Impact of Comb Cell Diameter on Nectar Evaporation Efficiency in Honey Bees
Simple Summary
Abstract
1. Introduction
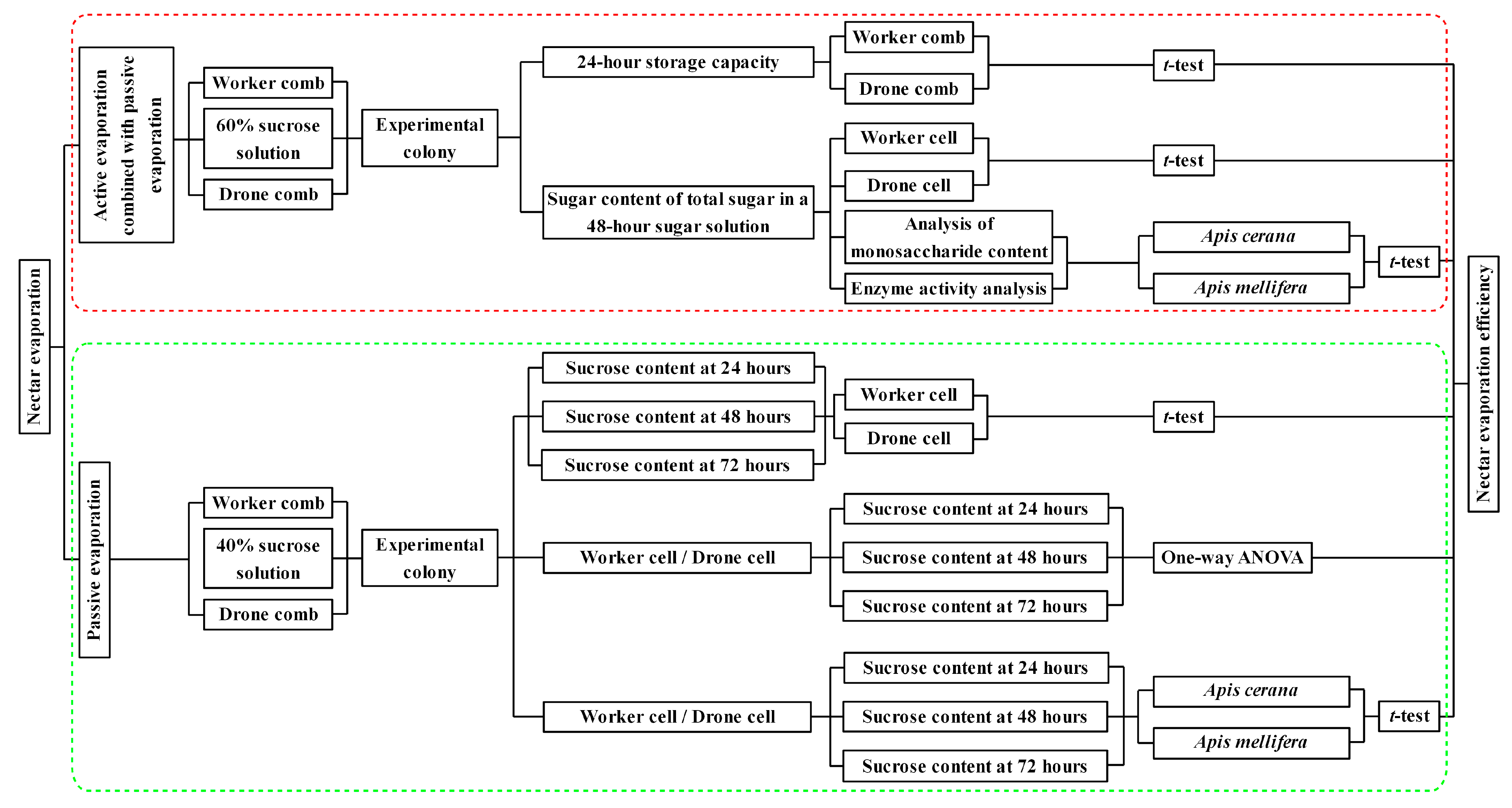
2. Materials and Methods
2.1. Active Evaporation Combined with Passive Evaporation
2.1.1. Establishing the Experimental Honey Bee Colonies
2.1.2. Sucrose Solution Feeding to Experimental Bee Colonies
2.1.3. Measurement of Sugar and Sucrose Content in 48-h Processed Solutions by Experimental Bee Colonies
2.1.4. Analysis of Sugar Content and Enzyme Activity
Analytical Methods for Sugar Content
Analytical Methods for Enzyme Activity
2.2. Passive Evaporation
2.2.1. Establishment of Experimental Honey Bee Colonies
2.2.2. Measurement of Sugar Content in Experimental Combs
2.3. Statistical Analysis
3. Results
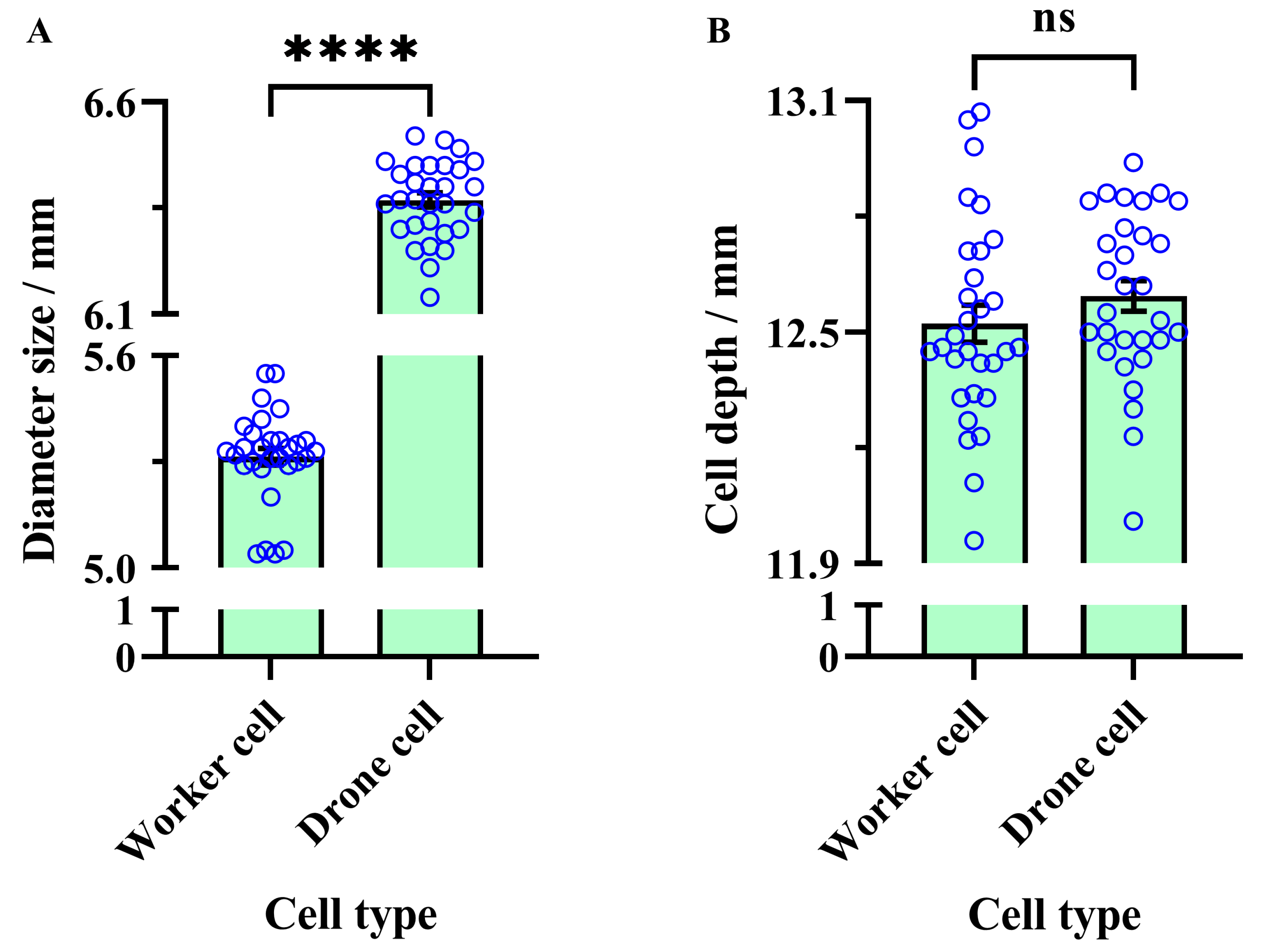
3.1. Cell Size
3.2. Active Evaporation Combined with Passive Evaporation
3.2.1. Weight of Sucrose Solution Stored in Worker and Drone Combs Within 24 h
3.2.2. Total Sugar Content of Sugar Solution Processed by Experimental Colonies in Different Cell Types for 48 h
3.2.3. Monosaccharide and Sucrose Content in Sugar Solution Processed by Experimental Bee Colonies for 48 h
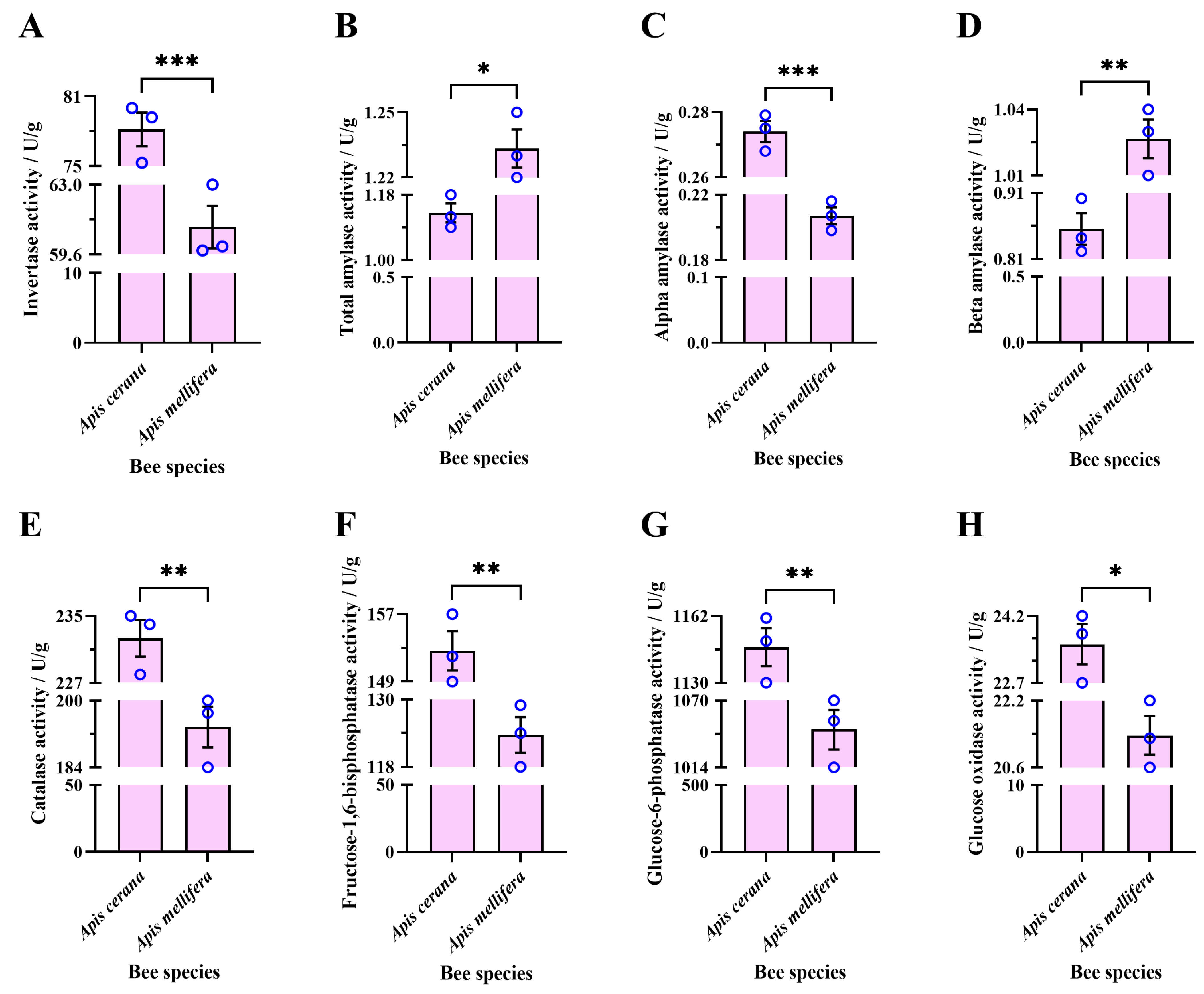
3.2.4. Enzyme Activity in Sugar Solution Processed by Experimental Bee Colonies for 48 h
3.3. Passive Evaporation
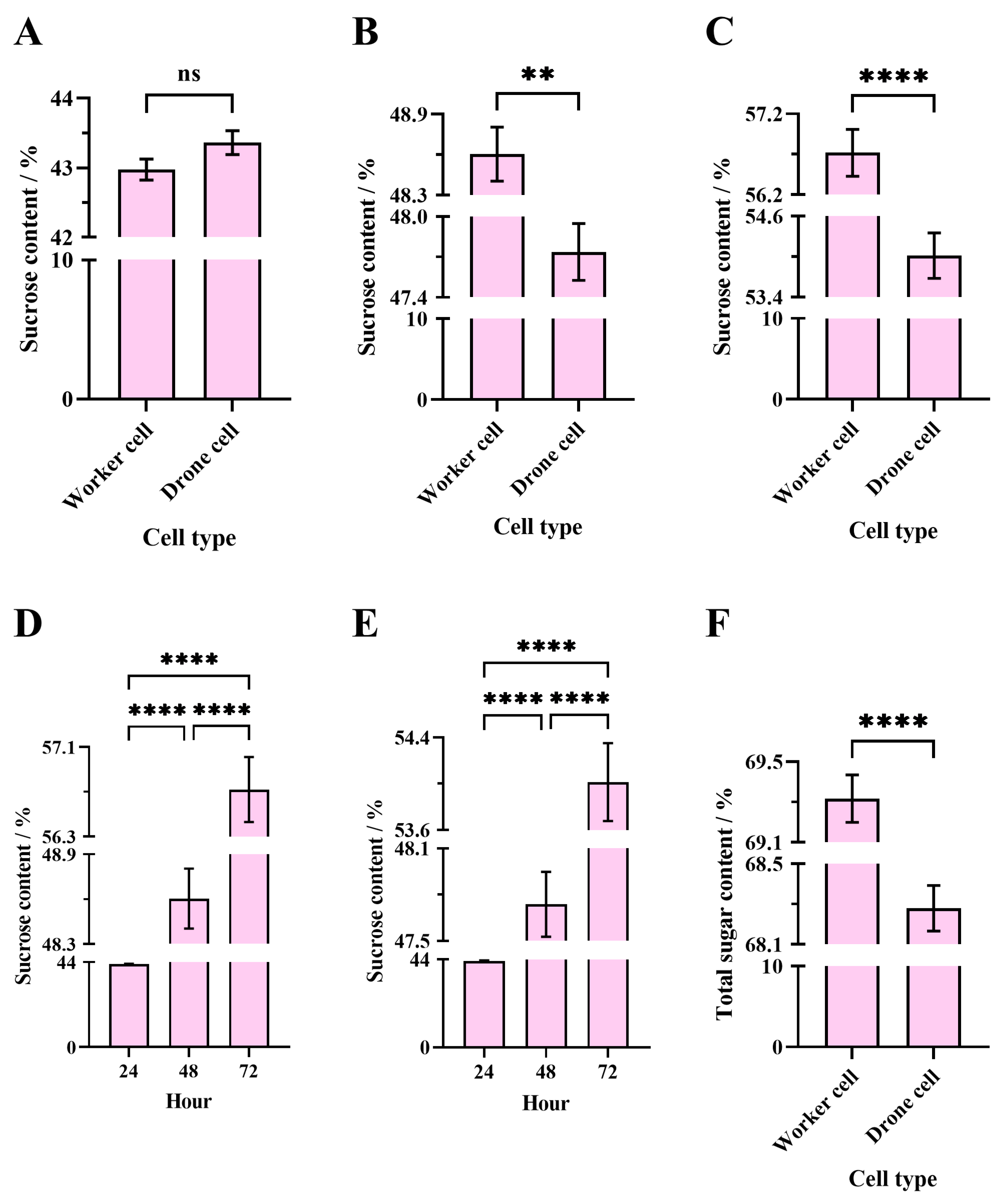
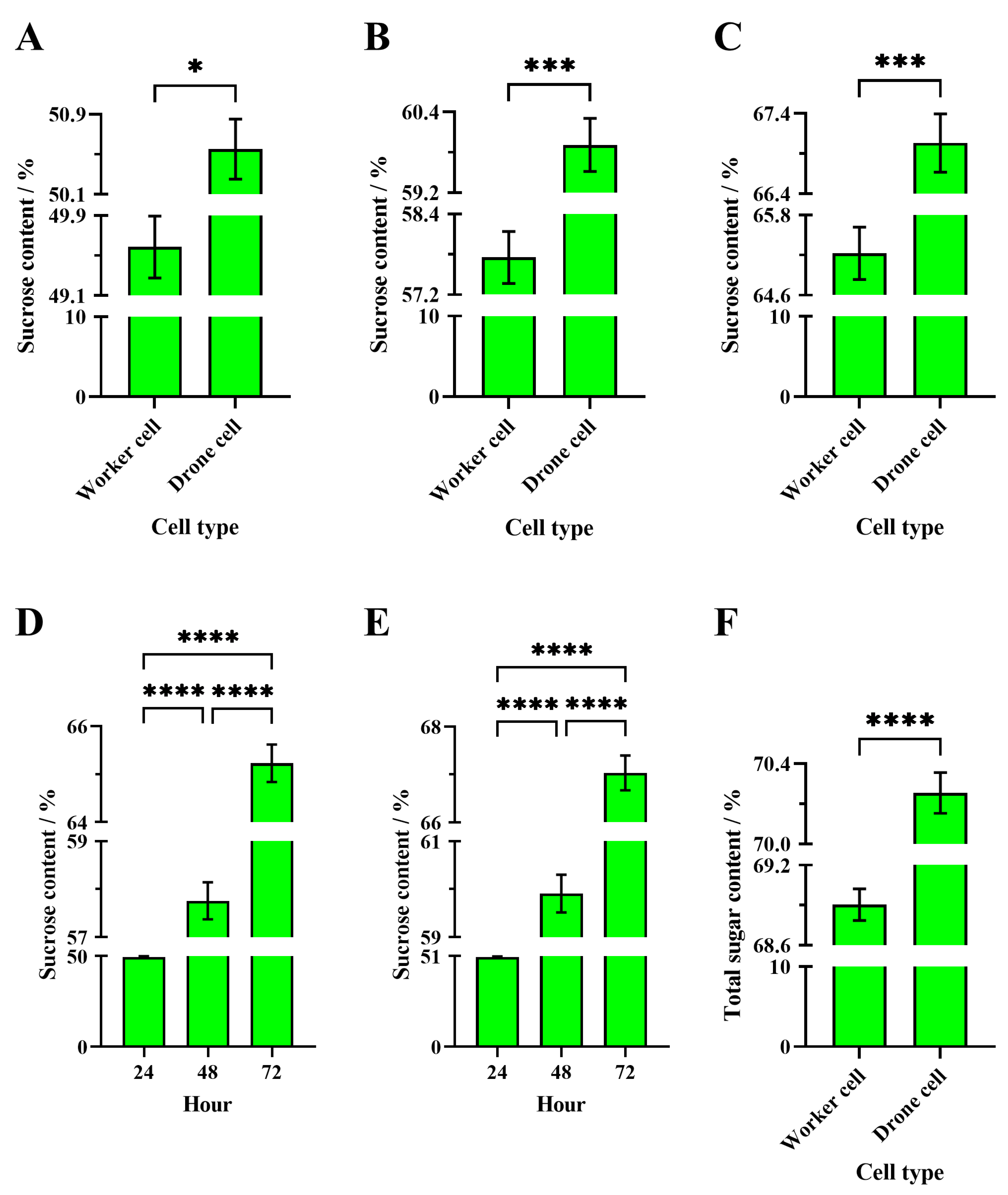
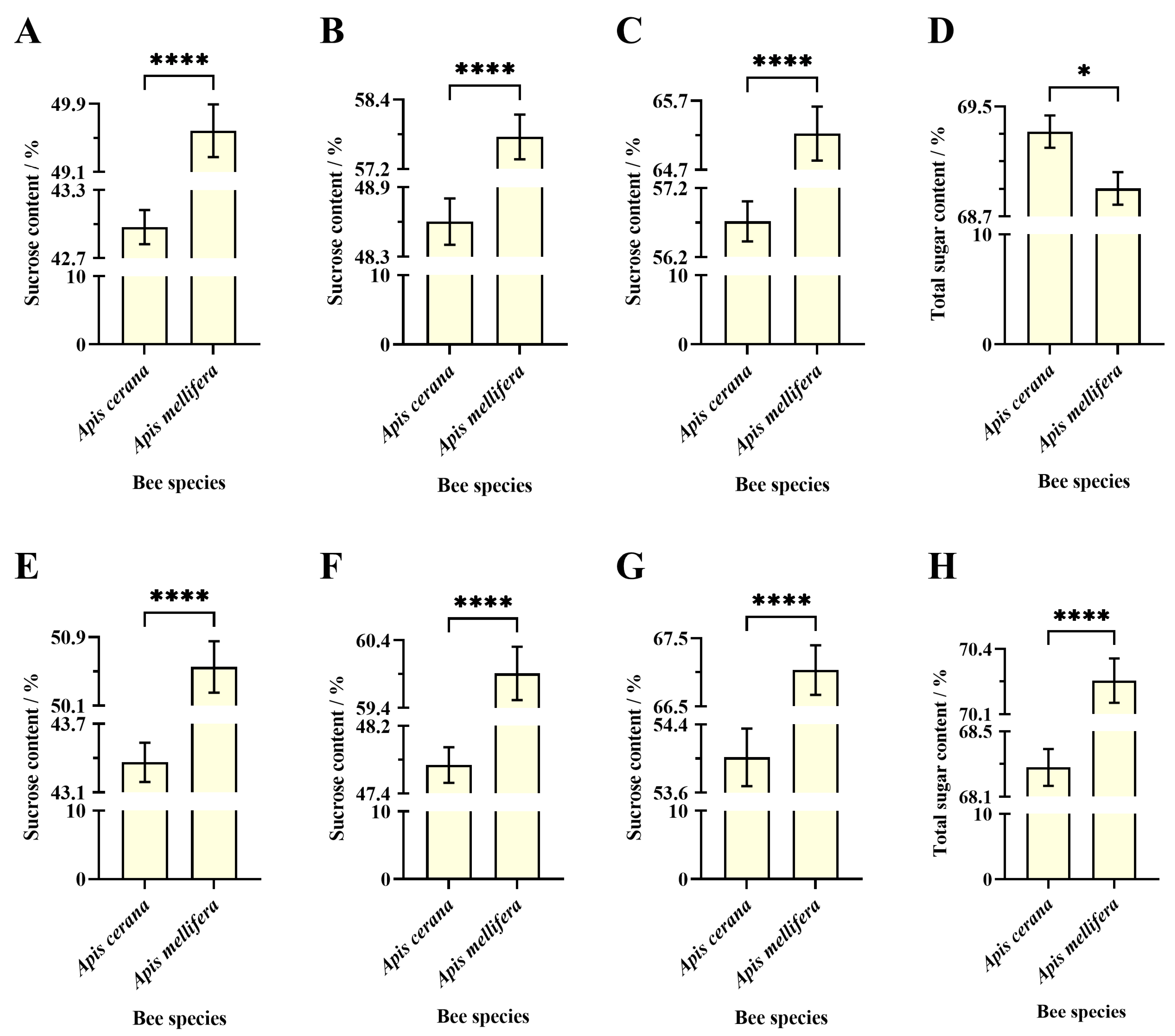
3.3.1. Sucrose Content in Different Cell Types for the Same Evaporation Time
Apis Cerana
Apis Mellifera
3.3.2. Sucrose Content in Same Cell Types at Different Evaporation Times
Apis Cerana
Apis Mellifera
3.3.3. Sucrose Content in Sucrose Solution Evaporated by Different Bee Species in the Same Cell Types
Worker Cell
Drone Cell
4. Discussion
4.1. The Significance of Bees Storing Honey in the Drone Cells
4.2. Difference in Nectar Evaporation Between Worker and Drone Cells
4.3. Difference in Concentrated Nectar Between EHB and WHB Colonies
4.4. Variability of Honey Concentration
4.5. Monosaccharide Content and Enzyme Activity Differences in Honey Brewed by EHB and WHB Colonies
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Crane, E. Honey from Honeybees and Other Insects. Ethol. Ecol. Evol. 1991, 3, 100–105. [Google Scholar] [CrossRef]
- Berenbaum, M.R.; Calla, B. Honey as a Functional Food for Apis mellifera. Annu. Rev. Entomol. 2021, 66, 185–208. [Google Scholar] [CrossRef] [PubMed]
- White, J.W., Jr. Honey. Adv. Food Res. 1978, 24, 287–374. [Google Scholar] [PubMed]
- Eyer, M.; Neumann, P.; Dietemann, V. A Look into the Cell: Honey Storage in Honey Bees, Apis mellifera. PLoS ONE 2016, 11, e0161059. [Google Scholar] [CrossRef] [PubMed]
- Nicolson, S.W.; Human, H.; Pirk, C.W.W. Honey Bees Save Energy in Honey Processing by Dehydrating Nectar before Returning to the Nest. Sci. Rep. 2022, 12, 16224. [Google Scholar] [CrossRef] [PubMed]
- Portman, Z.M.; Ascher, J.S.; Cariveau, D.P. Nectar Concentrating Behavior by Bees (Hymenoptera: Anthophila). Apidologie 2021, 52, 1169–1194. [Google Scholar] [CrossRef]
- Rösch, G.A. Untersuchungen über Die Arbeitsteilung Im Bienenstaat 1. Teil: Die Tätigkeiten Im Normalen Bienenstaate Und Ihre Beziehungen Zum Alter Der Arbeitsbienen. Z. Vgl. Physiol. 1925, 2, 571–631. [Google Scholar] [CrossRef]
- Lindauer, M. Ein Beitrag Zur Frage Der Arbeitsteilung Im Bienenstaat. Z. Vgl. Physiol. 1952, 34, 299–345. [Google Scholar] [CrossRef]
