Ecological Mercenaries: Why Aphids Remain Premier Models for the Study of Ecological Symbiosis
Simple Summary
Abstract
1. Introduction
2. Discovery of Aphid Facultative Symbionts
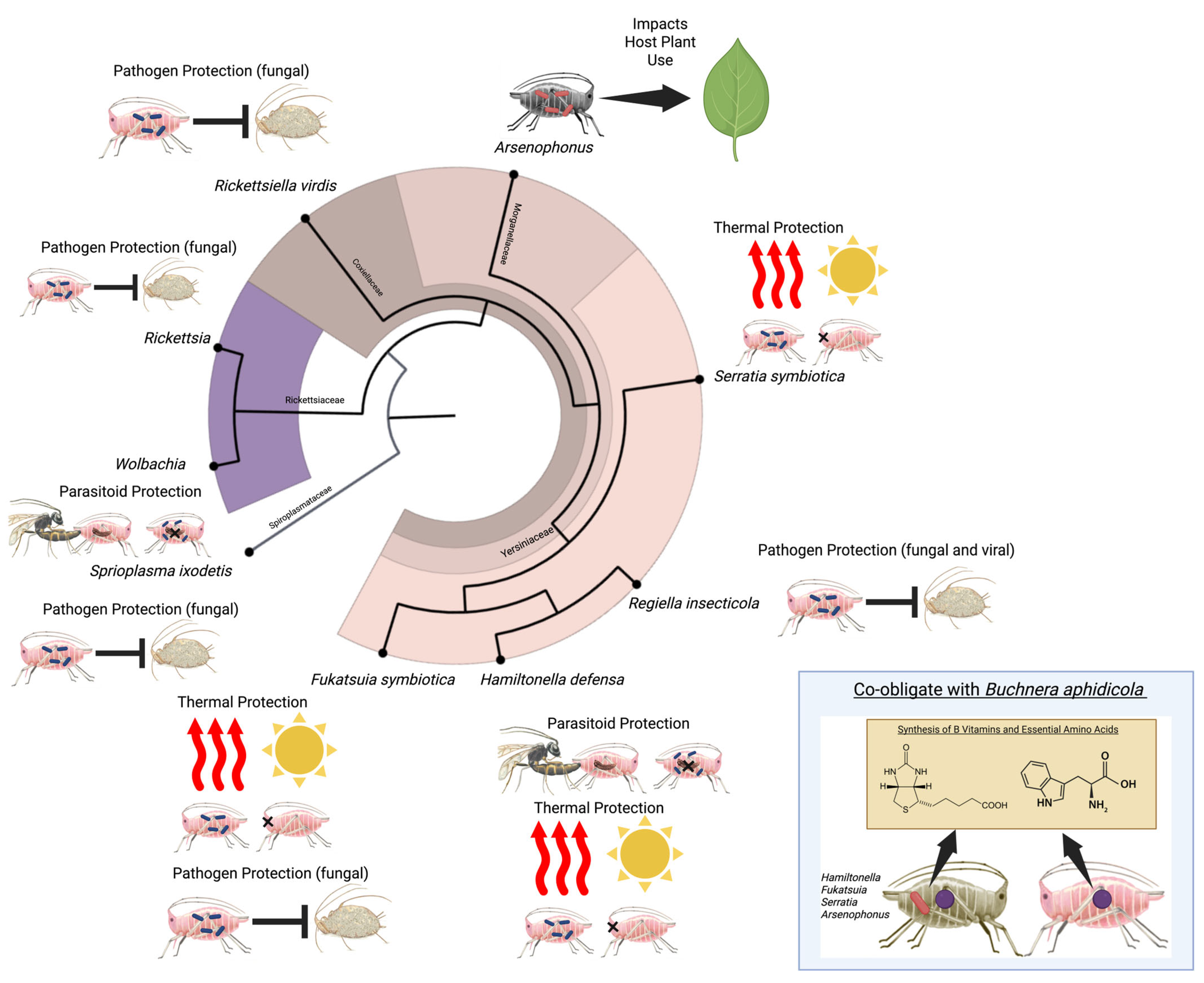
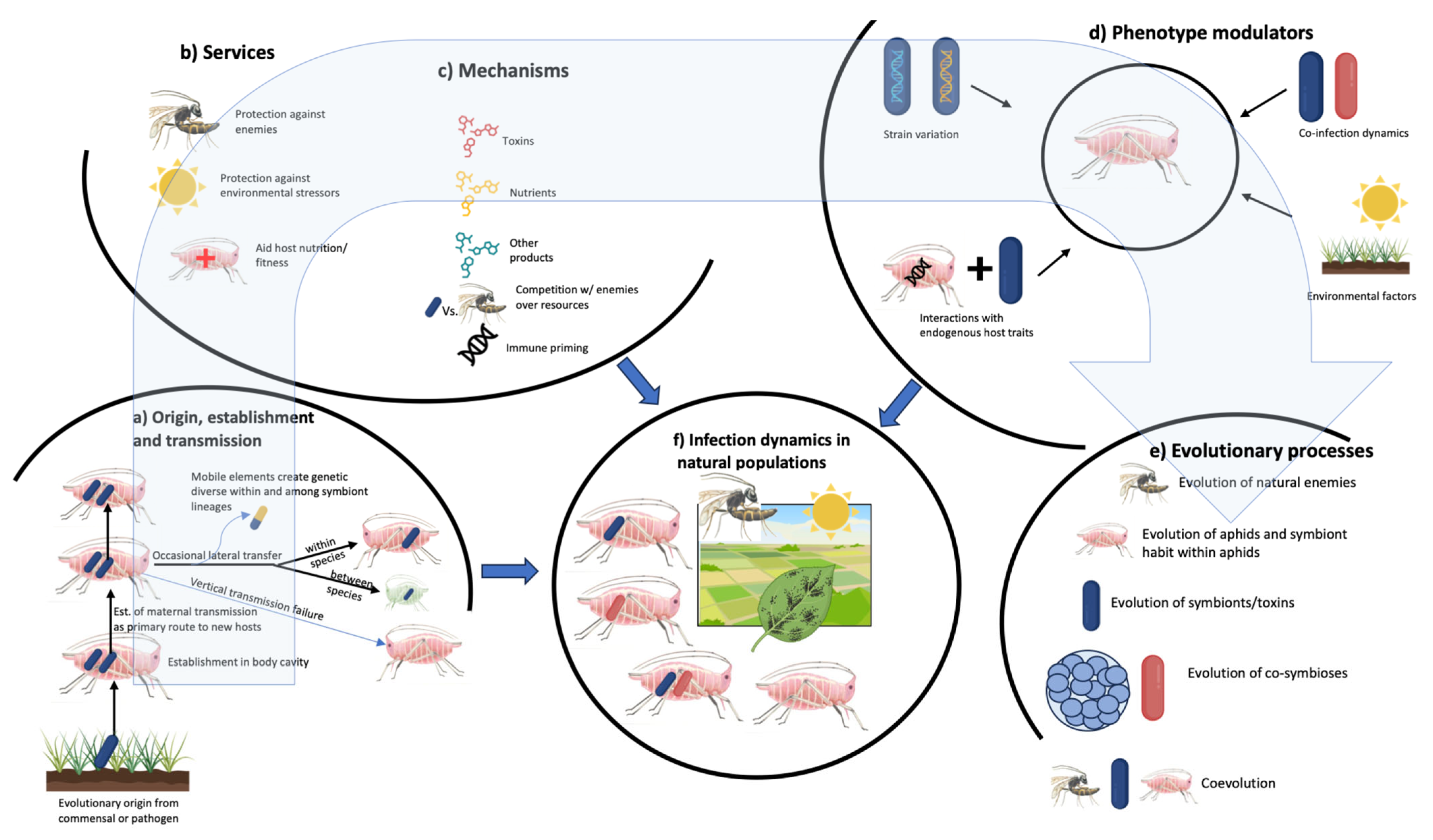
3. Aphid Facultative Symbionts Mediate Diverse Interactions
3.1. Experimental Foundations and Methodological Advances
3.2. Defensive Services Against Natural Enemies
3.3. Plant Interactions and Dietary Specialization
3.4. Thermal Tolerance and Climate Adaptation
3.5. Conditional Benefits Are Often Paired with Costs
3.6. Nutritional Supplementation and Co-Obligate Evolution
3.7. Future Directions and Research Priorities
4. Transmission and Tissue Tropism
4.1. Tissue Distribution and Cellular Mechanisms
4.2. Vertical and Horizontal Transmission Dynamics
4.3. Evolutionary Insights and Knowledge Gaps
5. Facultative Symbionts in Natural Populations
5.1. Survey Approaches and Distribution Patterns
5.2. Temporal Dynamics and Ecological Drivers
5.3. Knowledge Gaps and System Advantages
6. Mechanisms Underlying Symbiont-Mediated Phenotypes
6.1. Genomic Foundations and Mobile Elements
6.2. Advances in Cultivation and Sequencing Technologies
6.3. Molecular Mechanisms of Defense
6.4. Broader Defensive Strategies and Knowledge Gaps
7. Strain Variation, Co-Infections, and Interactions with Endogenous Host Traits Create Complex Phenotypic Landscapes
7.1. Strain-Level Variation Drives Phenotypic Diversity
7.2. Co-Infection Dynamics and Symbiont Interactions
7.3. Integration with Endogenous Aphid Defenses
7.4. Future Directions in Symbiont Complexity
8. Aphid Symbiont Systems: Cascading Effects, Coevolution, and Agricultural Applications
8.1. Ecological Network Effects
8.2. Coevolutionary Arms Races
8.3. Maintaining Symbiont Diversity
8.4. Agricultural Management Implications
9. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Douglas, A.E. Symbiosis as a general principle in eukaryotic evolution. Cold Spring Harb. Perspect. Biol. 2014, 6, a016113. [Google Scholar] [CrossRef]
- Russell, J.A.; Oliver, K.M. Mechanisms underlying microbial symbiosis. In Advances in Insect Physiology; Elsevier: Amsterdam, The Netherlands, 2020; Volume 58, pp. 1–25. [Google Scholar]
- Engel, P.; Moran, N.A. The gut microbiota of insects—Diversity in structure and function. FEMS Microbiol. Rev. 2013, 37, 699–735. [Google Scholar] [CrossRef]
- Kucuk, R. Gut bacteria in the holometabola: A review of obligate and facultative symbionts. J. Insect Sci. 2020, 20, 22. [Google Scholar] [CrossRef]
- Duron, O.; Bouchon, D.; Boutin, S.; Bellamy, L.; Zhou, L.; Engelstädter, J.; Hurst, G.D. The diversity of reproductive parasites among arthropods: Wolbachia do not walk alone. BMC Biol. 2008, 6, 27. [Google Scholar] [CrossRef]
- Vogel, K.J.; Coon, K.L. Functions and mechanisms of symbionts of insect disease vectors. In Advances in Insect Physiology; Elsevier: Amsterdam, The Netherlands, 2020; Volume 58, pp. 233–275. [Google Scholar]
- Hansen, A.K.; Pers, D.; Russell, J.A. Symbiotic solutions to nitrogen limitation and amino acid imbalance in insect diets. In Advances in Insect Physiology; Elsevier: Amsterdam, The Netherlands, 2020; Volume 58, pp. 161–205. [Google Scholar]
- Oliver, K.M.; Moran, N.A. Defensive symbionts in aphids and other insects. In Defensive Mutualism in Microbial Symbiosis; CRC Press: Boca Raton, FL, USA, 2009; pp. 147–166. [Google Scholar]
- Ng, J.C.; Perry, K.L. Transmission of plant viruses by aphid vectors. Mol. Plant Pathol. 2004, 5, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Van Emden, H.F.; Harrington, R. Aphids as Crop Pests; Cabi Publishing: Oxfordshire, UK, 2007. [Google Scholar]
- Moran, N.A.; McCutcheon, J.P.; Nakabachi, A. Genomics and evolution of heritable bacterial symbionts. Annu. Rev. Genet. 2008, 42, 165–190. [Google Scholar] [CrossRef]
- Buchner, P. Endosymbiosis of Animals with Plant Microorganisms; Interscience Publishers: New York, NY, USA, 1965. [Google Scholar]
- Woese, C.R. Bacterial evolution. Microbiol. Rev. 1987, 51, 221–271. [Google Scholar] [CrossRef] [PubMed]
- Munson, M.A.; Baumann, P.; Kinsey, M.G. Buchnera gen. nov. and Buchnera aphidicola sp. nov., a taxon consisting of the mycetocyte-associated, primary endosymbionts of aphids. Int. J. Syst. Evol. Microbiol. 1991, 41, 566–568. [Google Scholar] [CrossRef]
- Unterman, B.; Baumann, P.; McLean, D. Pea aphid symbiont relationships established by analysis of 16S rRNAs. J. Bacteriol. 1989, 171, 2970–2974. [Google Scholar] [CrossRef]
- Oliver, K.M.; Degnan, P.H.; Burke, G.R.; Moran, N.A. Facultative symbionts in aphids and the horizontal transfer of ecologically important traits. Annu. Rev. Entomol. 2010, 55, 247–266. [Google Scholar] [CrossRef]
- Guo, J.; Hatt, S.; He, K.; Chen, J.; Francis, F.; Wang, Z. Nine facultative endosymbionts in aphids. A review. J. Asia-Pacif. Entomol. 2017, 20, 794–801. [Google Scholar] [CrossRef]
- Fukatsu, T.; Tsuchida, T.; Nikoh, N.; Koga, R. Spiroplasma symbiont of the pea aphid, Acyrthosiphon pisum (Insecta: Homoptera). Appl. Environ. Microbiol. 2001, 67, 1284–1291. [Google Scholar] [CrossRef] [PubMed]
- Sandström, J.P.; Russell, J.A.; White, J.P.; Moran, N.A. Independent origins and horizontal transfer of bacterial symbionts of aphids. Mol. Ecol. 2001, 10, 217–228. [Google Scholar] [CrossRef] [PubMed]
- Tsuchida, T.; Koga, R.; Shibao, H.; Matsumoto, T.; Fukatsu, T. Diversity and geographic distribution of secondary endosymbiotic bacteria in natural populations of the pea aphid, Acyrthosiphon pisum. Mol. Ecol. 2002, 11, 2123–2135. [Google Scholar] [CrossRef]
- Russell, J.; Latorre, A.; Sabater-Muñoz, B.; Moya, A.; Moran, N. Side-stepping secondary symbionts: Widespread horizontal transfer across and beyond the Aphidoidea. Mol. Ecol. 2003, 12, 1061–1075. [Google Scholar] [CrossRef]
- Gómez-Valero, L.; Soriano-Navarro, M.; Pérez-Brocal, V.; Heddi, A.; Moya, A.; García-Verdugo, J.M.; Latorre, A. Coexistence of Wolbachia with Buchnera aphidicola and a secondary symbiont in the aphid Cinara cedri. J. Bacteriol. 2004, 186, 6626–6633. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, M.; Koga, R.; Tsuchida, T.; Meng, X.-Y.; Fukatsu, T. Rickettsia symbiont in the pea aphid Acyrthosiphon pisum: Novel cellular tropism, effect on host fitness, and interaction with the essential symbiont Buchnera. Appl. Environ. Microbiol. 2005, 71, 4069–4075. [Google Scholar] [CrossRef]
