Influences of Rearing Season, Host Plant, and Silkworm Species on Gut Bacterial Community
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect Culture Conditions and Sample Preparation
2.2. Total DNA Extraction and Purification
2.3. PCR Amplification and Sequencing
2.4. Data Processing
2.5. Statistical Analysis
3. Results
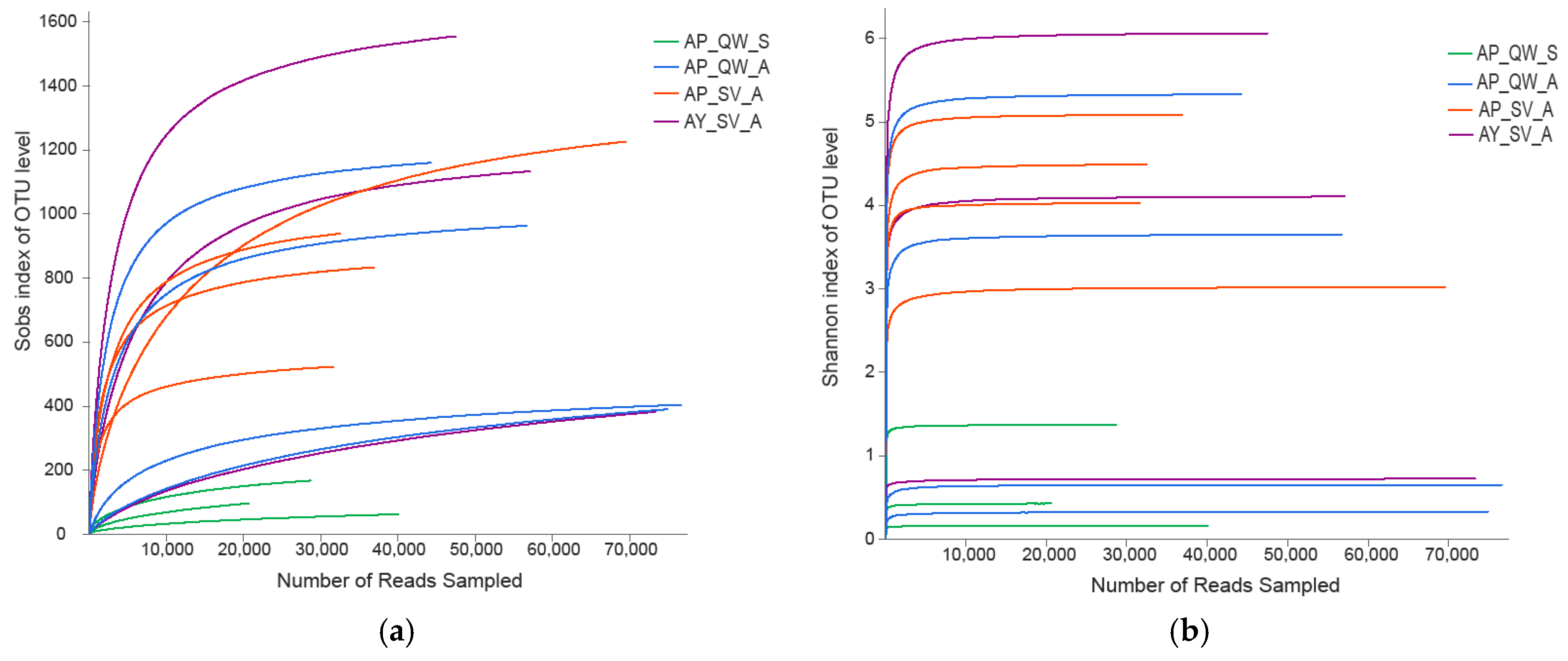
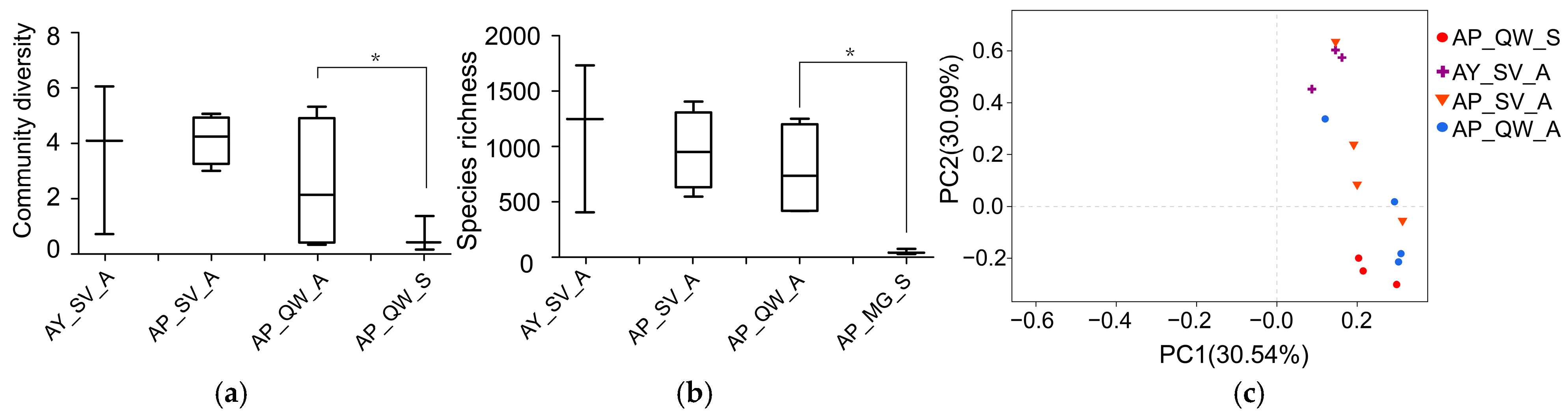
3.1. General Analyses of the Dataset
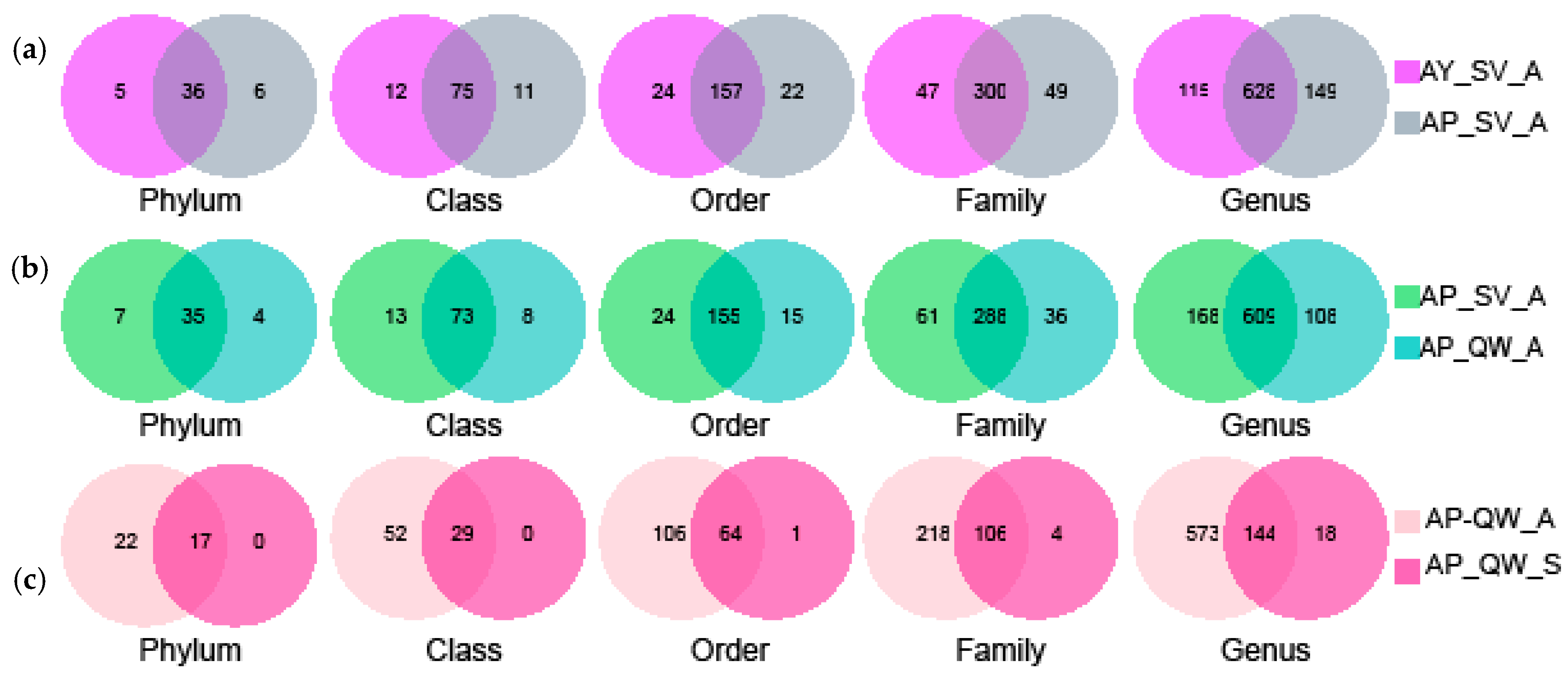
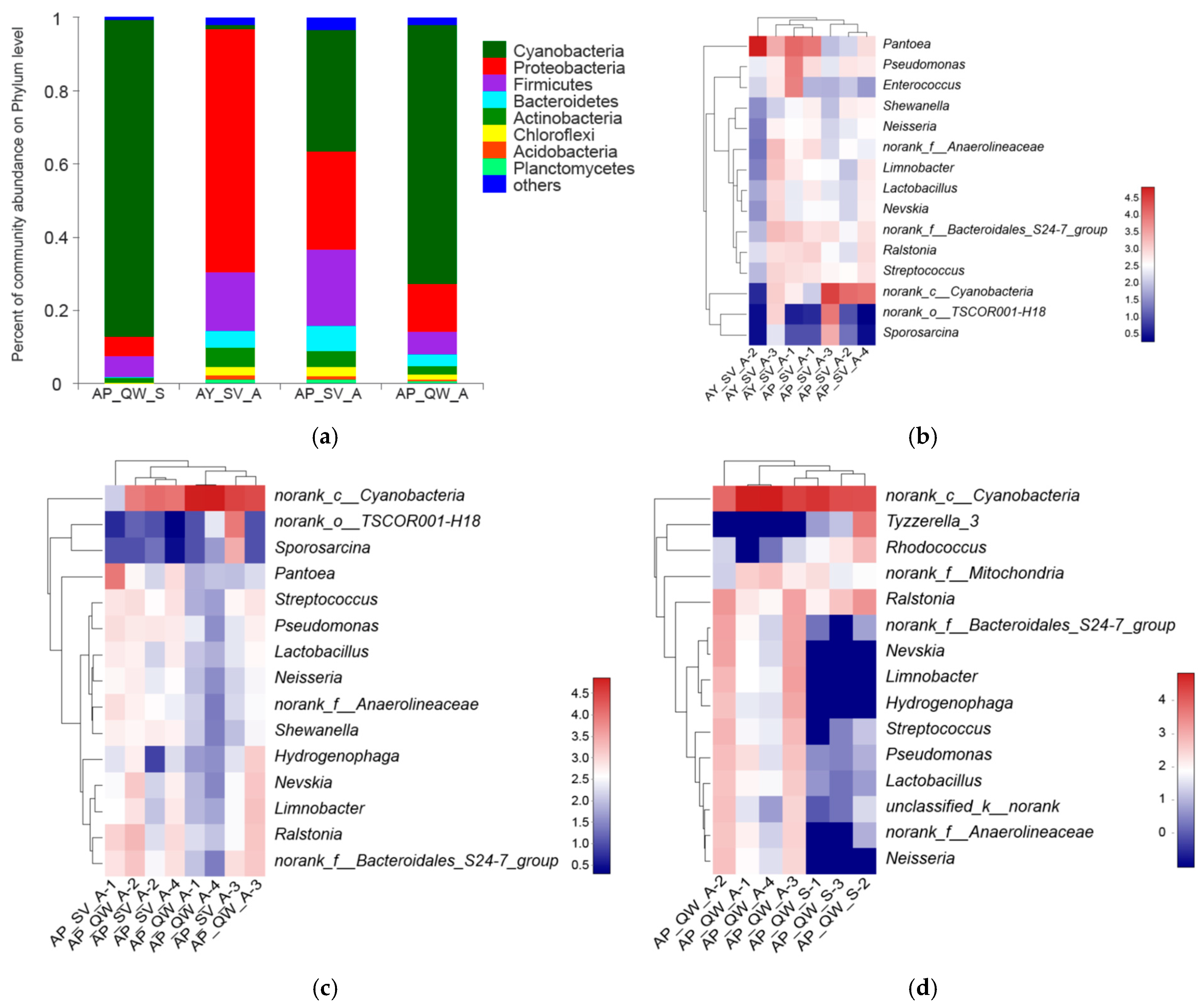
3.2. The Effect of Silkworm Species on the Gut Microbiota
3.3. The Effect of the Host Plants on the Gut Microbiota
3.4. Comparison of the Gut Microbiota Between Different Seasons
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, W.L.; Zhang, Z.Y.; Lin, L.; Terenius, O. Antheraea pernyi (Lepidoptera: Saturniidae) and its importance in sericulture, food consumption, and traditional Chinese medicine. J. Econ. Entomol. 2017, 110, 1404–1411. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.B.; Xia, R.X.; Li, Q.; Li, Y.P.; Cao, H.Y.; Liu, Y.Q. Genome-wide identification of detoxification genes in wild silkworm Antheraea pernyi and transcriptional response to coumaphos. Int. J. Mol. Sci. 2023, 24, 9775. [Google Scholar] [CrossRef] [PubMed]
- Hazarika, A.K.; Kalita, U. Human consumption of insects. Science 2023, 379, 140–141. [Google Scholar] [CrossRef] [PubMed]
- Van Huis, A.; Gasco, L. Insects as feed for livestock production. Science 2023, 379, 138–139. [Google Scholar] [CrossRef]
- Li, X.Y.; Liu, Y.C.; Zhang, R.S.; Chen, D.B.; Chen, M.M.; Li, Y.P.; Liu, Y.Q.; Qin, L. The mitochondrial genome of Qinghuang_1, the first modern improved strain of Chinese oak silkworm, Antheraea pernyi (Lepidoptera: Saturniidae). J. Insects Food Feed 2021, 7, 233–243. [Google Scholar] [CrossRef]
