Age-Stage, Two-Sex Life Table of Leptinotarsa decemlineata (Coleoptera: Chrysomelidae) Experiencing Cadmium Stress
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Rearing of Insects and Plants
2.2. Effect of Cadmium on Leptinotarsa decemlineata
2.3. Statistical Analysis
3. Results
3.1. Developmental Period, Longevity, and Fecundity of Leptinotarsa decemlineata in Response to Different Potencies
3.2. Population Parameters of Leptinotarsa decemlineata in Response to Different Potencies
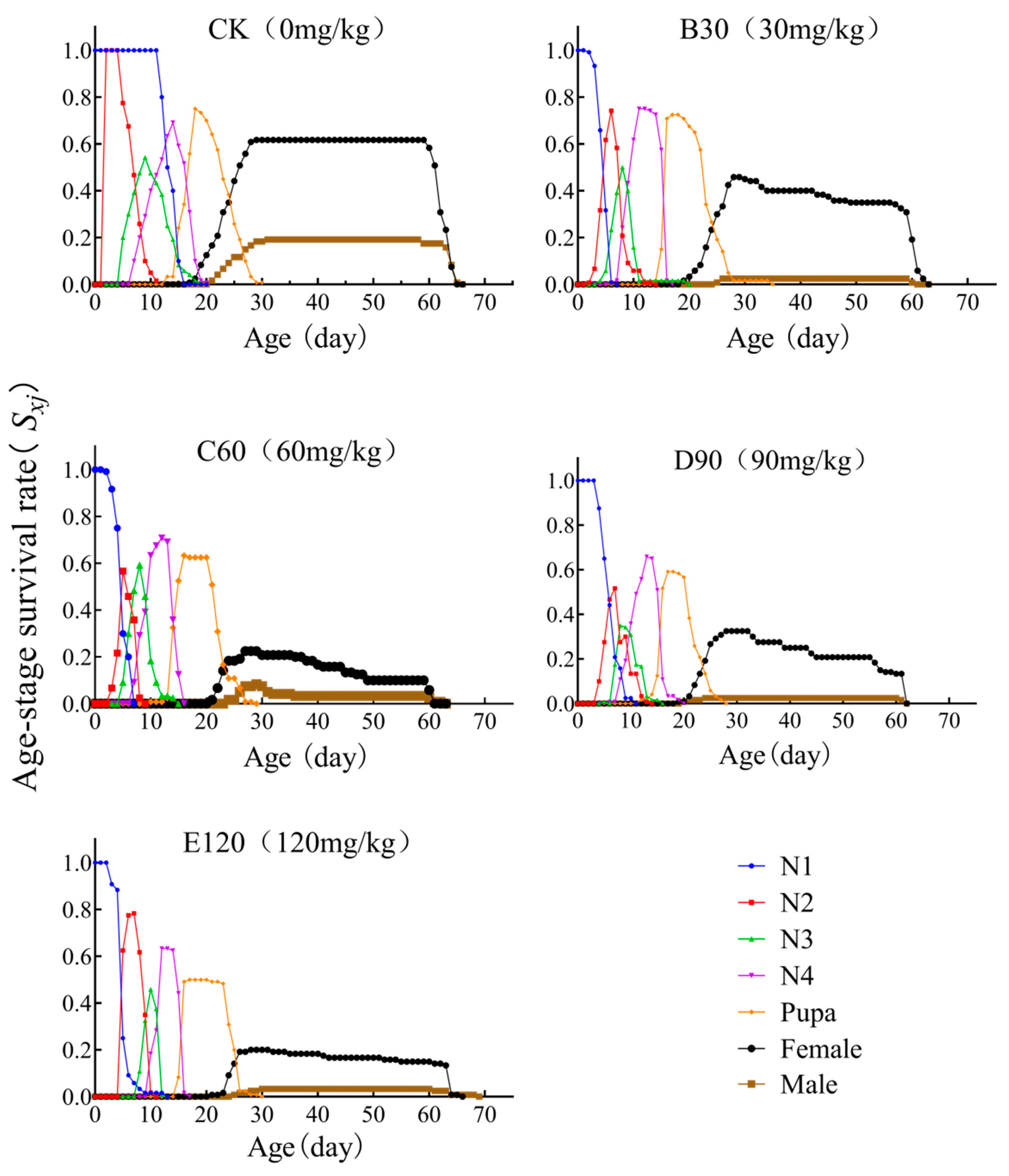
3.3. Age-Stage-Specific Survival Rate
