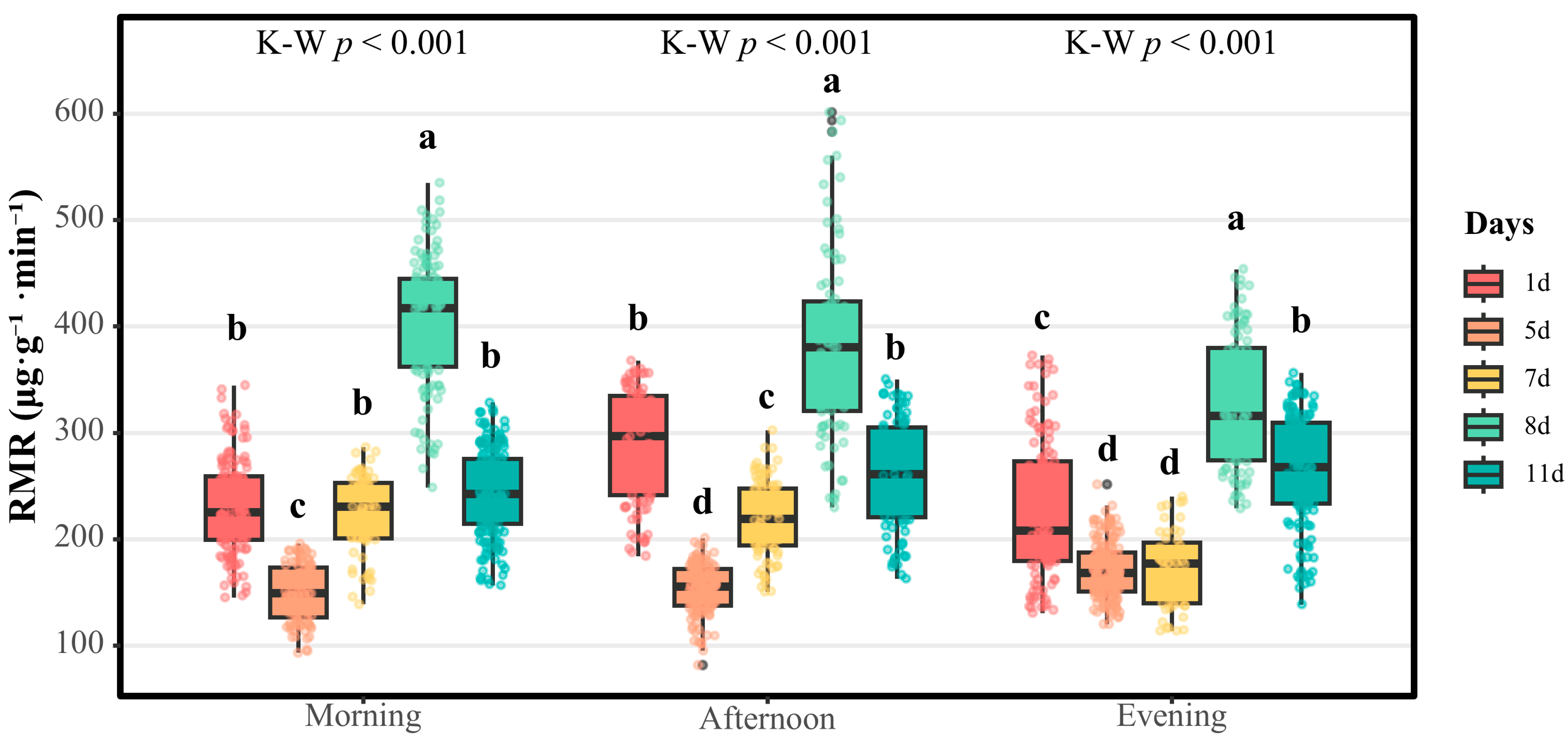
In striking contrast, male aphids exhibited high respiratory metabolic activity throughout the day and night, with their RMR being approximately twice that of females. This sexual dimorphism was particularly significant during the mating period (days 5–8), with the RMR reaching as high as 416.816 μg·g
−1·min
−1 on the 8th day (peak RMR). This sustained hypermetabolism is likely related to mate-searching behaviors, courtship displays, and intrasexual competition [
3,
20], with late-life maintenance of elevated RMRs (242.391–267.368 μg·g
−1·min
−1) potentially contributing to rapid post-mating senescence (3–5-day mortality window). The observed sexual RMR dichotomy reflects fundamental evolutionary trade-offs between female resource allocation strategies and male expenditure-driven reproductive tactics.