- Sun, S.W.; Huang, J.C.; Liu, Y.Q. The complete mitochondrial genome of the wild silkmoth Antheraea yamamai from Heilongjiang, China (Lepidoptera: Saturniidae). Mitochondrial DNA B 2021, 6, 2209–2211. [Google Scholar] [CrossRef]
- Kim, S.R.; Kwak, W.; Kim, H.; Caetano-Anolles, K.; Kim, K.Y.; Kim, S.B.; Choi, K.H.; Kim, S.W.; Hwang, J.S.; Kim, M.; et al. Genome sequence of the Japanese oak silk moth, Antheraea yamamai: The first draft genome in the family Saturniidae. Gigascience 2018, 7, gix113. [Google Scholar] [CrossRef]
- Yue, D.M.; Li, S.Y.; Zhang, J.; Wei, Q.; Zhao, M.N.; Wang, L.M. Analysis on nutrientional content and amino acid composition of Antheraea yamamai pupa. Sci. Seric. 2017, 43, 479–485. [Google Scholar]
- Ley, R.E.; Lozupone, C.A.; Hamady, M.; Knight, R.; Gordon, J.I. Worlds within worlds: Evolution of the vertebrate gut microbiota. Nat. Rev. Microbiol. 2008, 6, 776–788. [Google Scholar] [CrossRef]
- Flint, H.J.; Bayer, E.A.; Rincon, M.T.; Lamed, R.; White, B.A. Polysaccharide utilization by gut bacteria: Potential for new insights from genomic analysis. Nat. Rev. Microbiol. 2008, 6, 121–131. [Google Scholar] [CrossRef]
- Anand, A.A.; Vennison, S.J.; Sankar, S.G.; Prabhu, D.I.; Vasan, P.T.; Raghuraman, T.; Geoffrey, C.J.; Vendan, S.E. Isolation and characterization of bacteria from the gut of Bombyx mori that degrade cellulose, xylan, pectin and starch and their impact on digestion. J. Insect Sci. 2010, 10, 107. [Google Scholar] [CrossRef] [PubMed]
