Trapping Asian Citrus Psyllid (Diaphorina citri) on Adhesive-Coated New Shoots of Murraya paniculata
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Trapping Efficiency Evaluation
2.2.1. Laboratory Experimentation Evaluation
2.2.2. Field Experimentation Evaluation
2.3. Baseline ACP Sex Ratio
2.4. Assessment of ACP Directional Preference
2.5. Application in Monitoring ACP
2.6. Data Analysis
3. Results
3.1. Laboratory Experimentation
3.2. Field Experimentation
3.3. ACP Sex Ratio
3.4. ACP Directional Preference
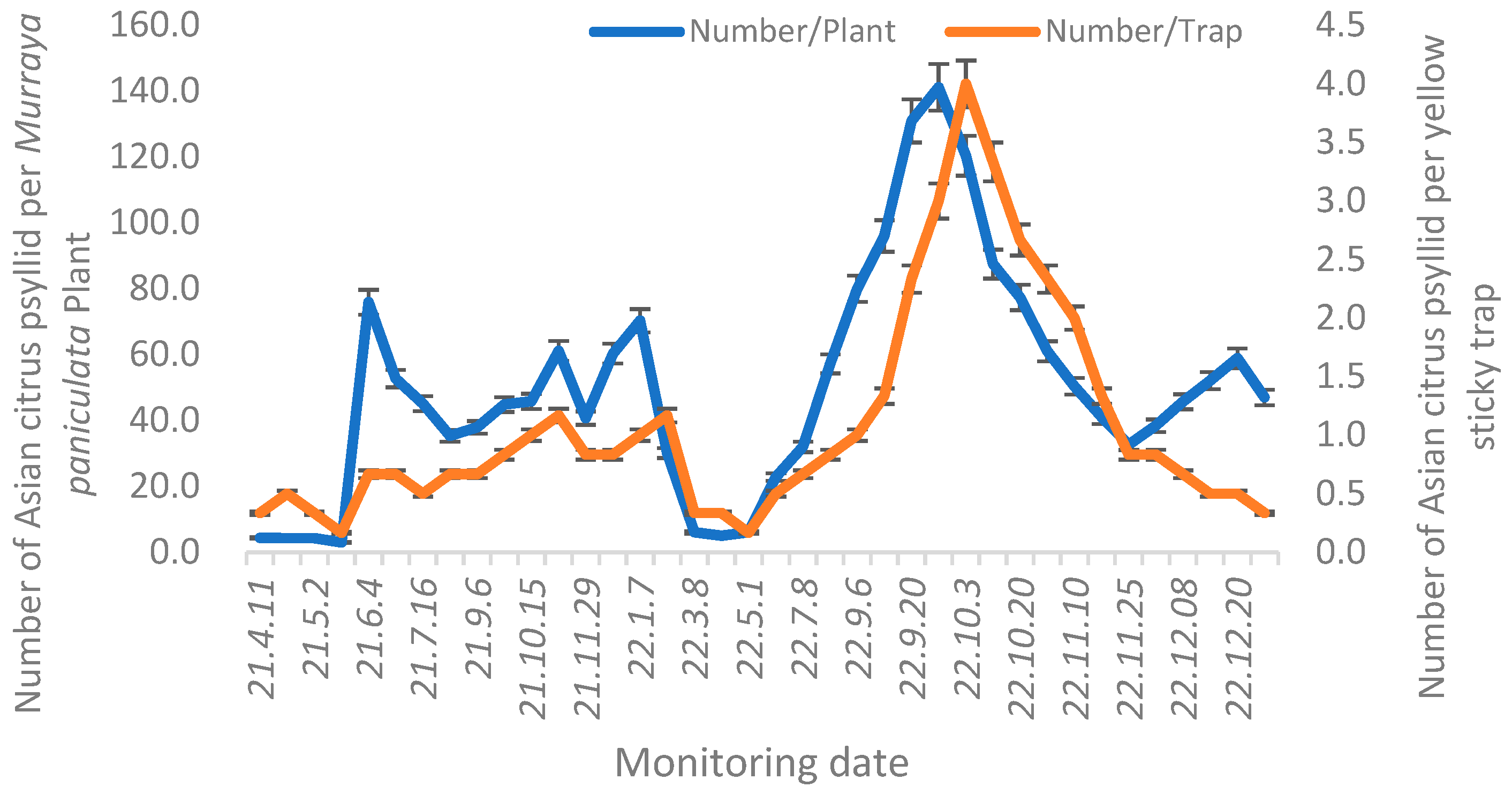
3.5. ACP Detection and Monitoring
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bové, J. Huanglongbing: A destructive, newly emerging, century-old disease of citrus. J. Plant Pathol. 2006, 88, 7–37. [Google Scholar]
- Hall, D.G.; Richardson, M.L.; Ammar, E.-D.; Halbert, S.E. Asian citrus psyllid, Diaphorina citri, vector of citrus huanglongbing disease. Entomol. Exp. Appl. 2013, 146, 207–223. [Google Scholar] [CrossRef]
- Chin, E.; Mishchuk, D.; Bruce, J.; Cilia, M.; Coaker, G.; Davis, C.; Jin, H.L.; Ma, W.B.; Seller, G.; Leveaque, C.; et al. An interdisciplinary approach to combat HLB. Citrograph 2014, 5, 28–34. [Google Scholar]
- Grafton-Cardwell, E.E.; Stelinski, L.L.; Stansly, P.A. Biology and management of Asian citrus psyllid, vector of the Huanglongbing pathogens. Annu. Rev. Entomol. 2013, 58, 413–432. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Zahid, M.; Khan, G.Z. Toxicity of botanic and synthetic pesticide residues to citrus psyllid Diaphorina citri Kuwayama and Chrysoperla carnea (Stephens). Pak. J. Zool. 2012, 44, 197–201. [Google Scholar]
- Tiwari, S.; Stelinski, L.L.; Rogers, M.E. Biochemical basis of organophosphate and carbamate resistance in Asian citrus psyllid. J. Econ. Entomol. 2012, 105, 9. [Google Scholar] [CrossRef]
- Kishk, A.; Stelinski, L.L.; Gowda, S.; Killiny, N. Citrus-mediated gene silencing of cytochrome P450 suppresses insecticide resistance and increases mortality in Diaphorina citri. Pest Manag. Sci. 2024, 80, 4980–4992. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Li, M.; Achal, V. A comprehensive review on environmental and human health impacts of chemical pesticide usage. Emerg. Contam. 2024, 11, 100410. [Google Scholar] [CrossRef]
- Grafton-Cardwell, E.E. Asian Citrus Psyllid; UCANR Publications: Oakland, CA, USA, 2006. [Google Scholar]
- Primo-Millo, E.; Agustí, M. Vegetative Growth. In The Genus Citrus; Woodhead Publishing: Cambridge, UK, 2020; pp. 193–217. [Google Scholar]