- Seeley, T.D. Adaptive Significance of the Age Polyethism Schedule in Honeybee Colonies. Behav. Ecol. Sociobiol. 1982, 11, 287–293. [Google Scholar] [CrossRef]
- Wilson, E.O. The Insect Societies; Harvard University Press: Cambridge, MA, USA, 1971. [Google Scholar]
- Michener, C.D. The Social Behavior of the Bees: A Comparative Study; The Belknap Press of Harvard University Press: Cambridge, MA, USA, 1974. [Google Scholar]
- Nixon, H.L.; Ribbands, C.R. Food Transmission within the Honeybee Community. Proc. R. Soc. Lond. Ser. B—Biol. Sci. 1952, 140, 43–50. [Google Scholar]
- Free, J.B. The Transmission of Food between Worker Honeybees. Br. J. Anim. Behav. 1957, 5, 41–47. [Google Scholar] [CrossRef]
- Free, J.B. The Transfer of Food between the Adult Members of a Honeybee Community. Bee World 1959, 40, 193–201. [Google Scholar] [CrossRef]
- Free, J.B.; Butler, C.G. An Analysis of the Factors Involved in the Formation of a Cluster of Honeybees. Behaviour 1955, 7, 304–316. [Google Scholar] [CrossRef]
- Doolittle, G.M. Large or Small Hives-Bees Unloading the Honey. Am. Bee J. 1890, 26, 49. [Google Scholar]
- Doolittle, G.M. Where Do the Field-Bees Deposit Their Loads. Am. Bee J. 1907, 47, 653–654. [Google Scholar]
- Von Frisch, K. Uber Die ’Sprache’ Der Bienen. Eine Tierpsychologische Untersuchung. Naturwissenschaften 1923, 11, 633–635. [Google Scholar]
- Lindauer, M. Temperaturregulierung Und Wasserhaushalt Im Bienenstaat. Z. Vgl. Physiol. 1954, 36, 391–432. [Google Scholar] [CrossRef]
- Frisch, K.V. The Dance Language and Orientation of Bees; Harvard University Press: Cambridge, MA, USA, 1967. [Google Scholar]
- Seeley, T.D. The Wisdom of the Hive: The Social Physiology of Honey Bee Colonies; Harvard University Press: Cambridge, MA, USA; London, UK, 1995. [Google Scholar]
- Park, W. The Storing and Ripening of Honey by Honeybees. J. Econ. Entomol. 1925, 18, 405–410. [Google Scholar] [CrossRef]
- Seeley, T.D. Social Foraging in Honey Bees: How Nectar Foragers Assess Their Colony’s Nutritional Status. Behav. Ecol. Sociobiol. 1989, 24, 181–199. [Google Scholar] [CrossRef]
- Prez, N.; Farina, W.M. Nectar-Receiver Behavior in Relation to the Reward Rate Experienced by Foraging Honeybees. Behav. Ecol. Sociobiol. 2004, 55, 574–582. [Google Scholar] [CrossRef]
- Grüter, C.; Farina, W.M. Nectar Distribution and Its Relation to Food Quality in Honeybee (Apis mellifera) Colonies. Insectes Sociaux 2007, 54, 87–94. [Google Scholar] [CrossRef]
- Crailsheim, K. Trophallactic Interactions in the Adult Honeybee (Apis mellifera L.). Apidologie 1998, 29, 97–112. [Google Scholar] [CrossRef]
- Pasedach-Poeverlein, K. Über Das „Spritzen“ Der Bienen Und über Die Konzentrationsänderung Ihres Honigblaseninhalts. Z. Vgl. Physiol. 1940, 28, 197–210. [Google Scholar] [CrossRef]
- Park, O.W. Studies on the Changes in Nectar Concentration Produced by the Honeybee, Apis mellifera Part I. Changes Which Occur Between the Flower and the Hive; Agricultural Experiment Station Iowa State College of Agriculture and Mechanic Arts: Ames, IA, USA, 1932; Volume 151. [Google Scholar]
- Oertel, E.; Fieger, E.A.; Williams, V.R.; Andrews, E.A. Inversion of Cane Sugar in the Honey Stomach of the Bee. J. Econ. Entomol. 1951, 44, 487–492. [Google Scholar] [CrossRef]
- Kleinhenz, M. Hot Bees in Empty Broodnest Cells: Heating from Within. J. Exp. Biol. 2003, 206, 4217–4231. [Google Scholar] [CrossRef] [PubMed]
- Esch, H.; Goller, F.; Heinrich, B. How Do Bees Shiver? Naturwissenschaften 1991, 78, 325–328. [Google Scholar] [CrossRef]
