Lethal Male Combat of Anastatus japonicus (Hymenoptera: Eupelmidae), an Egg Parasitoid of Lepidopterous and Hemipterous Pests
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect Colonies
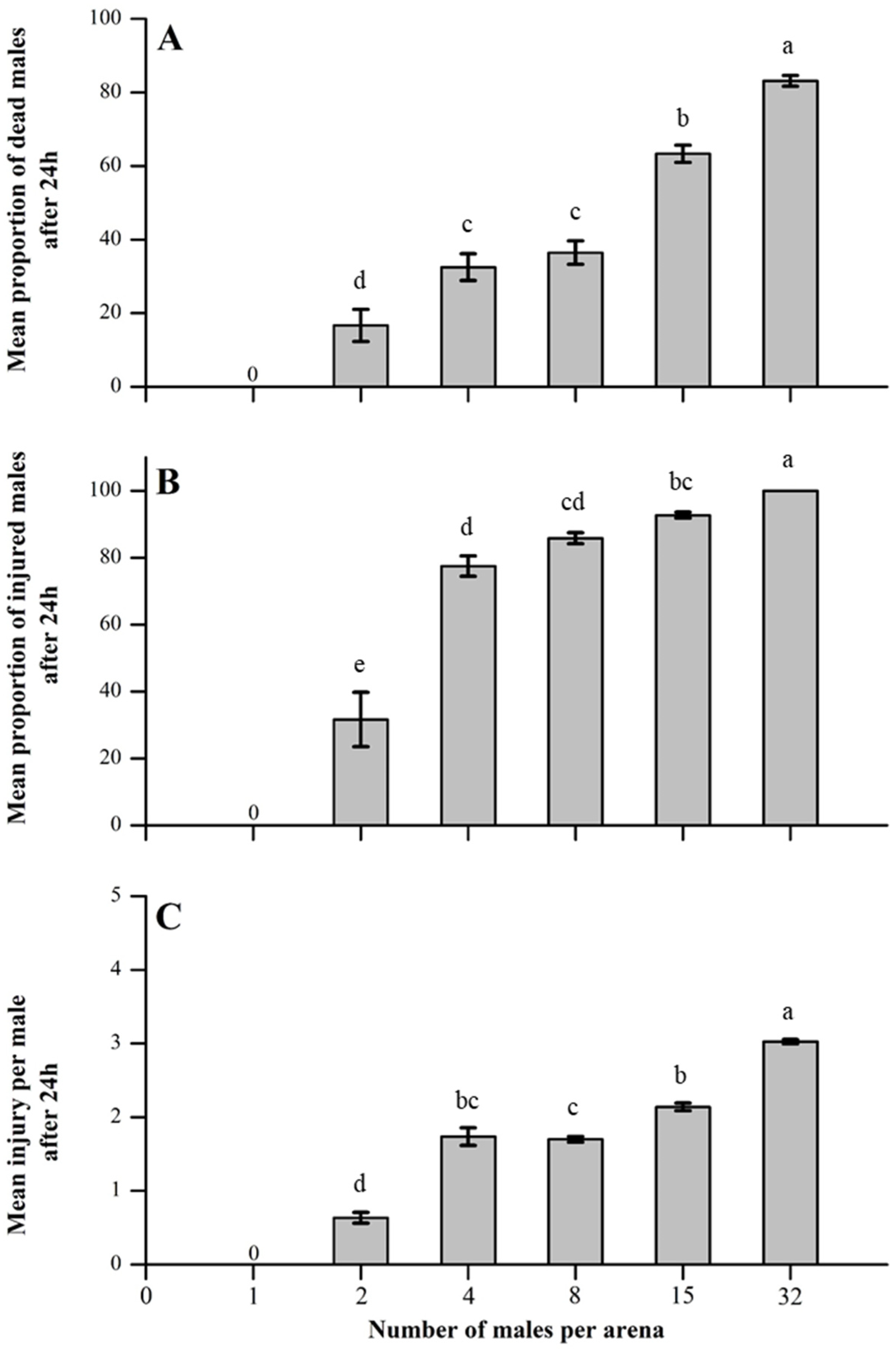
2.2. Male–Male Aggression as a Function of Male Density
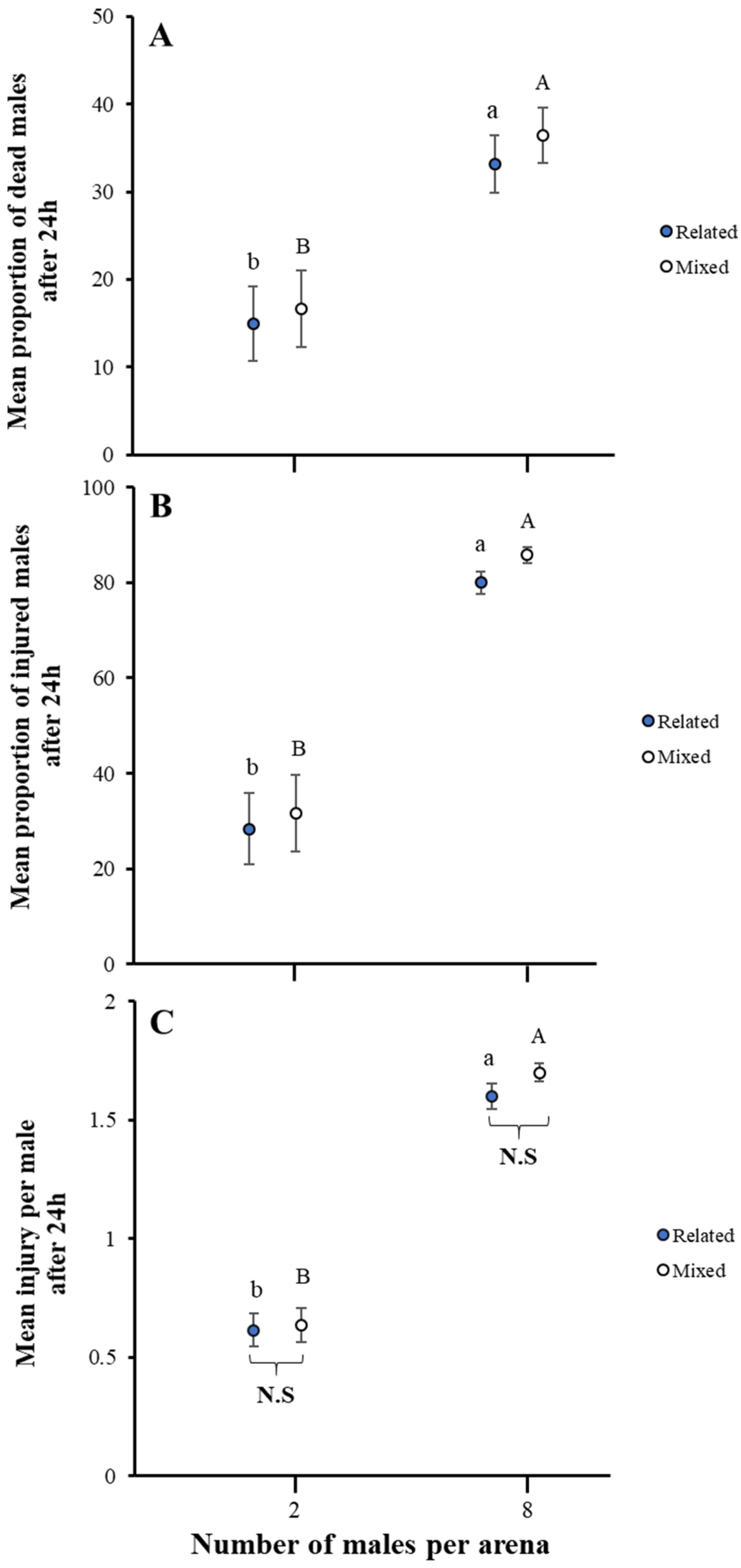