- Carvalho, E.V.; Cifuentes-Arenas, J.C.; Raiol-Junior, L.L.; Stuchi, E.S.; Girardi, E.A.; Lopes, S.A. Modeling seasonal flushing and shoot growth on different citrus scion-rootstock combinations. Sci. Hortic. 2021, 288, 110358. [Google Scholar] [CrossRef]
- Kalile, M.O.; Cardoso, A.C.; Pallini, A.; Fonseca, M.M.; Ferreira-Junior, T.A.; Janssen, A. A predatory mite that suppresses Diaphorina citri populations on plants with pollen and oviposition sites. Entomol. Exp. Appl. 2023, 171, 592–602. [Google Scholar] [CrossRef]
- Landis, D.A.; Wratten, S.D.; Gurr, G.M. Habitat management to conserve natural enemies of arthropod pests in agriculture. Annu. Rev. Entomol. 2000, 45, 175–201. [Google Scholar] [CrossRef]
- Gurr, G.M.; Scarratt, S.L.; Wratten, S.D.; Berndt, L.; Irvin, N. Ecological Engineering, Habitat Manipulation and Pest Management. In Ecological Engineering for Pest Management: Advances in Habitat Manipulation for Arthropods; CSIRO Publishing: Collingwood, Australia, 2004; pp. 1–12. [Google Scholar]
- Guo, L.; Muminov, M.A.; Wu, G.; Liang, X.; Li, C.; Meng, J.; Li, L.; Cheng, D.; Song, Y.; Gu, X.; et al. Large reductions in pesticides made possible by use of an insect-trapping lamp: A case study in a winter wheat-summer maize rotation system. Pest Manag. Sci. 2018, 74, 1728–1735. [Google Scholar] [CrossRef]
- Damsteegt, V.D.; Postnikova, E.N.; Stone, A.L.; Kuhlmann, M.; Wilson, C.; Sechler, A.; Schaad, N.W.; Brlansky, R.H.; Schneider, W.L. Murraya paniculata and related species as potential hosts and inoculum reservoirs of ‘Candidatus Liberibacter asiaticus’, causal agent of huanglongbing. Plant Dis. 2010, 94, 528–533. [Google Scholar] [CrossRef]
- Patt, J.M.; Sétamou, M. Responses of the Asian citrus psyllid to volatiles emitted by the flushing shoots of its rutaceous host plants. Environ. Entomol. 2010, 39, 618–624. [Google Scholar] [CrossRef]
- Hall, D.G.; Sétamou, M.; Mizell III, R.F. A comparison of sticky traps for monitoring Asian citrus psyllid (Diaphorina citri Kuwayama). Crop Prot. 2010, 29, 1341–1346. [Google Scholar] [CrossRef]
- Hernandez, F.J.; Shaw, R.F. Comparison of plankton net and light trap methodologies for sampling larval and juvenile fishes at offshore petroleum platforms and a coastal jetty off Louisiana. In American Fisheries Society Symposium; American Fisheries Society: Bethesda, MD, USA, 2003; pp. 15–38. [Google Scholar]
- Shelton, A.M.; Badenes-Perez, F.R. Concepts and applications of trap cropping in pest management. Annu. Rev. Entomol. 2006, 51, 285–308. [Google Scholar] [CrossRef] [PubMed]
- Cavanagh, A.; Hazzard, R.; Adler, L.S.; Boucher, J. Using trap crops for control of Acalymma vittatum (Coleoptera: Chrysomelidae) reduces insecticide use in butternut squash. J. Econ. Entomol. 2009, 102, 1101–1107. [Google Scholar] [CrossRef]