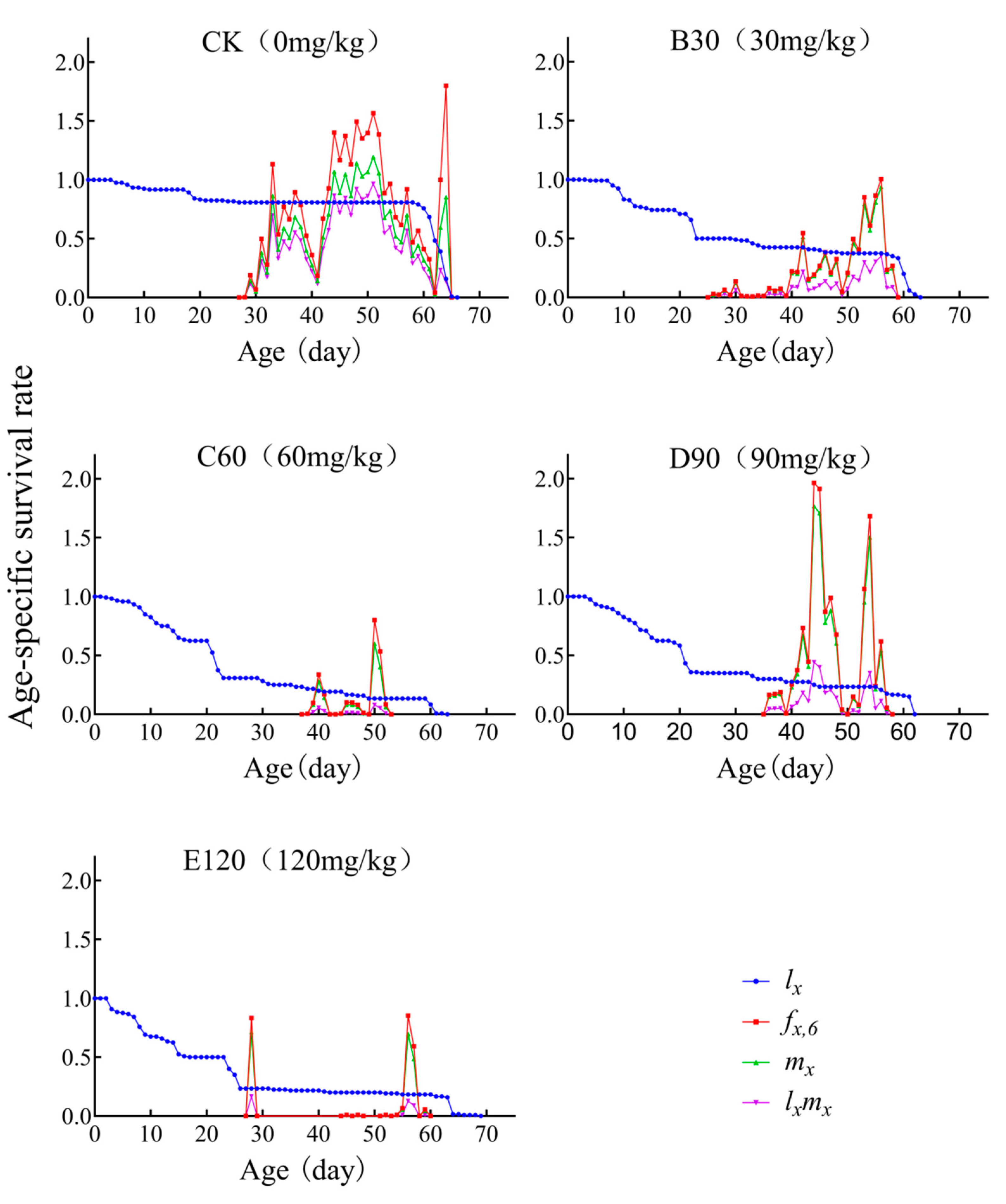
3.4. Age-Specific Survivability and Age-Stage-Specific Fecundity
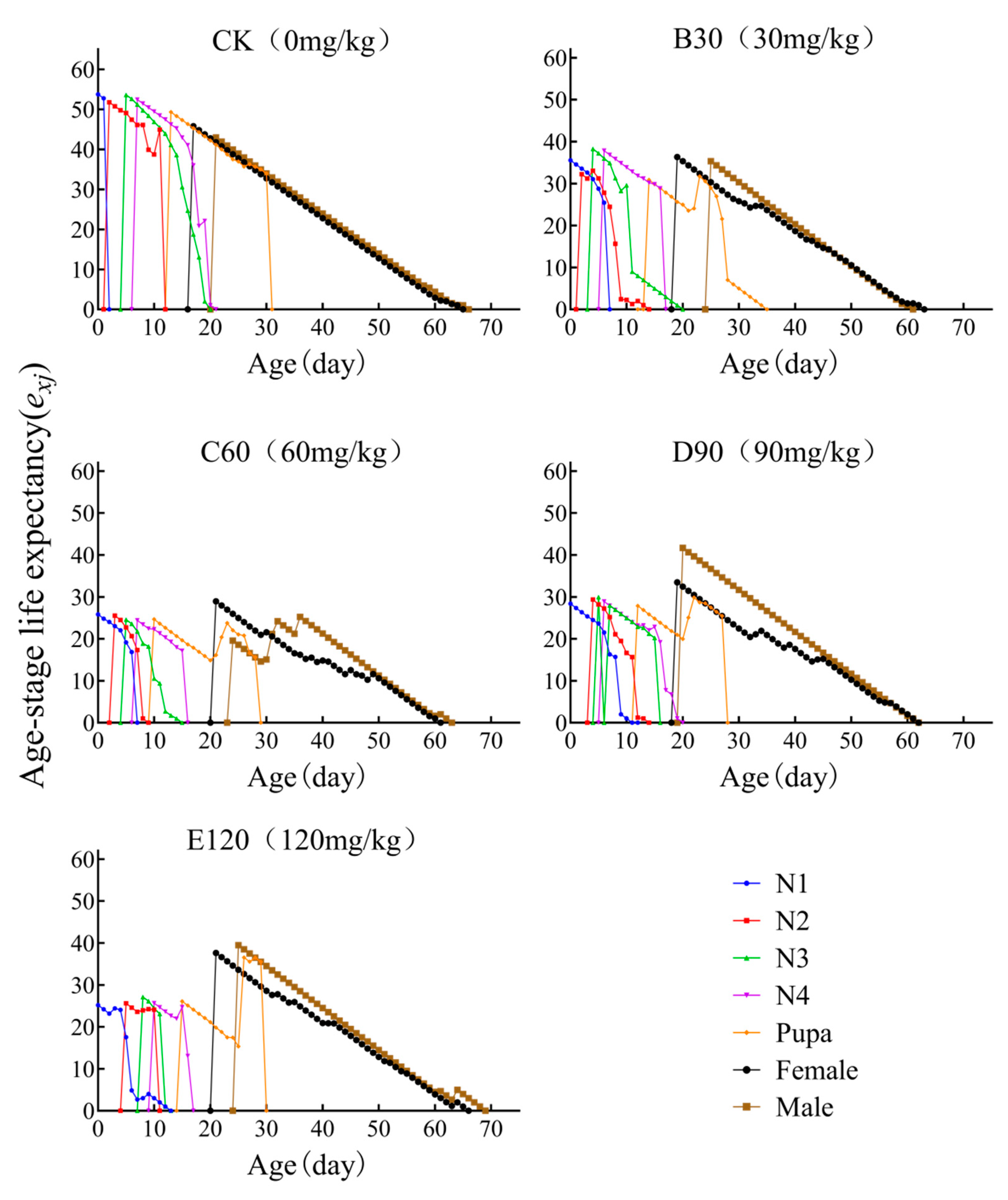
3.5. Age-Stage-Specific Life Expectancy
3.6. Age-Stage-Specific Reproductive Value
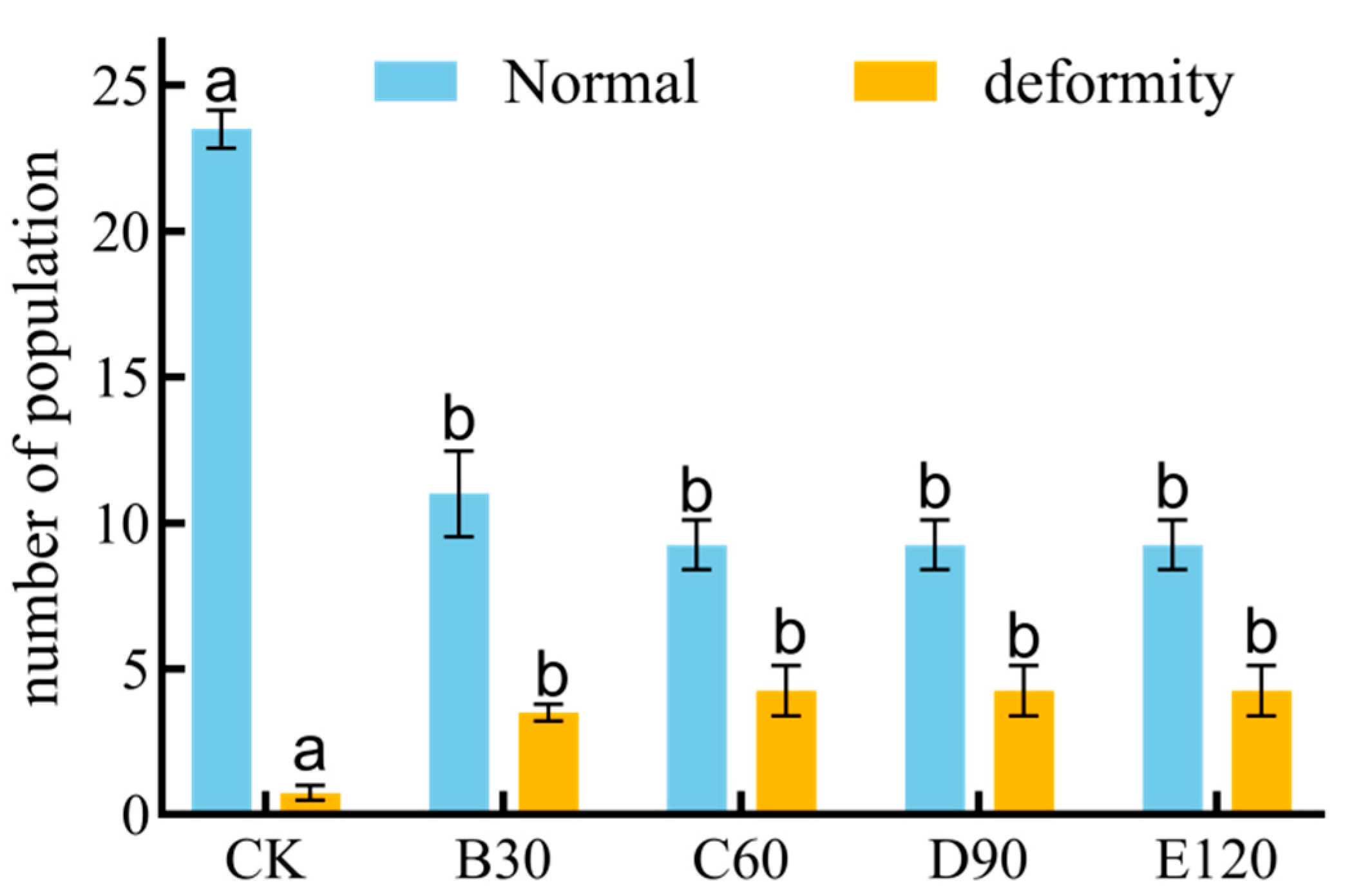
3.7. Deformity of Leptinotarsa decemlineata in Response to Different Potencies
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, X.; Yang, H.X.; Niu, F.S.; Sun, H.H.; Li, C. Impact of water stress on the demographic traits and population projection of Colorado potato beetle. Front. Physiol. 2023, 14, 1148129. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.H.; Liu, J.; Guan, Z.J.; Li, C. Duration of Low temperature exposure affects egg hatching of the Colorado potato beetle and emergence of overwintering adults. Insects 2021, 12, 12070609. [Google Scholar] [CrossRef]
- Liu, N.; Li, Y.C.; Zhang, R.Z. Invasion of Colorado potato beetle, Leptinotarsa decemlineata, in China: Dispersal, occurrence, and economic impact. Entomol. Exp. Appl. 2012, 143, 207–217. [Google Scholar] [CrossRef]
- Caselles, M.; Moral; Espinosa, P.; Murcia, P. Cadmium accumulation and distribution in cucumber plant. J. Plant Nutr. 2000, 23, 243–250. [Google Scholar] [CrossRef]
- Heinz, G.H.; Moriarty, F. Ecotoxicology, third edition: The study of pollutants in ecosystems. J. Wildl. Manag. 1984, 48, 1481. [Google Scholar] [CrossRef][Green Version]
- Li, Y.J. Environmental Problems and Countermeasures of Intensive Agriculture; China Agricultural Press: Beijing, China, 2001. [Google Scholar]
- National General Survey of Soil Contamination. China Environmental Protection Industry; Ministry of Agriculture and Rural Affairs of the People’s Republic of China: Beijing, China, 2014; pp. 10–11.