- Ezenwa, V.O.; Gerardo, N.M.; Inouye, D.W.; Mónica, M.; Xavier, J.B. Aminal behaviour and the microbiome. Science 2012, 338, 198–199. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Yang, B.; Huang, W.; Dobens, L.; Song, H.; Ling, E. Gut immunity in Lepidopteran insects. Dev. Comp. Immunol. 2016, 64, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Donkersley, P.; Rice, A.; Graham, R.I.; Wilson, K. Gut microbial community supplementation and reduction modulates African armyworm susceptibility to a baculovirus. FEMS Microbiol. Ecol. 2023, 99, fiac147. [Google Scholar] [CrossRef]
- Strano, C.P.; Malacrinò, A.; Campolo, O.; Palmeri, V. Influence of host plant on Thaumetopoea pityocampa gut bacterial community. Microb. Ecol. 2018, 75, 487–494. [Google Scholar] [CrossRef]
- Sun, Z.; Kumar, D.; Cao, G.; Zhu, L.; Liu, B.; Zhu, M.; Liang, Z.; Kuang, S.; Chen, F.; Feng, Y.; et al. Effects of transient high temperature treatment on the intestinal flora of the silkworm Bombyx mori. Sci. Rep. 2017, 7, 3349. [Google Scholar] [CrossRef]
- Li, G.N.; Xia, X.J.; Tang, W.C.; Zhu, Y. Intestinal microecology associated with fluoride resistance capability of the silkworm (Bombyx mori L.). Appl. Microbiol. Biotechnol. 2016, 100, 6715–6724. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, J.Y.; Wang, X.M.; Hu, H.L.; Xia, R.X.; Li, Q.; Zhu, X.W.; Wang, T.M.; Liu, Y.Q.; Qin, L. Comparison of bacterial communities between midgut and midgut contents in two silkworms, Antheraea pernyi and Bombyx mori. Sci. Rep. 2020, 10, 12966. [Google Scholar] [CrossRef]
- Tomasello, G.; Sorce, A.; Damiani, P.; Sinagra, E.; Carini, F. The importance of intestinal microbial flora (microbiota) and role of diet. Prog. Nutr. 2017, 19, 342–344. [Google Scholar] [CrossRef]
- Xu, N.; Tan, G.; Wang, H.; Gai, X. Effect of biochar additions to soil on nitrogen leaching, microbial biomass and bacterial community structure. Eur. J. Soil Biol. 2016, 74, 1–8. [Google Scholar] [CrossRef]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef] [PubMed]