- Sarkar, S.C.; Wang, E.; Wu, S.; Lei, Z. Application of trap cropping as companion plants for the management of agricultural pests: A review. Insects 2018, 9, 128. [Google Scholar] [CrossRef] [PubMed]
- Hall, D.G.; Hentz, M.G.; Patt, J.M. Behavioral assay on Asian citrus psyllid attraction to orange jasmine. J. Insect Behav. 2015, 28, 555–568. [Google Scholar] [CrossRef]
- de Carvalho, U.D.; Girardi, E.A.; Pacheco, C.A.; Primiano, I.V.; Kharfan, D.; Moreira, A.S.; Bassanezi, R.B. Topping sweet orange trees as Diaphorina citri bait on the farm edge for Huanglongbing management: Opportunities and limitations. Scientia Hortic. 2024, 338, 113612. [Google Scholar] [CrossRef]
- Davidson, M.M.; Nielsen, M.C.; Butler, R.C.; Vellekoop, R.; George, S.; Gunawardana, D.; Muir, C.A.; Teulon, D.A. The effect of adhesives and solvents on the capture and specimen quality of pest thrips on coloured traps. Crop Prot. 2015, 72, 108–111. [Google Scholar] [CrossRef]
- Monzo, C.; Arevalo, H.A.; Jones, M.M.; Vanaclocha, P.; Croxton, S.D.; Qureshi, J.A.; Stansly, P.A. Sampling methods for detection and monitoring of the Asian citrus psyllid (Hemiptera: Psyllidae). Environ. Entomol. 2015, 44, 780–788. [Google Scholar] [CrossRef]
- Liu, Y.; Dong, L.; Ran, D.; Wang, S.; Qu, R.; Zheng, L.; Peng, A.; He, Y.; Chen, S.; Zou, X. A Comparative Analysis of Three Rutaceae Species Reveals the Multilayered Mechanisms of Citrus in Response to Huanglongbing Disease. J. Plant Growth Regul. 2023, 42, 7564–7579. [Google Scholar] [CrossRef]
- Lee, J.A.; Halbert, S.E.; Dawson, W.O.; Robertson, C.J.; Keesling, J.E.; Singer, B.H. Asymptomatic spread of huanglongbing and implications for disease control. Proc. Natl. Acad. Sci. USA 2015, 112, 7605–7610. [Google Scholar] [CrossRef]
- Sule, H.; Muhamad, R.; Omar, D.; Hee, A. Life table and demographic parameters of Asian citrus psyllid Diaphorina citri on limau madu. J. Entomol. 2012, 9, 146–154. [Google Scholar] [CrossRef]
- Aubert, B.; Quilici, S. Monitoring adult psyllas on yellow traps in Reunion Island. In International Organization of Citrus Virologists Conference Proceedings (1957–2010); International Organization of Citrus Virologists: Riverside, CA, USA, 1988; Volume 10, p. 10. [Google Scholar]
- Mann, R.S.; Rouseff, R.L.; Smoot, J.; Rao, N.; Meyer, W.L.; Lapointe, S.L.; Robbins, P.S.; Cha, D.; Linn, C.E.; Webster, F.X.; et al. Chemical and behavioral analysis of the cuticular hydrocarbons from Asian citrus psyllid, Diaphorina citri. Insect Sci. 2013, 20, 367–378. [Google Scholar] [CrossRef]