- Zhang, X.; Kun, W.L.; Chen, J.K.; Liang, J.; Cui, L.J. Effects of Cadmium Stress on Seedlings Growth and Active Ingredients in Salvia miltiorrhiza. Plant Sci. J. 2013, 31, 583–589. [Google Scholar] [CrossRef]
- Fu, W.L.; Yi, Z.D.; Zhang, M. Toxicity effect of cadmium stress exposure to insects and defense mechanism of insects. Chin. J. Pharmacol. Toxicol. 2015, 29, 1001–1006. [Google Scholar]
- Xiong, M.X.; Wu, D.; Xu, X.N.; Zhen, M.Y.; Xing, T. Advances in Toxic Effects of Soil Heavy Metal Cadmium on Higher Plants. Asian J. Ecotoxicol. 2021, 16, 133–149. [Google Scholar]
- Song, B.; Chen, T.B.; Zheng, Y.M.; Huang, Z.C.; Zheng, G.D.; Luo, J.F. A survey of cadmium concentrations in vegetables and soils in Beijing and the potential risks to human health. J. Environ. Sci. 2006, 26, 1343–1353. [Google Scholar]
- Zhang, Y.Y. Temporal and Spatial Distribution and Risk Assessment of Cadmium in Farmland Soils in China Based on Bibliometrics. Master’s Thesis, Shanxi University, Taiyuan, China, 2019. [Google Scholar]
- Liu, Z.Z. Transfer of Heavy Metal Cadmium in Rice Paddy Field and Its Effects on the Growth and Reproductive Behaviors of Chilo suppressalis (Walker). Master’s Thesis, Jiangxi Agricultural University, Nanchang, China, 2020. [Google Scholar]
- Ding, S.L.; Liu, C.L.; Qian, Q.; Gao, Z.Y. Research Advances on Molecular Genetic Mechanism for Cadmium Absorption and Transportation in Rice. Chin. J. Rice Sci. 2019, 33, 383–390. [Google Scholar]
- Zhu, T.T.; Li, L.Y.; Duan, Q.X.; Liu, X.L.; Chen, M. Progress in our understanding of plant responses to the stress of heavy metal cadmium. Plant Signal. Behav. 2021, 16, 1836884. [Google Scholar] [CrossRef]
- Smeets, K.; Cuypers, A.; Lambrechts, A.; Semane, B.; Hoet, P.; Laere, A.V.; Vangronsveld, J. Induction of oxidative stress and antioxidative mechanisms in Phaseolus vulgaris after Cd application. Plant Physiol. Biochem. 2005, 43, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Srivastava, S.; Tripathi, R.D.; Govindarajan, R.; Kuriakose, S.V.; Prasad, M.N.V. Phytochelatin synthesis and response of antioxidants during cadmium stress in Bacopa monnieri L. Plant Physiol. Biochem. 2006, 44, 25–37. [Google Scholar] [CrossRef] [PubMed]
- Goncharuk, E.A.; Zagoskina, N.V. Heavy Metals, Their Phytotoxicity, and the Role of Phenolic Antioxidants in Plant Stress Responses with Focus on Cadmium: Review. Molecules 2023, 28, 3921. [Google Scholar] [CrossRef]
- Lysenko, E.A.; Klaus, A.A.; Pshybytko, N.L.; Kusnetsov, V.V. Cadmium accumulation in chloroplasts and its impact on chloroplastic processes in barley and maize. Photosynth. Res. 2015, 125, 291–303. [Google Scholar] [CrossRef] [PubMed]
- Muradoglu, F.; Gundogdu, M.; Ercisli, S.; Encu, T.; Balta, F.; Jaafar, H.Z.E.; Zia-Ul-Haq, M. Cadmium toxicity affects chlorophyll a and b content, antioxidant enzyme activities and mineral nutrient accumulation in strawberry. Biol. Res. 2015, 48, 11. [Google Scholar] [CrossRef]