- Moran, N.A.; Russell, J.A.; Koga, R.; Fukatsu, T. Evolutionary relationships of three new species of Enterobacteriaceae living as symbionts of aphids and other insects. Appl. Environ. Microbiol. 2005, 71, 3302–3310. [Google Scholar] [CrossRef]
- Guay, J.-F.; Boudreault, S.; Michaud, D.; Cloutier, C. Impact of environmental stress on aphid clonal resistance to parasitoids: Role of Hamiltonella defensa bacterial symbiosis in association with a new facultative symbiont of the pea aphid. J. Insect Physiol. 2009, 55, 919–926. [Google Scholar] [CrossRef]
- Tsuchida, T.; Koga, R.; Horikawa, M.; Tsunoda, T.; Maoka, T.; Matsumoto, S.; Simon, J.-C.; Fukatsu, T. Symbiotic bacterium modifies aphid body color. Science 2010, 330, 1102–1104. [Google Scholar] [CrossRef]
- Manzano-Marín, A.; Coeur d’acier, A.; Clamens, A.-L.; Cruaud, C.; Barbe, V.; Jousselin, E. Co-obligate symbioses have repeatedly evolved across aphids, but partner identity and nutritional contributions vary across lineages. Peer Community J. 2023, 3, e46. [Google Scholar] [CrossRef]
- Li, T.; Xiao, J.-H.; Xu, Z.-H.; Murphy, R.W.; Huang, D.-W. A possibly new Rickettsia-like genus symbiont is found in Chinese wheat pest aphid, Sitobion miscanthi (Hemiptera: Aphididae). J. Invertebr. Pathol. 2011, 106, 418–421. [Google Scholar] [CrossRef] [PubMed]
- McLean, A.H.; Godfray, H.C.J.; Ellers, J.; Henry, L.M. Host relatedness influences the composition of aphid microbiomes. Environ. Microbiol. Rep. 2019, 11, 808–816. [Google Scholar] [CrossRef] [PubMed]
- Zepeda-Paulo, F.; Romero, V.; Celis-Diez, J.L.; Lavandero, B. A newly discovered bacterial symbiont in the aphid microbiome identified through 16S rRNA sequencing. Symbiosis 2024, 93, 223–228. [Google Scholar] [CrossRef]
- Leonardo, T.E.; Muiru, G.T. Facultative symbionts are associated with host plant specialization in pea aphid populations. Proc. R. Soc. Lond. B Biol. Sci. 2003, 270 (Suppl. S2), S209–S212. [Google Scholar] [CrossRef]
- Ferrari, J.; Darby, A.C.; Daniell, T.J.; Godfray, H.C.J.; Douglas, A.E. Linking the bacterial community in pea aphids with host-plant use and natural enemy resistance. Ecol. Entomol. 2004, 29, 60–65. [Google Scholar] [CrossRef]
- Chen, D.-Q.; Purcell, A.H. Occurrence and transmission of facultative endosymbionts in aphids. Curr. Microbiol. 1997, 34, 220–225. [Google Scholar] [CrossRef]
- Koga, R.; Tsuchida, T.; Fukatsu, T. Changing partners in an obligate symbiosis: A facultative endosymbiont can compensate for loss of the essential endosymbiont Buchnera in an aphid. Proc. R. Soc. Lond. B Biol. Sci. 2003, 270, 2543–2550. [Google Scholar] [CrossRef]
- Chen, D.Q.; Montllor, C.B.; Purcell, A.H. Fitness effects of two facultative endosymbiotic bacteria on the pea aphid, Acyrthosiphon pisum, and the blue alfalfa aphid, A. kondoi. Entomol. Exp. Appl. 2000, 95, 315–323. [Google Scholar] [CrossRef]
- Montllor, C.B.; Maxmen, A.; Purcell, A.H. Facultative bacterial endosymbionts benefit pea aphids Acyrthosiphon pisum under heat stress. Ecol. Entomol. 2002, 27, 189–195. [Google Scholar] [CrossRef]
- Oliver, K.M.; Russell, J.A.; Moran, N.A.; Hunter, M.S. Facultative bacterial symbionts in aphids confer resistance to parasitic wasps. Proc. Natl. Acad. Sci. USA 2003, 100, 1803–1807. [Google Scholar] [CrossRef]
- Scarborough, C.L.; Ferrari, J.; Godfray, H. Aphid protected from pathogen by endosymbiont. Science 2005, 310, 1781. [Google Scholar] [CrossRef] [PubMed]
- Oliver, K.M.; Higashi, C.H. Variations on a protective theme: Hamiltonella defensa infections in aphids variably impact parasitoid success. Curr. Opin. Insect Sci. 2019, 32, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Vorburger, C. The evolutionary ecology of symbiont-conferred resistance to parasitoids in aphids. Insect Sci. 2014, 21, 251–264. [Google Scholar] [CrossRef] [PubMed]
- McLean, A.H.; Parker, B.J.; Hrček, J.; Kavanagh, J.C.; Wellham, P.A.; Godfray, H.C.J. Consequences of symbiont co-infections for insect host phenotypes. J. Anim. Ecol. 2018, 87, 478–488. [Google Scholar] [CrossRef]
- Vorburger, C.; Gehrer, L.; Rodriguez, P. A strain of the bacterial symbiont Regiella insecticola protects aphids against parasitoids. Biol. Lett. 2010, 6, 109–111. [Google Scholar] [CrossRef]
- Oliver, K.M.; Moran, N.A.; Hunter, M.S. Costs and benefits of a superinfection of facultative symbionts in aphids. Proc. R. Soc. Lond. B. Biol. Sci. 2006, 273, 1273–1280. [Google Scholar] [CrossRef]
- Schmid, M.; Sieber, R.; Zimmermann, Y.S.; Vorburger, C. Development, specificity and sublethal effects of symbiont-conferred resistance to parasitoids in aphids. Funct. Ecol. 2012, 26, 207–215. [Google Scholar] [CrossRef]
- Asplen, M.K.; Bano, N.; Brady, C.M.; Desneux, N.; Hopper, K.R.; Malouines, C.; Oliver, K.M.; White, J.A.; Heimpel, G.E. Specialisation of bacterial endosymbionts that protect aphids from parasitoids. Ecol. Entomol. 2014, 39, 736–739. [Google Scholar] [CrossRef]
- Leybourne, D.J.; Bos, J.I.; Valentine, T.A.; Karley, A.J. The price of protection: A defensive endosymbiont impairs nymph growth in the bird cherry-oat aphid, Rhopalosiphum padi. Insect Sci. 2020, 27, 69–85. [Google Scholar] [CrossRef]
- Łukasik, P.; Guo, H.; Van Asch, M.; Ferrari, J.; Godfray, H. Protection against a fungal pathogen conferred by the aphid facultative endosymbionts Rickettsia and Spiroplasma is expressed in multiple host genotypes and species and is not influenced by co-infection with another symbiont. J. Evol. Biol. 2013, 26, 2654–2661. [Google Scholar] [CrossRef]
- Heyworth, E.; Ferrari, J. A facultative endosymbiont in aphids can provide diverse ecological benefits. J. Evol. Biol. 2015, 28, 1753–1760. [Google Scholar] [CrossRef] [PubMed]
- McLean, A.; Hrček, J.; Parker, B.J.; Mathé-Hubert, H.; Kaech, H.; Paine, C.; Godfray, H.C.J. Multiple phenotypes conferred by a single insect symbiont are independent. Proc. R. Soc. Lond. B. Biol. Sci. 2020, 287, 20200562. [Google Scholar] [CrossRef]
- Higashi, C.H.; Patel, V.; Kamalaker, B.; Inaganti, R.; Bressan, A.; Russell, J.A.; Oliver, K.M. Another tool in the toolbox: Aphid-specific Wolbachia protect against fungal pathogens. Environ. Microbiol. 2024, 26, e70005. [Google Scholar] [CrossRef] [PubMed]
- Parker, B.J.; Spragg, C.J.; Altincicek, B.; Gerardo, N.M. Symbiont-mediated protection against fungal pathogens in pea aphids: A role for pathogen specificity? Appl. Environ. Microbiol. 2013, 79, 2455–2458. [Google Scholar] [CrossRef] [PubMed]