- Ayres, M.P.; Lombardero, M.J. Forest pests and their management in the Anthropocene. Can. J. For. Res. 2018, 48, 292–301. [Google Scholar] [CrossRef]
- Fragnière, A.L.; Bacher, S.; Kehrli, P. Identifying candidate host plants for trap crop against Drosophila suzukii in vineyards. J. Pest Sci. 2024, 97, 1975–1991. [Google Scholar] [CrossRef]
- Hall, D.G.; Hentz, M.G. Sticky trap and stem-tap sampling protocols for the Asian citrus psyllid (Hemiptera: Psyllidae). J. Econ. Entomol. 2010, 103, 541–549. [Google Scholar] [CrossRef]
- Hall, D.G.; Hentz, M.G.; Adair, R.C., Jr. Population ecology and phenology of Diaphorina citri (Hemiptera: Psyllidae) in two Florida citrus groves. Environ. Entomol. 2008, 37, 914–924. [Google Scholar] [CrossRef] [PubMed]
- Tsai, J.H.; Liu, Y.H. Biology of Diaphorina citri (Homoptera: Psyllidae) on four host plants. J. Econ. Entomol. 2000, 93, 1721–1725. [Google Scholar] [CrossRef]
- Badenes-Perez, R.F.; Shelton, M.A.; Nault, A.B. Evaluating Trap Crops for Diamondback Moth, Plutella xylostella (Lepidoptera: Plutellidae). J. Econ. Entomol. 2004, 97, 1365–1372. [Google Scholar] [CrossRef]
- Sétamou, M.; Flores, D.; French, J.V.; Hall, D.G. Dispersion patterns and sampling plans for Diaphorina citri (Hemiptera: Psyllidae) in citrus. J. Econ. Entomol. 2008, 101, 1478–1487. [Google Scholar] [CrossRef]
- Bayles, B.R.; Thomas, S.M.; Simmons, G.S.; Grafton-Cardwell, E.E.; Daugherty, M.P. Spatiotemporal dynamics of the Southern California Asian citrus psyllid (Diaphorina citri) invasion. PLoS ONE 2017, 12, e0173226. [Google Scholar] [CrossRef] [PubMed]
- Antolínez, C.A.; Martini, X.; Stelinski, L.L.; Rivera, M.J. Wind speed and direction drive assisted dispersal of Asian citrus psyllid. Environ. Entomol. 2022, 51, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Alves, M.N.; Cifuentes-Arenas, J.; Niñoles, R.; Raiol-Junior, L.L.; Carvalho, E.; Quirós-Rodriguez, I.; Ferro, J.A.; Licciardello, C.; Alquezar, B.; Carmona, L.; et al. Transcriptomic analysis of early stages of ‘Candidatus Liberibacter asiaticus’ infection in susceptible and resistant species after inoculation by Diaphorina citri feeding on young shoots. Front. Plant Sci. 2025, 16, 1502953. [Google Scholar] [CrossRef] [PubMed]

| Trap Number on Murraya paniculate | Mortality Rate | |||||
|---|---|---|---|---|---|---|
| 12 h | 24 h | 36 h | 12 h | 24 h | 36 h | |
| Shoot with spray | 13.33 ± 0.88 a | 16.33 ± 0.58 a | 18.00 ± 0.58 ab | 51.8% | 75.9% | 88.8% |
| Shoot without spray | 13.67 ± 0.33 a | 15.33 ± 0.33 a | 18.33 ± 0.33 a | 0% | 0% | 0% |
| No shoot with spray | 11.00 ± 0.58 b | 15.33 ± 0.88 a | 16.67 ± 0.33 bc | 49.07% | 63.41% | 77.31% |