- Heinrich, B. The Hot-Blooded Insects: Strategies and Mechanisms of Thermoregulation; Springer: Berlin/Heidelberg, Germany, 1993. [Google Scholar]
- Heinrich, B.; Esch, H. Thermoregulation in Bees. Am. Sci. 1994, 82, 164–170. [Google Scholar]
- Park, O.W. Further Studies on the Evaporation of Nectar. J. Econ. Entomol. 1928, 21, 882–887. [Google Scholar] [CrossRef]
- Gallup, E. Evaporating Nectar. Am. Bee J. 1868, 3, 171. [Google Scholar]
- Park, O.W. Water Carriers Versus Nectar Carriers. J. Econ. Entomol. 1926, 19, 656–664. [Google Scholar] [CrossRef]
- Nicolson, S.W.; Human, H. Bees Get a Head Start on Honey Production. Biol. Lett. 2008, 4, 299–301. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Heinrich, B. Keeping a Cool Head: Honeybee Thermoregulation. Science 1979, 205, 1269–1271. [Google Scholar] [CrossRef] [PubMed]
- Cooper, P.D.; Schaffer, W.M.; Buchmann, S.L. Temperature Regulation of Honey Bees (Apis mellifera) Foraging in the Sonoran Desert. J. Exp. Biol. 1985, 114, 1–15. [Google Scholar] [CrossRef]
- Heinrich, B. Mechanisms of Body-Temperature Regulation in Honeybees, Apis mellifera: I. Regulation of Head Temperature. J. Exp. Biol. 1980, 85, 61–72. [Google Scholar] [CrossRef]
- Brunnish, K. The Fable of Ripening of Honey by Evaporation. Am. Bee J. 1924, 64, 328–330. [Google Scholar]
- Reinhardt, J.F. Ventilating the Bee Colony to Facilitate the Honey Ripening Process. J. Econ. Entomol. 1939, 32, 654–660. [Google Scholar] [CrossRef]
- Park, O.W. Studies on the Evaporation of Nectar. J. Econ. Entomol. 1927, 20, 510–516. [Google Scholar] [CrossRef]
- Huber, F. New Observations Upon Bees. Dadant, C.P., Translator; American Bee Journal: Hamilton, IL, USA, 1926. [Google Scholar]
- Chadwick, P.C. Ventilation. Am. Bee J. 1922, 62, 158–159. [Google Scholar]
- Büdel, A. Le Microclimat De La Ruche. In Traité Biologie L’abeille; François Bourlière: Paris, France, 1968; Volume 4. [Google Scholar]
- Simpson, J. Nest Climate Regulation in Honey Bee Colonie: Honey Bees Control Their Domestic Environment by Methods Based on Their Habit of Clustering Together. Science 1961, 133, 1327–1333. [Google Scholar] [CrossRef]
- Bruman, P. Die Luftzirkulation Im Bienenstock. Z. Vgl. Physiol. 1928, 8, 366–370. [Google Scholar] [CrossRef]
- Gary, N.E. Activities and Behavior of Honey Bees. In Hive Honey Bee; Dadant & Sons: Hamilton, IL, USA, 1978; pp. 185–194. [Google Scholar]
- Frisch, K.V. Bees: Their Vision, Chemical Senses, and Language; Cornell University Press: Ithaca, NY, USA, 1971. [Google Scholar]
- Lindauer, M. Communication Among Social Bees; Harvard University Press: Cambridge, MA, USA, 1971. [Google Scholar]
- Hazelhoff, E.H. De Luchtverversching Van Een Bijenkast Gedurende Den Zomer. Maandschr. Voor Bijent. 1941, 44, 221–225. [Google Scholar]
- Lacher, V. Verhaltensreaktionen Der Bienenarbeiterin Bei Dressur Auf Kohlendioxid. Z. Vgl. Physiol. 1966, 54, 75–84. [Google Scholar] [CrossRef]
- Seeley, T.D. Atmospheric Carbon Dioxide Regulation in Honey-Bee (Apis mellifera) Colonies. J. Insect Physiol. 1974, 20, 2301–2305. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, D. Nectar, Humidity, Honey Bees (Apis mellifera) and Varroa in Summer: A Theoretical Thermofluid Analysis of the Fate of Water Vapour from Honey Ripening and Its Implications on the Control of Varroa Destructor. J. R. Soc. Interface 2019, 16, 20190048. [Google Scholar] [CrossRef] [PubMed]
- Hess, W.R. Die Temperaturregulierung Im Bienenvolk. Z. Vgl. Physiol. 1926, 4, 465–487. [Google Scholar] [CrossRef]