- Zhuang, P.; Zou, H.; Shu, W. Biotransfer of heavy metals along a soil-plant-insect-chicken food chain: Field study. J. Environ. Sci. 2009, 21, 849–853. [Google Scholar] [CrossRef]
- Wang, W.X.; Pan, J.F. The transfer of metals in marine food chains. Acta Ecol. Sin. 2004, 24, 599–604. [Google Scholar]
- Dawid, M.; Hajnalka, S.; Piotr, S.; Potts, S.G.; Michał, W. Survival, reproduction and population growth of the bee pollinator, Osmia rufa (Hymenoptera: Megachilidae), along gradients of heavy metal pollution. Insect Conserv. Divers. 2014, 7, 113–121. [Google Scholar]
- Dun, J.; Yan, S.C. Effects of Cd, Zn or Pb stress in Populus alba berolinensis on the development and reproduction of Lymantria dispar. Ecotoxicology 2017, 26, 1305–1313. [Google Scholar]
- Niu, C.Y.; Jiang, Y.; Lei, C.L.; Hu, C. Effects of cadmium on housefly: Influence on growth and development and metabolism during metamorphosis of housefly. Insect Sci. 2010, 9, 27–33. [Google Scholar]
- Liang, L.; Mao, X.; Guan, D.L.; Zhang, M. Effect of cadmium on the life span, reproductive capacity and the hereditability analysis of Drosophila melanogaster. J. Saf. Environ. 2013, 13, 4–8. [Google Scholar]
- Song, Y.Q.; Gao, H.H.; Zhang, L.; Zhao, H.Y. Effects of combined stress of heavy metal cadmium and zinc on the life table parameters and fecundity of English grain aphid Sitobion avenae (Hemiptera:Aphididae). J. Plant Prot. 2017, 44, 406–412. [Google Scholar]
- Zhang, Y.Y.; Hu, X.D.; Zheng, L.X.; Wei, H.Y. Effect of Cd2+ on development and male orientational behaviors to the conspecific female pheromones in the striped stem borer, Chilo suppressalis (Walker). J. Environ. Entomol. 2017, 39, 423–430. [Google Scholar]
- Kafel, A.; Nowak, A.; Bembenek, J.; Szczygie, J.; Nakonieczny, M.; Swiergosz-Kowalewska, R. The localisation of HSP70 and oxidative stress indices in heads of Spodoptera exigua larvae in a cadmium-exposed population. Ecotoxicol. Environ. Saf. 2012, 78, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Augustyniak, M.; Tarnawska, M.; Babczyńska, A.; Kafel, A.; Raszka, A.Z.; Adamek, B.; Płachetka-Bożek, A. Cross tolerance in beet armyworm: Long-term selection by cadmium broadens tolerance to other stressors. Ecotoxicology 2017, 26, 1408–1418. [Google Scholar] [CrossRef]
- Zhou, J.L.; Zhang, G.R.; Zhou, Q. Molecular characterization of cytochrome P450 CYP6B47 cDNAs and 5′-flanking sequence from Spodoptera litura (Lepidoptera: Noctuidae): Its response to lead stress. J. Insect Physiol. 2012, 58, 726–736. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.L.; Shu, Y.H.; Zhang, G.R.; Zhou, Q. Lead exposure improves the tolerance of Spodoptera litura (Lepidoptera: Noctuidae) to cypermethrin. Chemosphere 2012, 88, 507–513. [Google Scholar] [CrossRef] [PubMed]
- GB/T 23620-2009; Potato Beetle Outbreak Surveillance Protocols. General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China: Beijing, China. Standardization Administration of the People’s Republic of China: Beijing, China, 2009; p. 16.