- Cole, J.R.; Wang, Q.; Cardenas, E.; Fish, J.; Chai, B.; Farris, R.J.; Kulam-Syed-Mohideen, A.S.; McGarrell, D.M.; Marsh, T.; Garrity, G.M.; et al. The Ribosomal Database Project: Improved alignments and new tools for rRNA analysis. Nucleic Acids Res. 2009, 37, D141–D145. [Google Scholar] [CrossRef] [PubMed]
- Simpson, E.H. Measurement of diversity. Nature 1949, 163, 688. [Google Scholar] [CrossRef]
- Chao, A. Nonparametric estimation of the number of classes in a population. Scand. J. Stat. 1984, 11, 265–270. [Google Scholar]
- Chao, A.; Hwang, W.H.; Chen, Y.C.; Kuo, C.Y. Estimating the number of shared species in two communities. Stat. Sin. 2000, 10, 227–246. [Google Scholar]
- Kuczynski, J.; Stombaugh, J.; Walters, W.A.; González, A.; Caporaso, J.G.; Knight, R. Using QIIME to analyze 16S rRNA gene sequences from microbial communities. Curr. Protoc. Bioinform. 2011, 10, 7. [Google Scholar] [CrossRef]
- Lu, X.M.; Huang, S.K.; Wang, F.W.; Sun, Z.G.; Zhang, F.; Chen, S.L. Distribution of the Enterococci isolated from intestine of the pebrine infected silkworm. Sci. Seric. 2003, 29, 151–156. [Google Scholar]
- Xiang, H.; Li, M.W.; Zhao, Y.; Zhao, L.P.; Zhang, Y.H.; Huang, Y.P. Bacterial community in midgut of the silkworm larvae estimated by PCR/DGGE and 16S rDNA gene library analysis. Atca Entomol. Sin. 2007, 50, 222–233. [Google Scholar]
- Sun, Z.; Lu, Y.; Zhang, H.; Kumar, D.; Liu, B.; Gong, Y.; Zhu, M.; Zhu, L.; Liang, Z.; Kuang, S.; et al. Effects of BmCPV infection on silkworm Bombyx mori intestinal bacteria. PLoS ONE 2016, 11, e0146313. [Google Scholar] [CrossRef]