- Sudarsan, R.; Thompson, C.; Kevan, P.G.; Eberl, H.J. Flow Currents and Ventilation in Langstroth Beehives Due to Brood Thermoregulation Efforts of Honeybees. J. Theor. Biol. 2012, 295, 168–193. [Google Scholar] [CrossRef]
- Freudenstein, K. Lehrbuch der Bienenkunde, Teil 1: Das Wesen der Bienen; Pabst: Königsbrück, Germany, 1938. [Google Scholar]
- Winston, M.L. The Biology of the Honey Bee; Harvard University Press: Cambridge, MA, USA; London, UK, 1987. [Google Scholar]
- Hazelhoff, E.H. Ventilation in a Bee-Hive During Summer. Physiol. Comp. Oecologia 1954, 3, 343–364. [Google Scholar]
- Southwick, E.E.; Moritz, R.F.A. Social Control of Air Ventilation in Colonies of Honey Bees, Apis mellifera. J. Insect Physiol. 1987, 33, 623–626. [Google Scholar] [CrossRef]
- Oldroyd, B.P.; Wongsiri, S. Asian Honey Bees: Biology, Conservation, and Human Interactions; Harvard University Press: Cambridge, MA, USA, 2006. [Google Scholar]
- Punchihewa, R.W.K. Beekeeping for Honey Production in Sri Lanka; Sri Lanka Department of Agriculture: Kandy, Sri Lanka, 1994. [Google Scholar]
- Yang, S.; Deng, S.; Kuang, H.; Zhou, D.; Gong, X.; Dong, K. Evaluating and Comparing the Natural Cell Structure and Dimensions of Honey Bee Comb Cells of Chinese Bee, Apis cerana cerana (Hymenoptera: Apidae) and Italian Bee, Apis mellifera Ligustica (Hymenoptera: Apidae). J. Insect Sci. 2021, 21, 1. [Google Scholar] [CrossRef] [PubMed]
- Lichtenberg-Kraag, B. Evidence for Correlation between Invertase Activity and Sucrose Content During the Ripening Process of Honey. J. Apic. Res. 2014, 53, 364–373. [Google Scholar] [CrossRef]
- Yan, Z.; Zhou, Z.; Jiao, Y.; Huang, J.; Yu, Z.; Zhang, D.; Chen, Y.; Ni, D. Hot-Air Drying Significantly Improves the Quality and Functional Activity of Orange Black Tea Compared with Traditional Sunlight Drying. Foods 2023, 12, 1913. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhou, J.; Zhang, S.; Gao, X.; Yang, Y.; Hou, J.; Chen, G.; Tang, X.; Wu, J.; Yuan, L. Combined Metabolome and Transcriptome Analysis Elucidates Sugar Accumulation in Wucai (Brassica campestris L.). Int. J. Mol. Sci. 2023, 24, 4816. [Google Scholar] [CrossRef]
- Liang, J.; Wang, Z.; Ren, Y.; Jiang, Z.; Chen, H.; Hu, W.; Tang, M. The Alleviation Mechanisms of Cadmium Toxicity in Broussonetia Papyrifera by Arbuscular Mycorrhizal Symbiosis Varied with Different Levels of Cadmium Stress. J. Hazard. Mater. 2023, 459, 132076. [Google Scholar] [CrossRef]
- Zhou, W.-J.; Yang, H.-L.; Mei, J.; Chang, K.-K.; Lu, H.; Lai, Z.-Z.; Shi, J.-W.; Wang, X.-H.; Wu, K.; Zhang, T.; et al. Fructose-1,6-Bisphosphate Prevents Pregnancy Loss by Inducing Decidual Cox-2+ Macrophage Differentiation. Sci. Adv. 2022, 8, eabj2488. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.-r.; Ye, Y.-l.; Feng, Y.; Xu, T.-f.; Shen, Y.; Liu, J.; Huang, S.-l.; Shen, J.-h.; Leng, Y. Sl010110, a Lead Compound, Inhibits Gluconeogenesis Via Sirt2-P300-Mediated Pepck1 Degradation and Improves Glucose Homeostasis in Diabetic Mice. Acta Pharmacol. Sin. 2021, 42, 1834–1846. [Google Scholar] [CrossRef]
- Hu, J.; Feng, Y.; Li, B.; Wang, F.; Qian, Q.; Tian, W.; Niu, L.; Wang, X. Identification of Quality Markers for Cyanotis arachnoidea and Analysis of Its Physiological Mechanism Based on Chemical Pattern Recognition, Network Pharmacology, and Experimental Validation. PeerJ 2023, 11, e15948. [Google Scholar] [CrossRef] [PubMed]