| No shoot without spray | 12.33 ± 0.33 ab | 16.00 ± 1.00 a | 16.00 ± 0.58 c | 0% | 0% | 0% |
| 12 h Trap Number/10 Young Shoots | 12 h Mortality Rate | 24 h Trap Number/ 10 Young Shoots | 24 h Female Ratio | 24 h Mortality Rate | |
|---|---|---|---|---|---|
| Shoot with spray | 7.66 ± 0.88 a | 13.04% | 10.33 ± 0.88 a | 72.41% | 57.0% |
| Shoot without spray | 8.33 ± 0.88 a | 0.00% | 11.33 ± 0.67 a | 71.86% | 0.00% |
| No shoot with spray | 2.33 ± 0.33 b | 14.29% | 5.00 ± 0.58 b | 51.27% | 54.28% |
| No shoot without spray | 3.00 ± 0.58 b | 0.00% | 5.67 ± 0.67 b | 53.79% | 0.00% |
| Yellow sticky trap control | 2.67 ± 0.67 b | 100% | 6.24 ± 2.82 b | 51.67% | 100% |
| Survey Date | Female | Male | Sex Ratio (% Male) |
|---|---|---|---|
| 13 July 2023 | 150 | 290 | 65.91 |
| 20 July 2023 | 120 | 210 | 63.63 |
| 25 July 2023 | 86 | 195 | 69.40 |
| 26 July 2023 | 67 | 197 | 74.62 |
| 27 July 2023 | 80 | 201 | 71.53 |
| 6 August 2023 | 120 | 266 | 68.91 |
| 9 August 2023 | 130 | 230 | 63.89 |
| 10 August 2023 | 67 | 113 | 62.78 |
| Total | 820 | 1702 | 67.49 |
| Survey Date | East | West | South | North |
|---|---|---|---|---|
| 4 June 2023 | 11.25 ± 1.11 ab | 6.75 ± 0.75 c | 13.00 ± 0.41 a | 9.50 ± 0.65 b |
| 19 June 2023 | 7.00 ± 0.91 b | 6.50 ± 0.65 b | 9.75 ± 0.85 a | 6.00 ± 0.41 b |
| 16 July 2023 | 5.75 ± 0.85 bc | 6.25 ± 1.11 bc | 9.50 ± 0.65 a | 4.50 ± 0.64 c |
| 15 August 2023 | 4.75 ± 0.85 ab | 4.50 ± 0.65 ab | 5.75 ± 0.63 a | 3.00 ± 0.41 b |
| 6 September 2023 | 4.25 ± 0.75 ab | 4.00 ± 0.71 ab | 5.75 ± 0.48 a | 2.75 ± 0.48 b |
| Total | 26.00 ± 5.10 a | 22.20 ± 2.35 a | 34.00 ± 5.39 a | 20.20 ± 4.99 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, R.; Huang, Y.; Deng, G.; Zhu, C.; Wu, P.; Fan, Z.; Zeng, J. Trapping Asian Citrus Psyllid (Diaphorina citri) on Adhesive-Coated New Shoots of Murraya paniculata. Insects 2025, 16, 1011. https://doi.org/10.3390/insects16101011
Zhang R, Huang Y, Deng G, Zhu C, Wu P, Fan Z, Zeng J. Trapping Asian Citrus Psyllid (Diaphorina citri) on Adhesive-Coated New Shoots of Murraya paniculata. Insects. 2025; 16(10):1011. https://doi.org/10.3390/insects16101011
Chicago/Turabian StyleZhang, Ruimin, Yongjing Huang, Guiming Deng, Congyi Zhu, Pingzhi Wu, Zhengyan Fan, and Jiwu Zeng. 2025. "Trapping Asian Citrus Psyllid (Diaphorina citri) on Adhesive-Coated New Shoots of Murraya paniculata" Insects 16, no. 10: 1011. https://doi.org/10.3390/insects16101011
APA StyleZhang, R., Huang, Y., Deng, G., Zhu, C., Wu, P., Fan, Z., & Zeng, J. (2025). Trapping Asian Citrus Psyllid (Diaphorina citri) on Adhesive-Coated New Shoots of Murraya paniculata. Insects, 16(10), 1011. https://doi.org/10.3390/insects16101011