- Higashi, C.H.; Nichols, W.L.; Chevignon, G.; Patel, V.; Allison, S.E.; Kim, K.L.; Strand, M.R.; Oliver, K.M. An aphid symbiont confers protection against a specialized RNA virus, another increases vulnerability to the same pathogen. Mol. Ecol. 2023, 32, 936–950. [Google Scholar] [CrossRef]
- Hamilton, P.T.; Perlman, S.J. Host defense via symbiosis in Drosophila. PLoS Pathog. 2013, 9, e1003808. [Google Scholar] [CrossRef][Green Version]
- Simon, J.-C.; Carre, S.; Boutin, M.; Prunier–Leterme, N.; Sabater–Muñoz, B.; Latorre, A.; Bournoville, R. Host–based divergence in populations of the pea aphid: Insights from nuclear markers and the prevalence of facultative symbionts. Proc. R. Soc. Lond. B Biol. Sci. 2003, 270, 1703–1712. [Google Scholar] [CrossRef]
- Tsuchida, T.; Koga, R.; Fukatsu, T. Host plant specialization governed by facultative symbiont. Science 2004, 303, 1989. [Google Scholar] [CrossRef]
- Ferrari, J.; Scarborough, C.L.; Godfray, H.C.J. Genetic variation in the effect of a facultative symbiont on host-plant use by pea aphids. Oecologia 2007, 153, 323–329. [Google Scholar] [CrossRef]
- Leonardo, T.E. Removal of a specialization-associated symbiont does not affect aphid fitness. Ecol. Lett. 2004, 7, 461–468. [Google Scholar] [CrossRef]
- Wagner, S.M.; Martinez, A.J.; Ruan, Y.M.; Kim, K.L.; Lenhart, P.A.; Dehnel, A.C.; Oliver, K.M.; White, J.A. Facultative endosymbionts mediate dietary breadth in a polyphagous herbivore. Funct. Ecol. 2015, 29, 1402–1410. [Google Scholar] [CrossRef]
- Angelella, G.; Nalam, V.; Nachappa, P.; White, J.; Kaplan, I. Endosymbionts differentially alter exploratory probing behavior of a nonpersistent plant virus vector. Microb. Ecol. 2018, 76, 453–458. [Google Scholar] [CrossRef]
- Li, Q.; Fan, J.; Sun, J.; Zhang, Y.; Hou, M.; Chen, J. Anti-plant defense response strategies mediated by the secondary symbiont Hamiltonella defensa in the wheat aphid Sitobion miscanthi. Front. Microbiol. 2019, 10, 2419. [Google Scholar] [CrossRef]
- Skaljac, M.; Vogel, H.; Wielsch, N.; Mihajlovic, S.; Vilcinskas, A. Transmission of a protease-secreting bacterial symbiont among pea aphids via host plants. Front. Physiol. 2019, 10, 438. [Google Scholar] [CrossRef]
- Wang, Q.; Yuan, E.; Ling, X.; Zhu-Salzman, K.; Guo, H.; Ge, F.; Sun, Y. An aphid facultative symbiont suppresses plant defence by manipulating aphid gene expression in salivary glands. Plant Cell Environ. 2020, 43, 2311–2322. [Google Scholar] [CrossRef] [PubMed]
- Frago, E.; Mala, M.; Weldegergis, B.T.; Yang, C.; McLean, A.; Godfray, H.C.J.; Gols, R.; Dicke, M. Symbionts protect aphids from parasitic wasps by attenuating herbivore-induced plant volatiles. Nat. Commun. 2017, 8, 1860. [Google Scholar] [CrossRef] [PubMed]
- Oliver, K.; Higashi, C. Symbiosis in a rapidly changing world. In Microbes: The Foundation Stone of the Biosphere; Springer: Berlin/Heidelberg, Germany, 2021; pp. 263–296. [Google Scholar]
- Zhang, B.; Leonard, S.P.; Li, Y.; Moran, N.A. Obligate bacterial endosymbionts limit thermal tolerance of insect host species. Proc. Natl. Acad. Sci. USA 2019, 116, 24712–24718. [Google Scholar] [CrossRef] [PubMed]
- Russell, J.A.; Moran, N.A. Costs and benefits of symbiont infection in aphids: Variation among symbionts and across temperatures. Proc. R. Soc. Lond. B. Biol. Sci. 2006, 273, 603–610. [Google Scholar] [CrossRef]
- Burke, G.; Fiehn, O.; Moran, N. Effects of facultative symbionts and heat stress on the metabolome of pea aphids. ISME J. 2010, 4, 242–252. [Google Scholar] [CrossRef]
- Heyworth, E.R.; Smee, M.R.; Ferrari, J. Aphid facultative symbionts aid recovery of their obligate symbiont and their host after heat stress. Front. Ecol. Evol. 2020, 8, 56. [Google Scholar] [CrossRef]
- Ling, X.; Guo, H.; Di, J.; Xie, L.; Zhu-Salzman, K.; Ge, F.; Zhao, Z.; Sun, Y. A complete DNA repair system assembled by two endosymbionts restores heat tolerance of the insect host. Proc. Natl. Acad. Sci. USA 2024, 121, e2415651121. [Google Scholar] [CrossRef] [PubMed]
- Tougeron, K.; Iltis, C.; Rampnoux, E.; Goerlinger, A.; Dhondt, L.; Hance, T. Still standing: The heat protection delivered by a facultative symbiont to its aphid host is resilient to repeated thermal stress. Curr. Res. Insect Sci. 2023, 3, 100061. [Google Scholar] [CrossRef] [PubMed]
- Doremus, M.R.; Smith, A.H.; Kim, K.L.; Holder, A.J.; Russell, J.A.; Oliver, K.M. Breakdown of a defensive symbiosis, but not endogenous defences, at elevated temperatures. Mol. Ecol. 2018, 27, 2138–2151. [Google Scholar] [CrossRef] [PubMed]
- Higashi, C.H.; Barton, B.T.; Oliver, K.M. Warmer nights offer no respite for a defensive mutualism. J. Anim. Ecol. 2020, 89, 1895–1905. [Google Scholar] [CrossRef]
- Vorburger, C.; Ganesanandamoorthy, P.; Kwiatkowski, M. Comparing constitutive and induced costs of symbiont-conferred resistance to parasitoids in aphids. Ecol. Evol. 2013, 3, 706–713. [Google Scholar] [CrossRef]
- McLean, A.; Van Asch, M.; Ferrari, J.; Godfray, H. Effects of bacterial secondary symbionts on host plant use in pea aphids. Proc. R. Soc. Lond. B. Biol. Sci. 2011, 278, 760–766. [Google Scholar] [CrossRef]
- Cayetano, L.; Rothacher, L.; Simon, J.-C.; Vorburger, C. Cheaper is not always worse: Strongly protective isolates of a defensive symbiont are less costly to the aphid host. Proc. R. Soc. Lond. B. Biol. Sci. 2015, 282, 20142333. [Google Scholar] [CrossRef]
- Martinez, A.J.; Doremus, M.R.; Kraft, L.J.; Kim, K.L.; Oliver, K.M. Multi-modal defences in aphids offer redundant protection and increased costs likely impeding a protective mutualism. J. Anim. Ecol. 2018, 87, 464–477. [Google Scholar] [CrossRef]
- Vorburger, C.; Gouskov, A. Only helpful when required: A longevity cost of harbouring defensive symbionts. J. Evol. Biol. 2011, 24, 1611–1617. [Google Scholar] [CrossRef]
- Oliver, K.M.; Campos, J.; Moran, N.A.; Hunter, M.S. Population dynamics of defensive symbionts in aphids. Proc. R. Soc. Lond. B. Biol. Sci. 2008, 275, 293–299. [Google Scholar] [CrossRef]
- Skaljac, M.; Kirfel, P.; Grotmann, J.; Vilcinskas, A. Fitness costs of infection with Serratia symbiotica are associated with greater susceptibility to insecticides in the pea aphid Acyrthosiphon pisum. Pest Manage. Sci. 2018, 74, 1829–1836. [Google Scholar] [CrossRef]
- Leybourne, D.J.; Melloh, P.; Martin, E.A. Common facultative endosymbionts do not influence sensitivity of cereal aphids to pyrethroids. Agric. For. Entomol. 2023, 25, 344–354. [Google Scholar] [CrossRef]