- Casteel, D.B. The Manipulation of the Wax Scales of the Honey Bee; Forgotten Books: London, UK, 1912; Volume 161, pp. 1–13. [Google Scholar]
- Seeley, T.D.; Morse, R.A. The Nest of the Honey Bee (Apis mellifera L.). Insectes Sociaux 1976, 23, 495–512. [Google Scholar] [CrossRef]
- Free, J.B.; Williams, I.H. Factors Determining Food Storage and Brood Rearing in Honeybee (Apis mellifera L.) Comb. J. Entomol. Ser. A Gen. Entomol. 1974, 49, 47–63. [Google Scholar] [CrossRef]
- Soman, A.G. A Note on Apis Florea Storing Honey in Drone Cells. Bee World 1990, 71, 33–34. [Google Scholar] [CrossRef]
- Smith, M.L.; Ostwald, M.M.; Seeley, T.D. Adaptive Tuning of an Extended Phenotype: Honeybees Seasonally Shift Their Honey Storage to Optimize Male Production. Anim. Behav. 2015, 103, 29–33. [Google Scholar] [CrossRef]
- Taber, S.; Owens, C.D. Colony Founding and Initial Nest Design of Honey Bees, Apis mellifera L. Anim. Behav. 1970, 18, 625–632. [Google Scholar] [CrossRef]
- Hessberg, H.V. Om Den Fjerde Celleart (in Danish), Translated Title: On the Fourth Kind of Cell. Tidsskr. Biavl 1952, 86, 165–166. [Google Scholar]
- Mitchell, D.M. Honey Bee Engineering: Top Ventilation and Top Entrances. Am. Bee J. 2017, 157, 887–889. [Google Scholar]
- Johnson, B.R.; Baker, N. Adaptive Spatial Biases in Nectar Deposition in the Nests of Honey Bees. Insectes Sociaux 2007, 54, 351–355. [Google Scholar] [CrossRef]
- Martin, E.C. The Hygroscopic Properties of Honey. J. Econ. Entomol. 1939, 32, 660–663. [Google Scholar] [CrossRef]
- White, J.W.; Riethof, M.L.; Subers, M.H.; Kushnir, I. Composition of American Honeys; US Department of Agriculture: Washington, DC, USA, 1962. [Google Scholar]
- Fabian, F.W.; Quinet, R.I. A Study of the Cause of Honey Fermentation. Tech. Bull. Mich. Agric. Exp. Stn. 1928, 92, 1–41. [Google Scholar]
- Greenleaf, W.C. Fermented Honey. Am. Bee J. 1933, 73, 436. [Google Scholar]
- Doner, L.W. The Sugars of Honey—A Review. J. Sci. Food Agric. 1977, 28, 443–456. [Google Scholar] [CrossRef]
- Park, O.W. Variation in the Concentration of Floral Nectars. J. Econ. Entomol. 1930, 23, 440–441. [Google Scholar] [CrossRef]
- Balasubramanyam, M.V. Quantitative Physical Variations in Ripening of Honey of Indigenous Hive Bee Apis cerana indica. Int. J. Appl. Biol. Pharm. Technol. 2011, 2, 489–493. [Google Scholar]
- Balasubramanyam, M.V. Role of Invertase Enzyme in Ripening of Honey of Indigenous Hive Honeybee Apis cerana indica. J. Chem. Biol. Phys. Sci. 2011, 1, 322–327. [Google Scholar]
- Balasubramanyam, M.V. Role of Different Enzymes in Nectar to Honey Transformations in Indigenous Rockbee, Apis dorsata F. J. Chem. Biol. Phys. Sci. 2013, 4, 361–368. [Google Scholar]
- Balasubramanyam, M.V. Evaluation of Enzymatic Activity in the Transformation of Nectar into Honey in Indigenous Rockbee, Apis dorsata F. Asian J. Res. Zool. 2020, 3, 13–19. [Google Scholar] [CrossRef]
- Balasubramanyam, M.V. Factors Influencing the Transformation of Nectar to Honey in Apis cerana indica. Int. J. Biol. Innov. 2021, 3, 271–277. [Google Scholar] [CrossRef]
- Greco, M.K.; Lang, J.; Gallmann, P.; Priest, N.; Feil, E.; Crailsheim, K. Sugar Concentration Influences Decision Making in Apis mellifera L. Workers During Early-Stage Honey Storage Behaviour. Open J. Anim. Sci. 2013, 3, 210–218. [Google Scholar] [CrossRef][Green Version]
- Eyer, M.; Greco, M.K.; Lang, J.; Neumann, P.; Dietemann, V. No Spatial Patterns for Early Nectar Storage in Honey Bee Colonies. Insectes Sociaux 2016, 63, 51–59. [Google Scholar] [CrossRef][Green Version]