- Chi, H.; You, M.; Atlıhan, R.; Smith, C.L.; Kavousi, A.; Özgökçe, M.S.; Güncan, A.; Tuan, S.J.; Fu, J.W.; Xu, Y.Y.; et al. Age-Stage, two-sex life table: An introduction to theory, data analysis, and application. Entomol. Gen. 2020, 40, 103–124. [Google Scholar] [CrossRef]
- Chi, H. Life-Table Analysis Incorporating Both Sexes and Variable Development Rates Among Individuals. Environ. Entomol. 1988, 17, 26–34. [Google Scholar] [CrossRef]
- Liu, J.X.; Zhu, Z.Y.; Wang, S.; Wang, L.L.; Mao, X.F.; Zang, L.S.; Di, N. Accumulation and transfer of cadmium in tomato and its pest Frankliniella occidentalis. J. Environ. Entomol. 2023, 45, 673–681. [Google Scholar]
- Naikoo, M.I.; Raghib, F.; Dar, M.I.; Khan, F.A.; Hessini, K.; Ahmad, P. Uptake, accumulation and elimination of cadmium in a soil—Faba bean (Vicia faba)—Aphid (Aphis fabae)—Ladybird (Coccinella transversalis) food chain. Chemosphere 2021, 279, 130522. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.M.; Huang, J.N.; Wei, J.Q.; Liu, X.L.; Lu, J.; Baldwin, I.T.; Lou, Y.G.; Li, R. Low-level cadmium exposure influences rice resistance to herbivores by priming jasmonate signaling. Environ. Exp. Bot. 2021, 194, 104741. [Google Scholar] [CrossRef]
- Hladun, K.R.; Di, N.; Tong, X.L.; Trumble, J.T. Metal contaminant accumulation in the hive: Consequences for whole-colony health and brood production in the honey bee (Apis mellifera L.). Environ. Toxicol. Chem. 2016, 35, 322–329. [Google Scholar] [CrossRef] [PubMed]
- Martinez, E.A.; Moore, B.C.; Schaumloffel, J.; Dasgupta, N. Morphological abnormalities in Chironomus tentans exposed to cadmium-and copper-spiked sediments. Ecotoxicol. Environ. Saf. 2003, 55, 204–212. [Google Scholar] [CrossRef]
- Sun, H.X.; Shu, Y.H.; Tang, W.C.; Wang, Q.; Zhou, Q.; Zhang, G.R. Heavy metal Ni2+ continuous stress causes its accumulation and decreased survival in Spodoptera litura Fabricius. Sci. Bull. 2007, 52, 1413–1418. [Google Scholar] [CrossRef]
- Gao, H.H.; Zhao, H.Y.; Du, C.; Deng, M.M.; Du, E.X.; Hu, Z.Q.; Hu, X.S. Life table evaluation of survival and reproduction of the aphid, Sitobion avenae, exposed to cadmium. J. Insect Sci. 2012, 12, 44. [Google Scholar] [CrossRef] [PubMed]
- Shu, Q.H. The Study on Ecological Parameters Ofwheat and Sitobion avenae Under the Dualstress of Cadmium and Drought Stress. Master’s Thesis, Northwest A&F University, Xianyang, China, 2019. [Google Scholar]

| Developmental Period at Different Potency | |||||
|---|---|---|---|---|---|
| Developmental Stage | 0 mg/kg (CK) | 30 mg/kg (B30) | 60 mg/kg (C60) | 90 mg/kg (D90) | 120 mg/kg (E120) |
| 1st Instar | 2.00 ± 0.00 a | 4.91 ± 0.09 b | 5.19 ± 0.11 c | 6.30 ± 0.15 d | 6.51 ± 0.04 e |