- Ramírez-Cáceres, G.E.; Moya-Hernández, M.G.; Quilodrán, M.; Nespolo, R.F.; Ceballos, R.; Villagra, C.A.; Ramírez, C.C. Harbouring the secondary endosymbiont Regiella insecticola increases predation risk and reproduction in the cereal aphid Sitobion avenae. J. Pest Sci. 2019, 92, 1039–1047. [Google Scholar] [CrossRef]
- Wulff, J.A.; White, J.A. The endosymbiont Arsenophonus provides a general benefit to soybean aphid (Hemiptera: Aphididae) regardless of host plant resistance (Rag). Environ. Entomol. 2015, 44, 574–581. [Google Scholar] [CrossRef] [PubMed]
- Bennett, G.M.; Moran, N.A. Heritable symbiosis: The advantages and perils of an evolutionary rabbit hole. Proc. Natl. Acad. Sci. USA 2015, 112, 10169–10176. [Google Scholar] [CrossRef]
- Meseguer, A.S.; Manzano-Marín, A.; Coeur D’Acier, A.; Clamens, A.L.; Godefroid, M.; Jousselin, E. Buchnera has changed flatmate but the repeated replacement of co-obligate symbionts is not associated with the ecological expansions of their aphid hosts. Mol. Ecol. 2017, 26, 2363–2378. [Google Scholar] [CrossRef]
- Manzano-Marín, A.; Szabó, G.; Simon, J.C.; Horn, M.; Latorre, A. Happens in the best of subfamilies: Establishment and repeated replacements of co-obligate secondary endosymbionts within Lachninae aphids. Environ. Microbiol. 2017, 19, 393–408. [Google Scholar] [CrossRef]
- Russell, J.A.; Oliver, K.M.; Hansen, A.K. Band-aids for Buchnera and B vitamins for all. Mol. Ecol. 2017, 26, 2199–2203. [Google Scholar] [CrossRef]
- Monnin, D.; Jackson, R.; Kiers, E.T.; Bunker, M.; Ellers, J.; Henry, L.M. Parallel evolution in the integration of a co-obligate aphid symbiosis. Curr. Biol. 2020, 30, 1949–1957.e1946. [Google Scholar] [CrossRef]
- Renoz, F.; Lopes, M.R.; Gaget, K.; Duport, G.; Eloy, M.-C.; Geelhand de Merxem, B.; Hance, T.; Calevro, F. Compartmentalized into bacteriocytes but highly invasive: The puzzling case of the co-obligate symbiont Serratia symbiotica in the aphid Periphyllus lyropictus. Microbiol. Spectr. 2022, 10, e00457-22. [Google Scholar] [CrossRef]
- Tian, P.-P.; Zhang, Y.-L.; Huang, J.-L.; Li, W.-Y.; Liu, X.-D. Arsenophonus interacts with Buchnera to improve growth performance of aphids under amino acid stress. Microbiol. Spectr. 2023, 11, e01792-23. [Google Scholar] [CrossRef] [PubMed]
- Koga, R.; Meng, X.-Y.; Tsuchida, T.; Fukatsu, T. Cellular mechanism for selective vertical transmission of an obligate insect symbiont at the bacteriocyte–embryo interface. Proc. Natl. Acad. Sci. USA 2012, 109, E1230–E1237. [Google Scholar] [CrossRef] [PubMed]
- Tsuchida, T.; Koga, R.; Fujiwara, A.; Fukatsu, T. Phenotypic effect of “Candidatus Rickettsiella viridis,” a facultative symbiont of the pea aphid (Acyrthosiphon pisum), and its interaction with a coexisting symbiont. Appl. Environ. Microbiol. 2014, 80, 525–533. [Google Scholar] [CrossRef] [PubMed]
- Martinez, A.J.; Weldon, S.R.; Oliver, K.M. Effects of parasitism on aphid nutritional and protective symbioses. Mol. Ecol. 2014, 23, 1594–1607. [Google Scholar] [CrossRef]
- Parker, B.J.; Hrček, J.; McLean, A.H.; Brisson, J.A.; Godfray, H.C.J. Intraspecific variation in symbiont density in an insect–microbe symbiosis. Mol. Ecol. 2021, 30, 1559–1569. [Google Scholar] [CrossRef]
- Enders, L.S.; Miller, N.J. Stress-induced changes in abundance differ among obligate and facultative endosymbionts of the soybean aphid. Ecol. Evol. 2016, 6, 818–829. [Google Scholar] [CrossRef]
- Dykstra, H.R.; Weldon, S.R.; Martinez, A.J.; White, J.A.; Hopper, K.R.; Heimpel, G.E.; Asplen, M.K.; Oliver, K.M. Factors limiting the spread of the protective symbiont Hamiltonella defensa in Aphis craccivora aphids. Appl. Environ. Microbiol. 2014, 80, 5818–5827. [Google Scholar] [CrossRef]
- Ives, A.R.; Barton, B.T.; Penczykowski, R.M.; Harmon, J.P.; Kim, K.L.; Oliver, K.; Radeloff, V.C. Self-perpetuating ecological–evolutionary dynamics in an agricultural host–parasite system. Nat. Ecol. Evol. 2020, 4, 702–711. [Google Scholar] [CrossRef]
- Smith, A.H.; O’Connor, M.P.; Deal, B.; Kotzer, C.; Lee, A.; Wagner, B.; Joffe, J.; Woloszynek, S.; Oliver, K.M.; Russell, J.A. Does getting defensive get you anywhere?—Seasonal balancing selection, temperature, and parasitoids shape real-world, protective endosymbiont dynamics in the pea aphid. Mol. Ecol. 2021, 30, 2449–2472. [Google Scholar] [CrossRef]
- Moran, N.A.; Dunbar, H.E. Sexual acquisition of beneficial symbionts in aphids. Proc. Natl. Acad. Sci. USA 2006, 103, 12803–12806. [Google Scholar] [CrossRef]
- Peccoud, J.; Bonhomme, J.; Mahéo, F.; de La Huerta, M.; Cosson, O.; Simon, J.C. Inheritance patterns of secondary symbionts during sexual reproduction of pea aphid biotypes. Insect Sci. 2014, 21, 291–300. [Google Scholar] [CrossRef]
- Vorburger, C.; Siegrist, G.; Rhyner, N. Faithful vertical transmission but ineffective horizontal transmission of bacterial endosymbionts during sexual reproduction of the black bean aphid, Aphis fabae. Ecol. Entomol. 2017, 42, 202–209. [Google Scholar] [CrossRef]
- Li, Q.; Fan, J.; Sun, J.; Wang, M.-Q.; Chen, J. Plant-mediated horizontal transmission of Hamiltonella defensa in the wheat aphid Sitobion miscanthi. J. Agric. Food Chem. 2018, 66, 13367–13377. [Google Scholar] [CrossRef] [PubMed]
- Gehrer, L.; Vorburger, C. Parasitoids as vectors of facultative bacterial endosymbionts in aphids. Biol. Lett. 2012, 8, 613–615. [Google Scholar] [CrossRef] [PubMed]
- Łukasik, P.; Guo, H.; van Asch, M.; Henry, L.M.; Godfray, H.C.J.; Ferrari, J. Horizontal transfer of facultative endosymbionts is limited by host relatedness. Evolution 2015, 69, 2757–2766. [Google Scholar] [CrossRef]
- Niepoth, N.; Ellers, J.; Henry, L.M. Symbiont interactions with non-native hosts limit the formation of new symbioses. BMC Evol. Biol. 2018, 18, 27. [Google Scholar] [CrossRef]
- Henry, L.M.; Peccoud, J.; Simon, J.-C.; Hadfield, J.D.; Maiden, M.J.; Ferrari, J.; Godfray, H.C.J. Horizontally transmitted symbionts and host colonization of ecological niches. Curr. Biol. 2013, 23, 1713–1717. [Google Scholar] [CrossRef]
- Renoz, F.; Foray, V.; Ambroise, J.; Baa-Puyoulet, P.; Bearzatto, B.; Mendez, G.L.; Grigorescu, A.S.; Mahillon, J.; Mardulyn, P.; Gala, J.-L. At the gate of mutualism: Identification of genomic traits predisposing to insect-bacterial symbiosis in pathogenic strains of the aphid symbiont Serratia symbiotica. Front. Cell. Infect. Microbiol. 2021, 11, 660007. [Google Scholar] [CrossRef]