Under conditions of exogenous nutritional deprivation, females, as exclusive carriers of embryonic development, implement prioritized energy allocation through dynamic regulation of respiratory metabolism to ensure fundatrix embryogenesis. This adaptive mechanism aligns with the resource investment paradigm characteristic of K-selected species. Notably, the significant reduction in post-mating RMRs exemplifies their evolutionary “metabolic thrift” strategy, wherein deep RMR suppression extends nutrient reserve utilization cycles to guarantee complete fundatrix development. In contrast, males demonstrate respiratory metabolic patterns congruent with r-selected strategy parameters, emphasizing rapid metabolic turnover and short-term reproductive efficiency. This fundamental sexual dimorphism in metabolic regulation corresponds to the core premise of sexual selection theory: differential reproductive investment strategies, with males optimizing mating competition capacity and females prioritizing offspring provisioning [
4]. The observed dichotomy in respiratory physiology—sustained metabolic economy in females versus transient metabolic intensity in males—provides mechanistic evidence for sex-specific life-history strategies shaped by distinct evolutionary pressures. This metabolic specialization enables females to maintain developmental homeostasis during prolonged nutritional challenges, while males maximize immediate reproductive output through rapid energy mobilization.
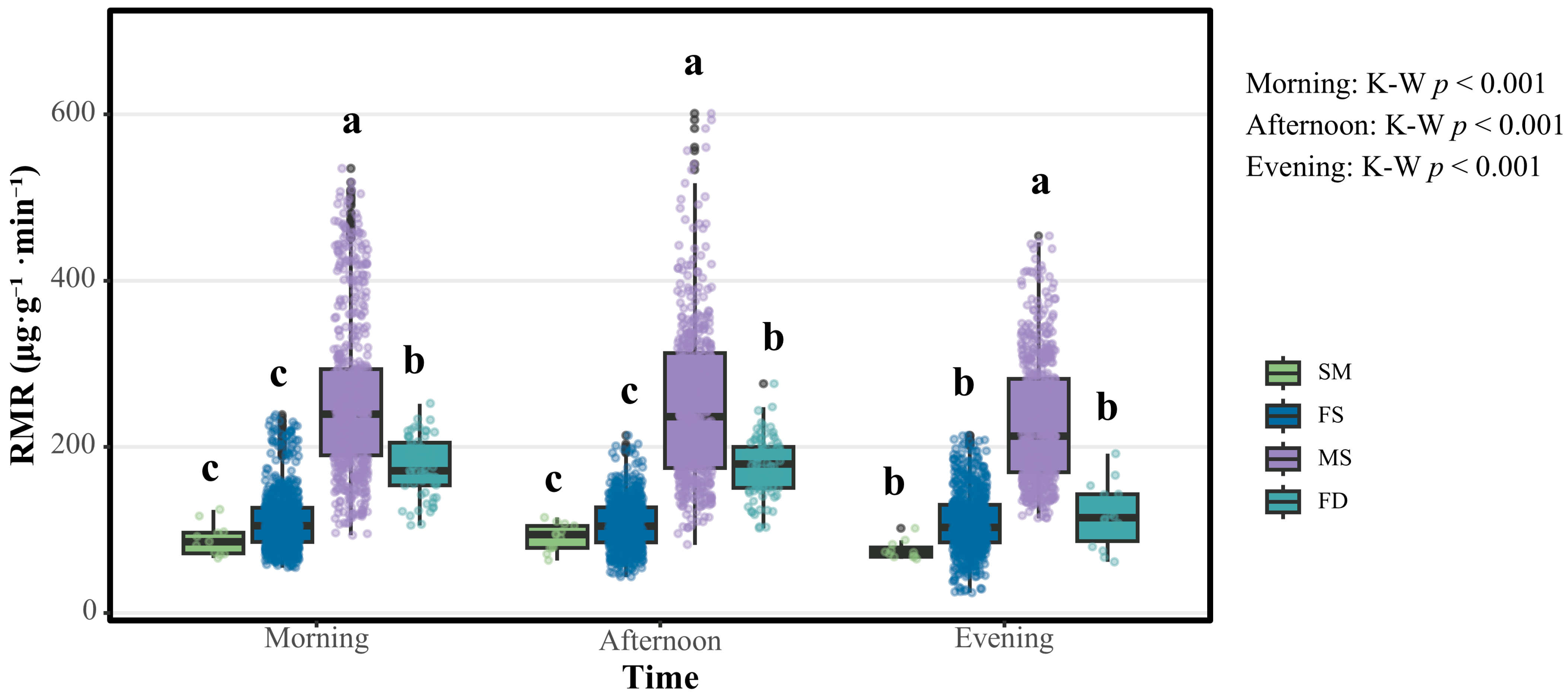
This study revealed significant intersexual and intergenerational variations in RMRs among
S. chinensis populations. Notably, males demonstrated the highest metabolic activity across diurnal and nocturnal cycles (the average value for their whole developmental stage was 212.385–239.047 μg·g
−1·min
−1), exhibiting statistically significant elevation compared to other morphotypes of
S. chinensis. The RMR hierarchy followed a distinct pattern: fundatrices ranked second (114.347–178.819 μg·g
−1·min
−1), followed by females (about 104.372 μg·g
−1·min
−1), with sexuparae displaying the lowest metabolic rates (72.427–94.032 μg·g
−1·min