- Yun, J.H.; Roh, S.W.; Whon, T.W.; Jung, M.J.; Kim, M.S.; Park, D.S.; Yoon, C.; Nam, Y.D.; Kim, Y.J.; Choi, J.H.; et al. Insect gut bacterial diversity determined by environmental habitat, diet, developmental stage, and phylogeny of host. Appl. Environ. Microbiol. 2014, 80, 5254–5264. [Google Scholar] [CrossRef]
- Jia, S.G.; Zhang, X.W.; Zhang, G.Y.; Yin, A.; Zhang, S.; Li, F.S.; Wang, L.; Zhao, D.J.; Yun, Q.Z.; Tala, T.; et al. Seasonally variable intestinal metagenomes of the red palm weevil (Rhynchophorus ferrugineus). Environ. Microbiol. 2013, 15, 3020–3029. [Google Scholar] [CrossRef] [PubMed]
- Sudakaran, S.; Salem, H.; Kost, C.; Kaltenpoth, M. Geographical and ecological stability of the symbiotic mid-gut microbiota in European firebugs, Pyrrhocoris apterus (Hemiptera, Pyrrhocoridae). Mol. Ecol. 2012, 21, 6134–6151. [Google Scholar] [CrossRef] [PubMed]
- Berasategui, A.; Axelsson, K.; Nordlander, G.; Schmidt, A.; Borg-Karlson, A.K.; Gershenzon, J.; Terenius, O.; Kaltenpoth, M. The gut microbiota of the pine weevil is similar across Europe and resembles that of other conifer-feeding beetles. Mol. Ecol. 2016, 25, 4014–4031. [Google Scholar] [CrossRef] [PubMed]
- Ding, T.; Melcher, U. Influences of plant species, season and location on leaf endophytic bacterial communities of non-cultivated plants. PLoS ONE 2016, 11, e0150895. [Google Scholar] [CrossRef]
- Broderick, N.A.; Raffa, K.F.; Goodman, R.M.; Handelsman, J. Census of the bacterial community of the gypsy moth larval midgut by using culturing and culture-independent methods. Appl. Environ. Microbiol. 2004, 70, 293–300. [Google Scholar] [CrossRef]
- Engel, P.; Moran, N.A. The gut microbiota of insects—Diversity in structure and function. FEMS Microbiol. Rev. 2013, 37, 699–735. [Google Scholar] [CrossRef]