- Russell, J.A.; Weldon, S.; Smith, A.H.; Kim, K.L.; Hu, Y.; Łukasik, P.; Doll, S.; Anastopoulos, I.; Novin, M.; Oliver, K.M. Uncovering symbiont-driven genetic diversity across North American pea aphids. Mol. Ecol. 2013, 22, 2045–2059. [Google Scholar] [CrossRef]
- Brady, C.M.; Asplen, M.K.; Desneux, N.; Heimpel, G.E.; Hopper, K.R.; Linnen, C.R.; Oliver, K.M.; Wulff, J.A.; White, J.A. Worldwide populations of the aphid Aphis craccivora are infected with diverse facultative bacterial symbionts. Microb. Ecol. 2014, 67, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Sepúlveda, D.A.; Zepeda-Paulo, F.; Ramírez, C.C.; Lavandero, B.; Figueroa, C.C. Diversity, frequency, and geographic distribution of facultative bacterial endosymbionts in introduced aphid pests. Insect Sci. 2017, 24, 511–521. [Google Scholar] [CrossRef]
- Jousselin, E.; Clamens, A.L.; Galan, M.; Bernard, M.; Maman, S.; Gschloessl, B.; Duport, G.; Meseguer, A.; Calevro, F.; Coeur D’Acier, A. Assessment of a 16S rRNA amplicon Illumina sequencing procedure for studying the microbiome of a symbiont-rich aphid genus. Mol. Ecol. Resour. 2016, 16, 628–640. [Google Scholar] [CrossRef]
- Xu, T.T.; Chen, J.; Jiang, L.Y.; Qiao, G.X. Diversity of bacteria associated with Hormaphidinae aphids (Hemiptera: Aphididae). Insect Sci. 2021, 28, 165–179. [Google Scholar] [CrossRef]
- Donner, S.H.; Slingerland, M.; Beekman, M.M.; Comte, A.; Dicke, M.; Zwaan, B.J.; Pannebakker, B.A.; Verhulst, E.C. Aphid populations are frequently infected with facultative endosymbionts. Environ. Microbiol. 2024, 26, e16599. [Google Scholar] [CrossRef]
- Augustinos, A.A.; Santos-Garcia, D.; Dionyssopoulou, E.; Moreira, M.; Papapanagiotou, A.; Scarvelakis, M.; Doudoumis, V.; Ramos, S.; Aguiar, A.F.; Borges, P.A. Detection and characterization of Wolbachia infections in natural populations of aphids: Is the hidden diversity fully unraveled? PLoS ONE 2011, 6, e28695. [Google Scholar] [CrossRef]
- Wang, Z.; Su, X.M.; Wen, J.; Jiang, L.Y.; Qiao, G.X. Widespread infection and diverse infection patterns of Wolbachia in Chinese aphids. Insect Sci. 2014, 21, 313–325. [Google Scholar] [CrossRef]
- Smith, A.H.; Łukasik, P.; O’Connor, M.P.; Lee, A.; Mayo, G.; Drott, M.T.; Doll, S.; Tuttle, R.; Disciullo, R.A.; Messina, A. Patterns, causes and consequences of defensive microbiome dynamics across multiple scales. Mol. Ecol. 2015, 24, 1135–1149. [Google Scholar] [CrossRef]
- Gimmi, E.; Wallisch, J.; Vorburger, C. Defensive symbiosis in the wild: Seasonal dynamics of parasitism risk and symbiont-conferred resistance. Mol. Ecol. 2023, 32, 4063–4077. [Google Scholar] [CrossRef]
- Rothacher, L.; Ferrer-Suay, M.; Vorburger, C. Bacterial endosymbionts protect aphids in the field and alter parasitoid community composition. Ecology 2016, 97, 1712–1723. [Google Scholar] [CrossRef]
- Doremus, M.R.; Oliver, K.M. Aphid heritable symbiont exploits defensive mutualism. Appl. Environ. Microbiol. 2017, 83, e03276-16. [Google Scholar] [CrossRef]
- Hrček, J.; McLean, A.H.; Godfray, H.C.J. Symbionts modify interactions between insects and natural enemies in the field. J. Anim. Ecol. 2016, 85, 1605–1612. [Google Scholar] [CrossRef]
- Moya, A.; Peretó, J.; Gil, R.; Latorre, A. Learning how to live together: Genomic insights into prokaryote-animal symbioses. Nat. Rev. Genet. 2008, 9, 218–229. [Google Scholar] [CrossRef] [PubMed]
- Burke, G.R.; Moran, N.A. Massive genomic decay in Serratia symbiotica, a recently evolved symbiont of aphids. Genome Biol. Evol. 2011, 3, 195–208. [Google Scholar] [CrossRef] [PubMed]
- Nikoh, N.; Koga, R.; Oshima, K.; Hattori, M.; Fukatsu, T. Genome sequence of “Candidatus Serratia symbiotica” strain IS, a facultative bacterial symbiont of the pea aphid Acyrthosiphon pisum. Microbiol. Resour. Announc. 2019, 8. [Google Scholar] [CrossRef] [PubMed]
- Degnan, P.H.; Yu, Y.; Sisneros, N.; Wing, R.A.; Moran, N.A. Hamiltonella defensa, genome evolution of protective bacterial endosymbiont from pathogenic ancestors. Proc. Natl. Acad. Sci. USA 2009, 106, 9063–9068. [Google Scholar] [CrossRef]
- Chevignon, G.; Boyd, B.M.; Brandt, J.W.; Oliver, K.M.; Strand, M.R. Culture-facilitated comparative genomics of the facultative symbiont Hamiltonella defensa. Genome Biol. Evol. 2018, 10, 786–802. [Google Scholar] [CrossRef]
- Yorimoto, S.; Hattori, M.; Kondo, M.; Shigenobu, S. Complex host/symbiont integration of a multi-partner symbiotic system in the eusocial aphid Ceratovacuna japonica. iScience 2022, 25, 105478. [Google Scholar] [CrossRef]
- Patel, V.; Lynn-Bell, N.; Chevignon, G.; Kucuk, R.A.; Higashi, C.H.; Carpenter, M.; Russell, J.A.; Oliver, K.M. Mobile elements create strain-level variation in the services conferred by an aphid symbiont. Environ. Microbiol. 2023, 25, 3333–3348. [Google Scholar] [CrossRef]
- Patel, V.; Chevignon, G.; Manzano-Marín, A.; Brandt, J.W.; Strand, M.R.; Russell, J.A.; Oliver, K.M. Cultivation-assisted genome of Candidatus Fukatsuia symbiotica; the enigmatic “X-type” symbiont of aphids. Genome Biol. Evol. 2019, 11, 3510–3522. [Google Scholar] [CrossRef]
- Maeda, G.P.; Kelly, M.K.; Sundar, A.; Moran, N.A. Intracellular defensive symbiont is culturable and capable of transovarial, vertical transmission. mBio 2024, 15, e03253-23. [Google Scholar] [CrossRef] [PubMed]
- Degnan, P.H.; Leonardo, T.E.; Cass, B.N.; Hurwitz, B.; Stern, D.; Gibbs, R.A.; Richards, S.; Moran, N.A. Dynamics of genome evolution in facultative symbionts of aphids. Environ. Microbiol. 2010, 12, 2060–2069. [Google Scholar] [CrossRef] [PubMed]
- Hansen, A.K.; Vorburger, C.; Moran, N.A. Genomic basis of endosymbiont-conferred protection against an insect parasitoid. Genome Res. 2012, 22, 106–114. [Google Scholar] [CrossRef]
- Nikoh, N.; Tsuchida, T.; Maeda, T.; Yamaguchi, K.; Shigenobu, S.; Koga, R.; Fukatsu, T. Genomic insight into symbiosis-induced insect color change by a facultative bacterial endosymbiont, ”Candidatus Rickettsiella viridis”. mBio 2018, 9. [Google Scholar] [CrossRef]
- El Karkouri, K.; Ghigo, E.; Raoult, D.; Fournier, P.-E. Genomic evolution and adaptation of arthropod-associated Rickettsia. Sci. Rep. 2022, 12, 3807. [Google Scholar] [CrossRef]
- Pontes, M.H.; Dale, C. Culture and manipulation of insect facultative symbionts. Trends Microbiol. 2006, 14, 406–412. [Google Scholar] [CrossRef]
- Kikuchi, Y.; Hosokawa, T.; Nikoh, N.; Meng, X.-Y.; Kamagata, Y.; Fukatsu, T. Host-symbiont co-speciation and reductive genome evolution in gut symbiotic bacteria of acanthosomatid stinkbugs. BMC Biol. 2009, 7, 2. [Google Scholar] [CrossRef]