- Mitchell, D. Thermal Efficiency Extends Distance and Variety for Honeybee Foragers: Analysis of the Energetics of Nectar Collection and Desiccation by Apis mellifera. J. R. Soc. Interface 2019, 16, 20180879. [Google Scholar] [CrossRef] [PubMed]
- DeGrandi-Hoffman, G.; Hagler, J. The Flow of Incoming Nectar through a Honey Bee (Apis mellifera L.) Colony as Revealed by a Protein Marker. Insectes Sociaux 2000, 47, 302–306. [Google Scholar] [CrossRef]
- Camazine, S. Self-Organizing Pattern Formation on the Combs of Honey Bee Colonies. Behav. Ecol. Sociobiol. 1991, 28, 61–76. [Google Scholar] [CrossRef]
- Park, O.W. Studies on the Rate at Which Honeybees Ripen Honey. J. Econ. Entomol. 1933, 26, 188–193. [Google Scholar] [CrossRef]
- Bicudo de Almeida-Muradian, L.; Monika Barth, O.; Dietemann, V.; Eyer, M.; Freitas, A.d.S.d.; Martel, A.-C.; Marcazzan, G.L.; Marchese, C.M.; Mucignat-Caretta, C.; Pascual-Maté, A.; et al. Standard Methods for Apis mellifera Honey Research. J. Apic. Res. 2020, 59, 1–62. [Google Scholar] [CrossRef]
- Siefert, P.; Buling, N.; Grünewald, B. Honey Bee Behaviours within the Hive: Insights from Long-Term Video Analysis. PLoS ONE 2021, 16, e0247323. [Google Scholar] [CrossRef] [PubMed]
- Nelson, J.M.; Cohn, D.J. Invertase in Honey. J. Biol. Chem. 1924, 61, 193–224. [Google Scholar] [CrossRef]
- Nelson, J.M.; Sottery, C.T. Influence of Glucose and Fructose on the Rate of Hydrolysis of Sucrose by Invertase from Honey. J. Biol. Chem. 1924, 62, 139–147. [Google Scholar] [CrossRef]
- Papadakis, P.E. Further Findings on Invertase from Honey. J. Biol. Chem. 1929, 83, 561–568. [Google Scholar] [CrossRef]
- Gorbach, G.; Schneiter, R. Zur Frage Der Verdaulichkeit Der Melezitose Durch Die Biene. Biochem. Z. 1938, 296, 367–372. [Google Scholar]
- White, J.W.; Maher, J. Transglucosidation by Honey Invertase. Arch. Biochem. Biophys. 1953, 42, 360–367. [Google Scholar] [CrossRef]
- Siddiqui, I.R. The Sugars of Honey. Adv. Carbohydr. Chem. Biochem. 1970, 25, 285–309. [Google Scholar]
- Maurizio, A. Zuckerabbau Unter Der Einwirkung Der Invertierenden Fermente in Pharynxdrüsen Und Mitteldarm Der Honigbiene (Apis mellifica L.): I. Sommerbienen Der Krainer- Und Nigra-Rasse. Insectes Sociaux 1957, 4, 225–243. [Google Scholar] [CrossRef]
- Snodgrass, R.E. Anatomy of the Honey Bee; Comstock Publishing Associates: New York, NY, USA, 1956. [Google Scholar]
- Maurixio, A. Zuckerabbau Unter Der Einwirkung Der Invertierenden Fermente in PharynxdrÜsen Und Mitteldarm Der Honigbiene (Apis mellifica L.). 5.-Einfluss Von Alter Und ErnÄhrung Der Bienen Auf Die FermentaktivitÄt Der PharynxdrÜsen. Les Ann. L’abeille 1962, 5, 215–232. [Google Scholar] [CrossRef][Green Version]
- Rinaudo, M.T.; Ponzetto, C.; Vidano, C.; Marletto, F. The Origin of Honey Saccharase. Comp. Biochem. Physiol. Part B Comp. Biochem. 1973, 46, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Gothe, F. Experimentelle Studien über Eigenschaften Und Wirkungsweise Der Honigdiastase: Sowie Die Beurteilung Des Honigs Auf Grund Seines Diastasegehaltes. Z. Unters. Nahr.-Genußmittel Sowie Gebrauchsgegenstände 1914, 28, 286–305. [Google Scholar] [CrossRef]
- Sarin, E. Über Fermente Der Verdauungsorgane Der Honigbiene. Biochem. Z. 1923, 135, 59–74. [Google Scholar]
- Babacan, S.; Pivarnik, L.F.; Rand, A.G. Honey Amylase Activity and Food Starch Degradation. J. Food Sci. 2002, 67, 1625–1630. [Google Scholar] [CrossRef]