| 2nd Instar | 5.34 ± 0.17 a | 2.41 ± 0.13 c | 1.71 ± 0.06 d | 2.43 ± 0.13 c | 4.19 ± 0.08 b |
| 3rd Instar | 4.20 ± 0.14 a | 2.07 ± 0.05 c | 2.25 ± 0.09 b | 2.11 ± 0.12 bc | 1.95 ± 0.11 d |
| 4th Instar | 5.54 ± 0.19 b | 6.54 ± 0.11 a | 5.66 ± 0.14 b | 5.79 ± 0.20 c | 5.73 ± 0.10 d |
| Pupal stage | 7.34 ± 0.23 a | 8.79 ± 0.28 c | 9.68 ± 0.32 e | 8.26 ± 0.33 b | 9.46 ± 0.27 d |
| Adult stage | 39.18 ± 0.21 a | 31.12 ± 1.44 c | 24.00 ± 2.13 e | 29.36 ± 1.70 d | 34.36 ± 1.82 b |
| TPOP | 31.83 ± 0.17 a | 36.43 ± 0.78 b | 39.75 ± 0.65 d | 39.45 ± 0.63 c | 53.52 ± 1.46 e |
| Fecundity | 74.00 ± 1.2 a | 55.00 ± 0.72 b | 27.00 ± 0.43 c | 22.00 ± 1.63 d | 21.00 ± 1.01 e |
| Total longevity | 53.76 ± 1.77 a | 35.54 ± 1.93 b | 25.82 ± 1.57 d | 28.37 ± 1.81 c | 25.18 ± 1.89 e |
| Population Parameters of Different Potencies | |||||
|---|---|---|---|---|---|
| Population Parameters | 0 mg/kg (CK) | 30 mg/kg (B30) | 60 mg/kg (C60) | 90 mg/kg (D90) | 120 mg/kg (E120) |
| Intrinsic rate of increase (r) (d−1) | 0.618 ± 0.130 a | 0.2173 ± 0.304 b | 0.214 ± 0.00 b | 0.018 ± 0.00 c | 0.0026 ± 0.001 c |
| Finite rate of increase (λ) (d−1) | 1.063 ± 2.265 a | 1.021 ± 3.067 b | 1.0216 ± 4.855 b | 0.981 ± 8.579 c | 0.793 ± 9.139 c |
| Net reproductive rate (R0) | 16.74 ± 1.41 a | 2.93 ± 0.44 b | 2.76 ± 0.61 b | 0.41 ± 0.19 c | 0.28 ± 0.11 c |
| Gross reproduction rate (GRR) | 21.773 ± 1.59 a | 11.369 ± 1.82 b | 7.521 ± 0.79 c | 2.030 ± 0.85 d | 1.812 ± 0.61 e |
| Mean generation time (T) (d) | 49.427 ± 0.502 a | 47.477 ± 7.073 a | 47.549 ± 0.729 a | 47.406 ± 0.729 a | 45.545 ± 0.368 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, B.; Zhang, J.; Hu, Y.; Zhang, Y.; Wang, J.; Li, C. Age-Stage, Two-Sex Life Table of Leptinotarsa decemlineata (Coleoptera: Chrysomelidae) Experiencing Cadmium Stress. Insects 2025, 16, 73. https://doi.org/10.3390/insects16010073
He B, Zhang J, Hu Y, Zhang Y, Wang J, Li C. Age-Stage, Two-Sex Life Table of Leptinotarsa decemlineata (Coleoptera: Chrysomelidae) Experiencing Cadmium Stress. Insects. 2025; 16(1):73. https://doi.org/10.3390/insects16010073
Chicago/Turabian StyleHe, Bingyu, Jiebo Zhang, Yang Hu, Yi Zhang, Jianan Wang, and Chao Li. 2025. "Age-Stage, Two-Sex Life Table of Leptinotarsa decemlineata (Coleoptera: Chrysomelidae) Experiencing Cadmium Stress" Insects 16, no. 1: 73. https://doi.org/10.3390/insects16010073
APA StyleHe, B., Zhang, J., Hu, Y., Zhang, Y., Wang, J., & Li, C. (2025). Age-Stage, Two-Sex Life Table of Leptinotarsa decemlineata (Coleoptera: Chrysomelidae) Experiencing Cadmium Stress. Insects, 16(1), 73. https://doi.org/10.3390/insects16010073