- Sabri, A.; Leroy, P.; Haubruge, E.; Hance, T.; Frere, I.; Destain, J.; Thonart, P. Isolation, pure culture and characterization of Serratia symbiotica sp. nov., the R-type of secondary endosymbiont of the black bean aphid Aphis fabae. Int. J. Syst. Evol. Microbiol. 2011, 61, 2081–2088. [Google Scholar] [CrossRef]
- Elston, K.M.; Perreau, J.; Maeda, G.P.; Moran, N.A.; Barrick, J.E. Engineering a culturable Serratia symbiotica strain for aphid paratransgenesis. Appl. Environ. Microbiol. 2021, 87, e02245-20. [Google Scholar] [CrossRef]
- Moran, N.A.; Degnan, P.H.; Santos, S.R.; Dunbar, H.E.; Ochman, H. The players in a mutualistic symbiosis: Insects, bacteria, viruses, and virulence genes. Proc. Natl. Acad. Sci. USA 2005, 102, 16919–16926. [Google Scholar] [CrossRef] [PubMed]
- Degnan, P.H.; Moran, N.A. Evolutionary genetics of a defensive facultative symbiont of insects: Exchange of toxin-encoding bacteriophage. Mol. Ecol. 2008, 17, 916–929. [Google Scholar] [CrossRef]
- Degnan, P.H.; Moran, N.A. Diverse phage-encoded toxins in a protective insect endosymbiont. Appl. Environ. Microbiol. 2008, 74, 6782–6791. [Google Scholar] [CrossRef] [PubMed]
- Oliver, K.M.; Perlman, S.J. Toxin-mediated protection against natural enemies by insect defensive symbionts. In Advances in Insect Physiology; Elsevier: Amsterdam, The Netherlands, 2020; Volume 58, pp. 277–316. [Google Scholar]
- Boyd, B.M.; Chevignon, G.; Patel, V.; Oliver, K.M.; Strand, M.R. Evolutionary genomics of APSE: A tailed phage that lysogenically converts the bacterium Hamiltonella defensa into a heritable protective symbiont of aphids. Virol. J. 2021, 18, 219. [Google Scholar] [CrossRef] [PubMed]
- Rouïl, J.; Jousselin, E.; Coeur d’Acier, A.; Cruaud, C.; Manzano-Marín, A. The protector within: Comparative genomics of APSE phages across aphids reveals rampant recombination and diverse toxin arsenals. Genome Biol. Evol. 2020, 12, 878–889. [Google Scholar] [CrossRef] [PubMed]
- Van Der Wilk, F.; Dullemans, A.M.; Verbeek, M.; Van Den Heuvel, J.F. Isolation and characterization of APSE-1, a bacteriophage infecting the secondary endosymbiont of Acyrthosiphon pisum. Virology 1999, 262, 104–113. [Google Scholar] [CrossRef][Green Version]
- Oliver, K.M.; Degnan, P.H.; Hunter, M.S.; Moran, N.A. Bacteriophages encode factors required for protection in a symbiotic mutualism. Science 2009, 325, 992–994. [Google Scholar] [CrossRef]
- Lynn-Bell, N.L.; Strand, M.R.; Oliver, K.M. Bacteriophage acquisition restores protective mutualism. Microbiology 2019, 165, 985–989. [Google Scholar] [CrossRef]
- Brandt, J.W.; Chevignon, G.; Oliver, K.M.; Strand, M.R. Culture of an aphid heritable symbiont demonstrates its direct role in defence against parasitoids. Proc. R. Soc. Lond. B. Biol. Sci. 2017, 284, 20171925. [Google Scholar] [CrossRef]
- Paredes, J.C.; Herren, J.K.; Schüpfer, F.; Lemaitre, B. The role of lipid competition for endosymbiont-mediated protection against parasitoid wasps in Drosophila. mBio 2016, 7. [Google Scholar] [CrossRef]
- Gerardo, N.M.; Parker, B.J. Mechanisms of symbiont-conferred protection against natural enemies: An ecological and evolutionary framework. Curr. Opin. Insect Sci. 2014, 4, 8–14. [Google Scholar] [CrossRef]
- Hamilton, P.T.; Peng, F.; Boulanger, M.J.; Perlman, S.J. A ribosome-inactivating protein in a Drosophila defensive symbiont. Proc. Natl. Acad. Sci. USA 2016, 113, 350–355. [Google Scholar] [CrossRef] [PubMed]
- Ballinger, M.J.; Perlman, S.J. Generality of toxins in defensive symbiosis: Ribosome-inactivating proteins and defense against parasitic wasps in Drosophila. PLoS Pathog. 2017, 13, e1006431. [Google Scholar] [CrossRef] [PubMed]
- Hrdina, A.; Serra Canales, M.; Arias-Rojas, A.; Frahm, D.; Iatsenko, I. The endosymbiont Spiroplasma poulsonii increases Drosophila melanogaster resistance to pathogens by enhancing iron sequestration and melanization. mBio 2024, 15, e00936-24. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.L.; Bayles, D.O. The contribution of cytolethal distending toxin to bacterial pathogenesis. Crit. Rev. Microbiol. 2006, 32, 227–248. [Google Scholar] [CrossRef]
- Guerra, L.; Cortes-Bratti, X.; Guidi, R.; Frisan, T. The biology of the cytolethal distending toxins. Toxins 2011, 3, 172–190. [Google Scholar] [CrossRef]
- Bezine, E.; Vignard, J.; Mirey, G. The cytolethal distending toxin effects on Mammalian cells: A DNA damage perspective. Cells 2014, 3, 592–615. [Google Scholar] [CrossRef]
- Verster, K.I.; Cinege, G.; Lipinszki, Z.; Magyar, L.B.; Kurucz, É.; Tarnopol, R.L.; Ábrahám, E.; Darula, Z.; Karageorgi, M.; Tamsil, J.A. Evolution of insect innate immunity through domestication of bacterial toxins. Proc. Natl. Acad. Sci. USA 2023, 120, e2218334120. [Google Scholar] [CrossRef]
- Oliver, K.M. Flies co-opt bacterial toxins for use in defense against parasitoids. Proc. Natl. Acad. Sci. USA 2023, 120, e2304493120. [Google Scholar] [CrossRef]
- Rancès, E.; Ye, Y.H.; Woolfit, M.; McGraw, E.A.; O’Neill, S.L. The relative importance of innate immune priming in Wolbachia-mediated dengue interference. PLoS Pathog. 2012, 8, e1002548. [Google Scholar] [CrossRef]
- Kim, J.K.; Lee, J.B.; Huh, Y.R.; Am Jang, H.; Kim, C.-H.; Yoo, J.W.; Lee, B.L. Burkholderia gut symbionts enhance the innate immunity of host Riptortus pedestris. Dev. Comp. Immunol. 2015, 53, 265–269. [Google Scholar] [CrossRef]
- Laughton, A.M.; Garcia, J.R.; Gerardo, N.M. Condition-dependent alteration of cellular immunity by secondary symbionts in the pea aphid, Acyrthosiphon pisum. J. Insect Physiol. 2016, 86, 17–24. [Google Scholar] [CrossRef]
- Nichols, H.L.; Goldstein, E.B.; Saleh Ziabari, O.; Parker, B.J. Intraspecific variation in immune gene expression and heritable symbiont density. PLoS Pathog. 2021, 17, e1009552. [Google Scholar] [CrossRef]
- Luo, C.; Belghazi, M.; Schmitz, A.; Lemauf, S.; Desneux, N.; Simon, J.C.; Poirié, M.; Gatti, J.L. Hosting certain facultative symbionts modulates the phenoloxidase activity and immune response of the pea aphid Acyrthosiphon pisum. Insect Sci. 2021, 28, 1780–1799. [Google Scholar] [CrossRef] [PubMed]
- ter Braak, B.; Laughton, A.M.; Altincicek, B.; Parker, B.J.; Gerardo, N.M. Exposure to bacterial signals does not alter pea aphids’ survival upon a second challenge or investment in production of winged offspring. PLoS ONE 2013, 8, e73600. [Google Scholar] [CrossRef] [PubMed]