- Vansell, G.H.; Freeborn, S.B. Preliminary Report on the Investigations of the Source of Diastase in Honey. J. Econ. Entomol. 1929, 22, 922–926. [Google Scholar] [CrossRef]
- Lothrop, R.E.; Paine, H.S. Diastatic Activity of Some American Honeys. Ind. Eng. Chem. 1931, 23, 71–74. [Google Scholar] [CrossRef]
- Fiehe, J. Über Die Herkunft Der Honigdiastase. Z. Unters. Lebensm. 1932, 63, 329–331. [Google Scholar] [CrossRef]
- Gorbach, G. Zur Kenntnis Der Stärkeverdaung Durch Die Biene. Ii Mitteilung in Der Reihe: Ernährungsphysiologische Studien an Der Biene. Forschungsdienst 1942, 13, 67–78. [Google Scholar]
- Stadelmeier, M.; Bergner, K.-G. Proteine Des Bienenhonigs Vii. Eigenschaften Und Herkunft Der Honigamylase. Z. Lebensm.-Unters.-Forsch 1986, 182, 196–199. [Google Scholar] [CrossRef]
- Lotmar, R. Abbau Und Verwertung Von Stärke Und Dextrin Durch Die Honigbiene. Arch. Bienenkd 1935, 16, 195–204. [Google Scholar]
- Ammon, R. Der Ursprung Der Diastase Des Bienenhonigs. Biochem. Z. 1949, 319, 295–299. [Google Scholar]
- Rinaudo, M.T.; Ponzetto, C.; Vidano, C.; Marletto, F. The Origin of Honey Amylase. Comp. Biochem. Physiol. Part B Comp. Biochem. 1973, 46, 253–256. [Google Scholar] [CrossRef] [PubMed]
- Arnold, G.; Delage-Darchen, B. Nouvelles Donnees Sur L’equipment Enzymatique Des Glandes Salivaires De L’ouvriere D’apis mellifica (Hymenoptere Apide). Ann. Sci. Nat. Zool. Biol. Anim. 1978, 20, 401–422. [Google Scholar]
- Costa, R.A.C.; Da Cruz-Landim, C. Enzymes Present in the Thoracic Gland Extracts from Workers and Males of Apis mellifera (Hymenoptera: Apidae). Sociobiology 2001, 37, 563–569. [Google Scholar]
- Maurizio, A. Zuckerabbau Unter Der Einwirkung Der Invertierenden Fermente in Pharynxdruesen Und Mitteldarm Der Honigbiene (Apis mellifica L.) 4. Sommerbienen Der Italienischen, Kaukasischen Und Griechischen Rasse. Insectes Sociaux 1962, 9, 39–72. [Google Scholar] [CrossRef]
- Delage-Darchen, B.; Ramos de Conconi, J.; Cuadriello Aguilar, I. Comparaison Entre L’Équipement Enzymatique Des Glandes Salivaires Et De L’intestin Moyen De Diverses EspÈces D’abeilles Sociales. Apidologie 1982, 13, 265–273. [Google Scholar] [CrossRef]
- Hrassnigg, N.; Brodschneider, R.; Fleischmann, P.H.; Crailsheim, K. Unlike Nectar Foragers, Honeybee Drones (Apis mellifera) Are Not Able to Utilize Starch as Fuel for Flight. Apidologie 2005, 36, 547–557. [Google Scholar] [CrossRef][Green Version]
- Mindt, B. Untersuchungen über Das Leben Der Drohnen, Insbesondere Ernährung Und Geschlechtsreife. Z. Bienenforsch. 1962, 6, 9–33. [Google Scholar]
- Auzinger, A. Über Fermente Im Honig Und Den Wert Ihres Nachweises Für Die Honigbeurteilung. Eur. Food Res. Technol. 1910, 19, 65–83. [Google Scholar] [CrossRef]
- Gothe, F. Die Fermente Des Honigs. Zeitschrift Untersuchung Nahrungs-und Genußmittel 1914, 28, 273–286. [Google Scholar] [CrossRef]
- Gillette, C.C. Honey Catalase. J. Econ. Entomol. 1931, 24, 605–606. [Google Scholar] [CrossRef]
- Schepartz, A.I. Honey Catalase: Occurrence and Some Kinetic Properties. J. Apic. Res. 1966, 5, 167–176. [Google Scholar] [CrossRef]
- Dustmann, J.H. Über Die Katalaseaktivität in Bienenhonig Aus Der Tracht Der Heidekrautgewächse (Ericaceae). Z. Lebensm.-Unters.-Forsch 1971, 145, 294–295. [Google Scholar] [CrossRef]
- Giri, K.V. The Chemical Composition and Enzyme Content of Indian Honey. Madras Agric. J. 1938, 25, 68–72. [Google Scholar]
- Zalewski, W. Fosfatazy W Miodach. Pszczel. Zesz. Nauk 1965, 9, 1–2. [Google Scholar]