- Colman, D.R.; Toolson, E.C.; Takacs-Vesbach, C.D. Do diet and taxonomy influence insect gut bacterial communities? Mol. Ecol. 2012, 21, 5124–5137. [Google Scholar] [CrossRef]
- Coutinho, T.A.; Wingfield, M.J. Ralstonia solanacearum and R. pseudosolanacearum on Eucalyptus: Opportunists or Primary Pathogens? Front. Plant Sci. 2017, 8, 761. [Google Scholar] [CrossRef]
- Salcedo-Porras, N.; Umaña-Diaz, C.; Bitencourt, B.O.R.; Lowenberger, C. The role of bacterial symbionts in Triatomines: An evolutionary perspective. Microorganisms 2020, 8, 1438. [Google Scholar] [CrossRef]
- Hosokawa, T.; Ishii, Y.; Nikoh, N.; Fujie, M.; Satoh, N.; Fukatsu, T. Obligate bacterial mutualists evolving from environmental bacteria in natural insect populations. Nat. Microbiol. 2016, 1, 15011. [Google Scholar] [CrossRef]
| Sample | Sequences | Phylum | Class | Order | Family | Genus | OTUs | |
|---|---|---|---|---|---|---|---|---|
| AP_QW_S (spring) | 1 | 40,091 | 9 | 14 | 27 | 39 | 50 | 54 |
| 2 | 29,184 | 15 | 26 | 58 | 83 | 124 | 150 | |
| 3 | 20,842 | 14 | 19 | 41 | 63 | 76 | 89 | |
| Total | 90,117 | 17 | 29 | 65 | 110 | 162 | 206 | |
| AP_QW_A (autumn) | 1 | 77,966 | 22 | 43 | 86 | 145 | 249 | 328 |
| 2 | 47,763 | 31 | 67 | 144 | 264 | 528 | 819 | |
| 3 | 59,491 | 32 | 67 | 136 | 237 | 485 | 714 | |
| 4 | 75,785 | 20 | 38 | 87 | 145 | 258 | 338 | |
| Total | 261,005 | 39 | 81 | 170 | 324 | 717 | 1167 | |
| AP_SV_A (autumn) | 1 | 39,036 | 31 | 67 | 135 | 235 | 449 | 626 |
| 2 | 33,723 | 22 | 48 | 95 | 163 | 298 | 406 | |
| 3 | 73,121 | 27 | 62 | 138 | 263 | 580 | 892 | |
| 4 | 34,677 | 32 | 63 | 137 | 245 | 477 | 697 | |
| Total | 180,557 | 42 | 86 | 179 | 349 | 777 | 1281 | |
| AY_SV_A (autumn) | 1 | 60,194 | 31 | 59 | 136 | 257 | 524 | 824 |
| 2 | 74,352 | 18 | 35 | 76 | 138 | 242 | 322 | |