- Caragata, E.P.; Rancès, E.; Hedges, L.M.; Gofton, A.W.; Johnson, K.N.; O’Neill, S.L.; McGraw, E.A. Dietary cholesterol modulates pathogen blocking by Wolbachia. PLoS Pathog. 2013, 9, e1003459. [Google Scholar] [CrossRef] [PubMed]
- Oliver, K.M.; Noge, K.; Huang, E.M.; Campos, J.M.; Becerra, J.X.; Hunter, M.S. Parasitic wasp responses to symbiont-based defense in aphids. BMC Biol. 2012, 10, 11. [Google Scholar] [CrossRef]
- Martinez, A.J.; Kim, K.L.; Harmon, J.P.; Oliver, K.M. Specificity of multi-modal aphid defenses against two rival parasitoids. PLoS ONE 2016, 11, e0154670. [Google Scholar] [CrossRef]
- Rouchet, R.; Vorburger, C. Strong specificity in the interaction between parasitoids and symbiont-protected hosts. J. Evol. Biol. 2012, 25, 2369–2375. [Google Scholar] [CrossRef]
- Cayetano, L.; Vorburger, C. Symbiont-conferred protection against Hymenopteran parasitoids in aphids: How general is it? Ecol. Entomol. 2015, 40, 85–93. [Google Scholar] [CrossRef]
- Hopper, K.R.; Kuhn, K.L.; Lanier, K.; Rhoades, J.H.; Oliver, K.M.; White, J.A.; Asplen, M.K.; Heimpel, G.E. The defensive aphid symbiont Hamiltonella defensa affects host quality differently for Aphelinus glycinis versus Aphelinus atriplicis. Biol. Control 2018, 116, 3–9. [Google Scholar] [CrossRef]
- McLean, A.H.; Godfray, H.C.J. Evidence for specificity in symbiont-conferred protection against parasitoids. Proc. R. Soc. Lond. B. Biol. Sci. 2015, 282, 20150977. [Google Scholar] [CrossRef] [PubMed]
- Dennis, A.B.; Patel, V.; Oliver, K.M.; Vorburger, C. Parasitoid gene expression changes after adaptation to symbiont-protected hosts. Evolution 2017, 71, 2599–2617. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Monnin, D.; Lee, R.A.; Henry, L.M. Local adaptation to hosts and parasitoids shape Hamiltonella defensa genotypes across aphid species. Proc. R. Soc. Lond. B. Biol. Sci. 2022, 289, 20221269. [Google Scholar] [CrossRef]
- Käch, H.; Mathé-Hubert, H.; Dennis, A.B.; Vorburger, C. Rapid evolution of symbiont-mediated resistance compromises biological control of aphids by parasitoids. Evol. Appl. 2018, 11, 220–230. [Google Scholar] [CrossRef] [PubMed]
- McLean, A.H.; Godfray, H.C.J. The outcome of competition between two parasitoid species is influenced by a facultative symbiont of their aphid host. Funct. Ecol. 2017, 31, 927–933. [Google Scholar] [CrossRef]
- Kraft, L.J.; Kopco, J.; Harmon, J.P.; Oliver, K.M. Aphid symbionts and endogenous resistance traits mediate competition between rival parasitoids. PLoS ONE 2017, 12, e0180729. [Google Scholar] [CrossRef]
- Sanders, D.; Kehoe, R.; van Veen, F.F.; McLean, A.; Godfray, H.C.J.; Dicke, M.; Gols, R.; Frago, E. Defensive insect symbiont leads to cascading extinctions and community collapse. Ecol. Lett. 2016, 19, 789–799. [Google Scholar] [CrossRef]
- Weldon, S.; Strand, M.; Oliver, K. Phage loss and the breakdown of a defensive symbiosis in aphids. Proc. R. Soc. Lond. B. Biol. Sci. 2013, 280, 20122103. [Google Scholar] [CrossRef]
- Goldstein, E.B.; de Anda Acosta, Y.; Henry, L.M.; Parker, B.J. Variation in density, immune gene suppression, and coinfection outcomes among strains of the aphid endosymbiont Regiella insecticola. Evolution 2023, 77, 1704–1711. [Google Scholar] [CrossRef]
- Smee, M.R.; Raines, S.A.; Ferrari, J. Genetic identity and genotype × genotype interactions between symbionts outweigh species level effects in an insect microbiome. ISME J. 2021, 15, 2537–2546. [Google Scholar] [CrossRef]
- Zytynska, S.E.; Weisser, W.W. The natural occurrence of secondary bacterial symbionts in aphids. Ecol. Entomol. 2016, 41, 13–26. [Google Scholar] [CrossRef]
- Rock, D.I.; Smith, A.H.; Joffe, J.; Albertus, A.; Wong, N.; O’Connor, M.; Oliver, K.M.; Russell, J.A. Context-dependent vertical transmission shapes strong endosymbiont community structure in the pea aphid, Acyrthosiphon pisum. Mol. Ecol. 2018, 27, 2039–2056. [Google Scholar] [CrossRef]
- Weldon, S.; Russell, J.; Oliver, K. More is not always better: Coinfections with defensive symbionts generate highly variable outcomes. Appl. Environ. Microbiol. 2020, 86, e02537-19. [Google Scholar] [CrossRef]
- Leclair, M.; Pons, I.; Mahéo, F.; Morlière, S.; Simon, J.-C.; Outreman, Y. Diversity in symbiont consortia in the pea aphid complex is associated with large phenotypic variation in the insect host. Evol. Ecol. 2016, 30, 925–941. [Google Scholar] [CrossRef]
- Leclair, M.; Polin, S.; Jousseaume, T.; Simon, J.C.; Sugio, A.; Morliere, S.; Fukatsu, T.; Tsuchida, T.; Outreman, Y. Consequences of coinfection with protective symbionts on the host phenotype and symbiont titres in the pea aphid system. Insect Sci. 2017, 24, 798–808. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Hoban, J.; Joffe, J.; Smith, A.H.; Carpenter, M.; Marcelis, T.; Patel, V.; Lynn-Bell, N.; Oliver, K.M.; Russell, J.A. Cryptic community structure and metabolic interactions among the heritable facultative symbionts of the pea aphid. J. Evol. Biol. 2023, 36, 1712–1730. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, M.; Peng, L.; Smith, A.H.; Joffe, J.; O’Connor, M.; Oliver, K.M.; Russell, J.A. Frequent drivers, occasional passengers: Signals of symbiont-driven seasonal adaptation and hitchhiking in the pea aphid, Acyrthosiphon pisum. Insects 2021, 12, 805. [Google Scholar] [CrossRef]
- Polin, S.; Le Gallic, J.-F.; Simon, J.-C.; Tsuchida, T.; Outreman, Y. Conditional reduction of predation risk associated with a facultative symbiont in an insect. PLoS ONE 2015, 10, e0143728. [Google Scholar] [CrossRef]
- Mathé-Hubert, H.; Kaech, H.; Ganesanandamoorthy, P.; Vorburger, C. Evolutionary costs and benefits of infection with diverse strains of Spiroplasma in pea aphids. Evolution 2019, 73, 1466–1481. [Google Scholar] [CrossRef]
- Henter, H.J.; Via, S. The potential for coevolution in a host-parasitoid system. I. Genetic variation within an aphid population in susceptibility to a parasitic wasp. Evolution 1995, 49, 427–438. [Google Scholar] [CrossRef]
- Ferrari, J.; Müller, C.B.; Kraaijeveld, A.R.; Godfray, H.C.J. Clonal variation and covariation in aphid resistance to parasitoids and a pathogen. Evolution 2001, 55, 1805–1814. [Google Scholar] [CrossRef] [PubMed]
- Parker, B.J.; Garcia, J.R.; Gerardo, N.M. Genetic variation in resistance and fecundity tolerance in a natural host-pathogen interaction. Evolution 2014, 68, 2421–2429. [Google Scholar] [CrossRef] [PubMed]
- Hrček, J.; Parker, B.J.; McLean, A.H.; Simon, J.-C.; Mann, C.M.; Godfray, H.C.J. Hosts do not simply outsource pathogen resistance to protective symbionts. Evolution 2018, 72, 1488–1499. [Google Scholar] [CrossRef] [PubMed]