−1). This RMR pattern appears to be evolutionarily conserved through functional adaptation. The heightened respiratory metabolic demand in males correlates with energy-intensive reproductive behaviors, including mate location and copulatory activities. Conversely, females maintain reduced respiratory metabolic states to optimize energy allocation for sustained oviposition and embryonic development. Fundatrices’ elevated RMRs likely facilitate host plant colonization through enhanced locomotor capacity. The respiratory metabolic suppression observed in sexuparae suggests dual adaptive strategies: (1) the conservation of energetic reserves for migratory flight requirements and (2) the extension of reproductive longevity through RMR modulation, thereby ensuring sufficient production of sexual offspring [
20].
Circadian analysis of the RMRs revealed conserved diurnal oscillation patterns across all four aphid morphotypes, characterized by elevated diurnal and reduced nocturnal respiratory metabolic activity. However, quantitative differentials emerged: fundatrices exhibited statistically significant nocturnal metabolic suppression compared to their diurnal levels. This differential regulation appears to be evolutionarily conserved through photoperiod-mediated physiological modulation. The observed RMR downregulation corresponds with reduced nocturnal host-seeking behaviors. Such chronobiological adaptation likely optimizes energy conservation strategies while maintaining essential physiological functions under suboptimal environmental conditions [
21].
This investigation addresses critical knowledge gaps regarding the respiratory metabolism of S. chinensis while elucidating its evolutionary adaptation strategy for nutrient allocation through respiratory metabolic regulation under non-trophic conditions. Our findings establish this species as a novel experimental system for studying extreme environmental adaptation mechanisms in insects, providing a theoretical foundation for optimizing sexual aphid conservation protocols in gallnut cultivation systems. However, this study did not delineate the molecular architecture of respiratory metabolic pathway regulation in S. chinensis—a critical knowledge gap that could be elucidated through integrated metabolomic profiling and flux analysis. Furthermore, the influences of environmental factors (such as thermal and hygric) on the RMRs of sexuales require systematic evaluation via controlled laboratory simulations, which would enable environmental parameter optimization and refinement in artificial rearing systems.