| 3 | 52,244 | 40 | 84 | 170 | 313 | 644 | 1065 | |
| Total | 186,790 | 41 | 87 | 181 | 347 | 743 | 1274 | |
| Sample | Chao | Shannon | Ace | Simpson | |
|---|---|---|---|---|---|
| AP_QW_S (spring) | 1 | 84.21 | 0.16 | 93.81 | 0.96 |
| 2 | 213.00 | 1.37 | 205.73 | 0.45 | |
| 3 | 136.05 | 0.42 | 147.67 | 0.87 | |
| AP_QW_A (autumn) | 1 | 458.22 | 0.65 | 449.11 | 0.86 |
| 2 | 1206.67 | 5.33 | 1190.60 | 0.04 | |
| 3 | 1014.56 | 3.65 | 993.39 | 0.23 | |
| 4 | 509.99 | 0.33 | 524.79 | 0.93 | |
| AP_SV_A (autumn) | 1 | 870.04 | 5.08 | 860.77 | 0.04 |
| 2 | 555.39 | 4.02 | 547.38 | 0.16 | |
| 3 | 1345.86 | 3.01 | 1321.72 | 0.25 | |
| 4 | 985.58 | 4.48 | 975.85 | 0.12 | |
| AY_SV_A (autumn) | 1 | 1192.98 | 4.10 | 1174.13 | 0.07 |
| 2 | 528.04 | 0.72 | 546.26 | 0.68 | |
| 3 | 1631.35 | 6.06 | 1605.97 | 0.01 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, C.; Hao, Y.; Yang, J.; Zhang, J.; Wang, H.; Liu, Y. Influences of Rearing Season, Host Plant, and Silkworm Species on Gut Bacterial Community. Insects 2025, 16, 47. https://doi.org/10.3390/insects16010047
Chen C, Hao Y, Yang J, Zhang J, Wang H, Liu Y. Influences of Rearing Season, Host Plant, and Silkworm Species on Gut Bacterial Community. Insects. 2025; 16(1):47. https://doi.org/10.3390/insects16010047
Chicago/Turabian StyleChen, Chang, Yujuan Hao, Jiaqi Yang, Jingyu Zhang, Huan Wang, and Yanqun Liu. 2025. "Influences of Rearing Season, Host Plant, and Silkworm Species on Gut Bacterial Community" Insects 16, no. 1: 47. https://doi.org/10.3390/insects16010047
APA StyleChen, C., Hao, Y., Yang, J., Zhang, J., Wang, H., & Liu, Y. (2025). Influences of Rearing Season, Host Plant, and Silkworm Species on Gut Bacterial Community. Insects, 16(1), 47. https://doi.org/10.3390/insects16010047