- Verster, K.I.; Wisecaver, J.H.; Karageorgi, M.; Duncan, R.P.; Gloss, A.D.; Armstrong, E.E.; Price, D.K.; Menon, A.R.; Ali, Z.M.; Whiteman, N.K. Horizontal transfer of bacterial cytolethal distending toxin B genes to insects. Mol. Biol. Evol. 2019, 36, 2105–2110. [Google Scholar] [CrossRef]
- Le Trionnaire, G.; Tanguy, S.; Hudaverdian, S.; Gléonnec, F.; Richard, G.; Cayrol, B.; Monsion, B.; Pichon, E.; Deshoux, M.; Webster, C. An integrated protocol for targeted mutagenesis with CRISPR-Cas9 system in the pea aphid. Insect Biochem. Mol. Biol. 2019, 110, 34–44. [Google Scholar] [CrossRef]
- Ghodke, A.B.; Good, R.T.; Golz, J.F.; Russell, D.A.; Edwards, O.; Robin, C. Extracellular endonucleases in the midgut of Myzus persicae may limit the efficacy of orally delivered RNAi. Sci. Rep. 2019, 9, 11898. [Google Scholar] [CrossRef]
- Elston, K.M.; Maeda, G.P.; Perreau, J.; Barrick, J.E. Addressing the challenges of symbiont-mediated RNAi in aphids. PeerJ 2023, 11, e14961. [Google Scholar] [CrossRef]
- McLean, A.H.; Parker, B.J.; Hrček, J.; Henry, L.M.; Godfray, H.C.J. Insect symbionts in food webs. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2016, 371, 20150325. [Google Scholar] [CrossRef]
- Ye, Z.; Vollhardt, I.M.; Parth, N.; Rubbmark, O.; Traugott, M. Facultative bacterial endosymbionts shape parasitoid food webs in natural host populations: A correlative analysis. J. Anim. Ecol. 2018, 87, 1440–1451. [Google Scholar] [CrossRef]
- Erickson, D.M.; Wood, E.A.; Oliver, K.M.; Billick, I.; Abbot, P. The effect of ants on the population dynamics of a protective symbiont of aphids, Hamiltonella defensa. Ann. Entomol. Soc. Am. 2012, 105, 447–453. [Google Scholar] [CrossRef]
- Dion, E.; Polin, S.E.; Simon, J.-C.; Outreman, Y. Symbiont infection affects aphid defensive behaviours. Biol. Lett. 2011, 7, 743–746. [Google Scholar] [CrossRef] [PubMed]
- Attia, S.; Renoz, F.; Pons, I.; Louâpre, P.; Foray, V.; Piedra, J.-M.; Sanané, I.; Le Goff, G.; Lognay, G.; Hance, T. The aphid facultative symbiont Serratia symbiotica influences the foraging behaviors and the life-history traits of the parasitoid Aphidius ervi. Entomol. Gen. 2022, 42, 21–23. [Google Scholar] [CrossRef]
- Rouchet, R.; Vorburger, C. Experimental evolution of parasitoid infectivity on symbiont-protected hosts leads to the emergence of genotype specificity. Evolution 2014, 68, 1607–1616. [Google Scholar] [CrossRef] [PubMed]
- Hafer, N.; Vorburger, C. Diversity begets diversity: Do parasites promote variation in protective symbionts? Curr. Opin. Insect Sci. 2019, 32, 8–14. [Google Scholar] [CrossRef]
- Wu, T.; Rodrigues, A.A.; Fayle, T.M.; Henry, L.M. Defensive symbiont genotype distributions are linked to parasitoid attack networks. Ecol. Lett. 2025, 28, e70082. [Google Scholar] [CrossRef]
- Rossbacher, S.; Vorburger, C. Prior adaptation of parasitoids improves biological control of symbiont-protected pests. Evol. Appl. 2020, 13, 1868–1876. [Google Scholar] [CrossRef]
- Hafer-Hahmann, N.; Vorburger, C. Positive association between the diversity of symbionts and parasitoids of aphids in field populations. Ecosphere 2021, 12, e03355. [Google Scholar] [CrossRef]
- Leclair, M.; Buchard, C.; Mahéo, F.; Simon, J.-C.; Outreman, Y. A link between communities of protective endosymbionts and parasitoids of the pea aphid revealed in unmanipulated agricultural systems. Front. Ecol. Evol. 2021, 9, 618331. [Google Scholar] [CrossRef]
- Hafer-Hahmann, N.; Vorburger, C. Parasitoid species diversity has no effect on protective symbiont diversity in experimental host-parasitoid populations. Ecol. Evol. 2024, 14, e11090. [Google Scholar] [CrossRef]
- Monticelli, L.S.; Outreman, Y.; Frago, E.; Desneux, N. Impact of host endosymbionts on parasitoid host range—From mechanisms to communities. Curr. Opin. Insect Sci. 2019, 32, 77–82. [Google Scholar] [CrossRef]
- Gimmi, E.; Vorburger, C. High specificity of symbiont-conferred resistance in an aphid-parasitoid field community. J. Evol. Biol. 2024, 37, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Vorburger, C.; Rouchet, R. Are aphid parasitoids locally adapted to the prevalence of defensive symbionts in their hosts? BMC Evol. Biol. 2016, 16, 271. [Google Scholar] [CrossRef] [PubMed]
- Oliver, K.M.; Smith, A.H.; Russell, J.A. Defensive symbiosis in the real world—Advancing ecological studies of heritable, protective bacteria in aphids and beyond. Funct. Ecol. 2014, 28, 341–355. [Google Scholar] [CrossRef]
- Desneux, N.; Asplen, M.K.; Brady, C.M.; Heimpel, G.E.; Hopper, K.R.; Luo, C.; Monticelli, L.; Oliver, K.M.; White, J.A. Intraspecific variation in facultative symbiont infection among native and exotic pest populations: Potential implications for biological control. Biol. Control 2018, 116, 27–35. [Google Scholar] [CrossRef]
- Nell, L.A.; Kishinevsky, M.; Bosch, M.J.; Sinclair, C.; Bhat, K.; Ernst, N.; Boulaleh, H.; Oliver, K.M.; Ives, A.R. Dispersal stabilizes coupled ecological and evolutionary dynamics in a host-parasitoid system. Science 2024, 383, 1240–1244. [Google Scholar] [CrossRef]
- Yu, H.; Guo, J.; Wu, X.; Liang, J.; Fan, S.; Du, H.; Zhao, S.; Li, Z.; Liu, G.; Xiao, Y. Haplotype-resolved genome assembly provides insights into the genetic basis of green peach aphid resistance in peach. Curr. Biol. 2025, 35, 2614–2629.e2615. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kucuk, R.A.; Trendle, B.R.; Jones, K.C.; Makarenko, A.; Patel, V.; Oliver, K.M. Ecological Mercenaries: Why Aphids Remain Premier Models for the Study of Ecological Symbiosis. Insects 2025, 16, 1000. https://doi.org/10.3390/insects16101000
Kucuk RA, Trendle BR, Jones KC, Makarenko A, Patel V, Oliver KM. Ecological Mercenaries: Why Aphids Remain Premier Models for the Study of Ecological Symbiosis. Insects. 2025; 16(10):1000. https://doi.org/10.3390/insects16101000
Chicago/Turabian StyleKucuk, Roy A., Benjamin R. Trendle, Kenedie C. Jones, Alina Makarenko, Vilas Patel, and Kerry M. Oliver. 2025. "Ecological Mercenaries: Why Aphids Remain Premier Models for the Study of Ecological Symbiosis" Insects 16, no. 10: 1000. https://doi.org/10.3390/insects16101000
APA StyleKucuk, R. A., Trendle, B. R., Jones, K. C., Makarenko, A., Patel, V., & Oliver, K. M. (2025). Ecological Mercenaries: Why Aphids Remain Premier Models for the Study of Ecological Symbiosis. Insects, 16(10), 1000. https://doi.org/10.3390/insects16101000