- Čaušević, B.; Haurdic, B.; Jašić, M.; Bašić, M. Enzimatic Activities in Honey. In Proceedings of the Drugi Kongres O Pčelarstvu I Pčelinjim Proizvodima sa medđunarodnim učešćem, Gradačac, Bosnia and Herzegovina, 20 August 2017; Jašić, M., Ed.; pp. 55–61. [Google Scholar]
- Gauhe, A. Über Ein Glukoseoxydierendes Enzym in Der Pharynxdrüse Der Honigbiene. Z. Vgl. Physiol. 1940, 28, 211–253. [Google Scholar] [CrossRef]
- White, J.W.; Subers, M.H.; Schepartz, A.I. The Identification of Inhibine. Am. Bee J. 1962, 102, 430–431. [Google Scholar]
- Schepartz, A.I.; Subers, M.H. The Glucose Oxidase of Honey I. Purification and Some General Properties of the Enzyme. Biochim. Biophys. Acta (BBA)-Spec. Sect. Enzymol. Subj. 1964, 85, 228–237. [Google Scholar] [CrossRef]
- EdelhÄusere, M.; Bergner, K.-G. Proteine Des Bienenhonigs Viii. Honigsaccharase, Isolierung, Chromatographisches Verhalten Und Eigenschaften. Z. Lebensm. -Unters. Und-Forsch 1987, 184, 189–194. [Google Scholar]
- Sancho, M.T.; Muniategui, S.; Huidobro, J.F.; Simal Lozano, J. Aging of Honey. J. Agric. Food Chem. 1992, 40, 134–138. [Google Scholar] [CrossRef]
- Huang, Z.Y.; Otis, G.W. Factors Determining Hypopharyngeal Gland Activity of Worker Honey Bees (Apis mellifera L.). Insectes Sociaux 1989, 36, 264–276. [Google Scholar] [CrossRef]
- Huang, Z.Y.; Otis, G.W.; Teal, P.E.A. Nature of Brood Signal Activating the Protein Synthesis of Hypopharyngeal Gland in Honey Bees, Apis mellifera (Apidae: Hymenoptera). Apidologie 1989, 20, 455–464. [Google Scholar] [CrossRef]
- Simpson, J.; Riedel, I.B.M.; Wilding, N. Invertase in the Hypopharyngeal Glands of the Honeybee. J. Apic. Res. 1968, 7, 29–36. [Google Scholar] [CrossRef]
- Lipp, J.; Vorwohl, G.; Lipp, G. Der Honig, E Auflage: 3., Vollst. Neubearb; Erwu, U., Aufl.; Verlag Eugen Ulmer: Stuttgart, Germany, 1994. [Google Scholar]
- Böhm, D.; Horn, H. The Relationship between the Yield, Moisture, Proline and the Enzyme Activities Invertase and Diastase in Honey. Dtsch. Lebensm.-Rundsch. Z. Leb. Leb 2004, 100, 88–92. [Google Scholar]
- Sipos, E. The Latest Problems of Honey Evaluation. In Proceedings of the XIX International Beekeeping Congress, Prague, Czech Republic, 7–11 August 1963; pp. 643–646. [Google Scholar]
- Lensky, Y. Les échanges De Nourriture Liquide Entre Abeilles Aux Températures élevées. Insectes Sociaux 1961, 8, 361–368. [Google Scholar] [CrossRef]
- Pershad, S. Analyse De DiffÉrents Facteurs Conditionnant Les Échanges Alimentaires Dans Une Colonie D’abeilles Apis mellifica L. Au Moyen Du Radio-Isotope P32. Ann. L’abeille 1967, 10, 139–197. [Google Scholar] [CrossRef][Green Version]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, S.; Meng, Q.; Ye, T.; Wang, J.; Zhao, W.; Tian, Y.; Dong, K. Impact of Comb Cell Diameter on Nectar Evaporation Efficiency in Honey Bees. Insects 2025, 16, 71. https://doi.org/10.3390/insects16010071
Yang S, Meng Q, Ye T, Wang J, Zhao W, Tian Y, Dong K. Impact of Comb Cell Diameter on Nectar Evaporation Efficiency in Honey Bees. Insects. 2025; 16(1):71. https://doi.org/10.3390/insects16010071
Chicago/Turabian StyleYang, Shunhua, Qingxin Meng, Tao Ye, Jianming Wang, Wenzheng Zhao, Yakai Tian, and Kun Dong. 2025. "Impact of Comb Cell Diameter on Nectar Evaporation Efficiency in Honey Bees" Insects 16, no. 1: 71. https://doi.org/10.3390/insects16010071
APA StyleYang, S., Meng, Q., Ye, T., Wang, J., Zhao, W., Tian, Y., & Dong, K. (2025). Impact of Comb Cell Diameter on Nectar Evaporation Efficiency in Honey Bees. Insects, 16(1), 71. https://doi.org/10.3390/insects16010071