2.3. Male–Male Aggression as a Function of Related Males
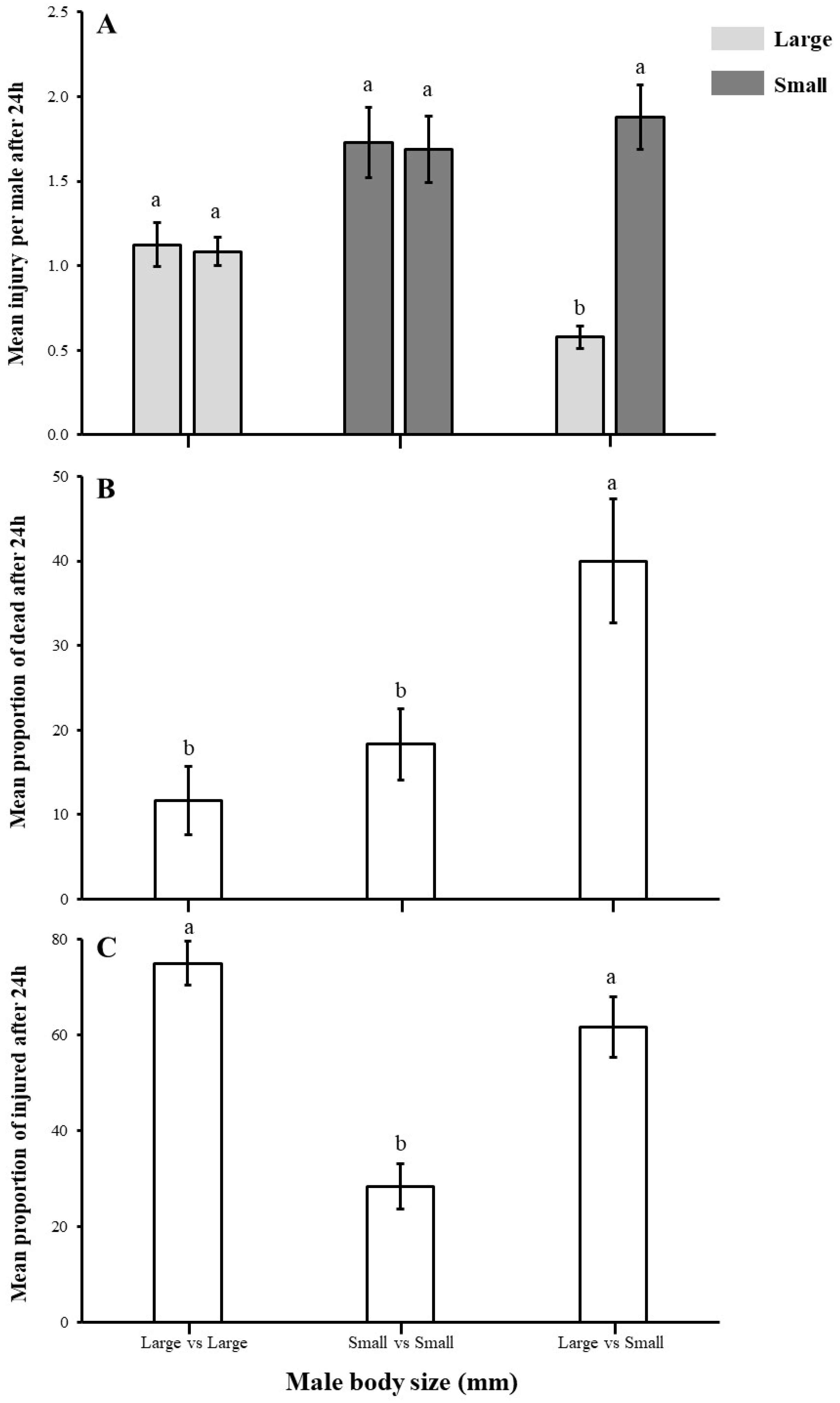
2.4. Male Aggression as a Function of Body Size
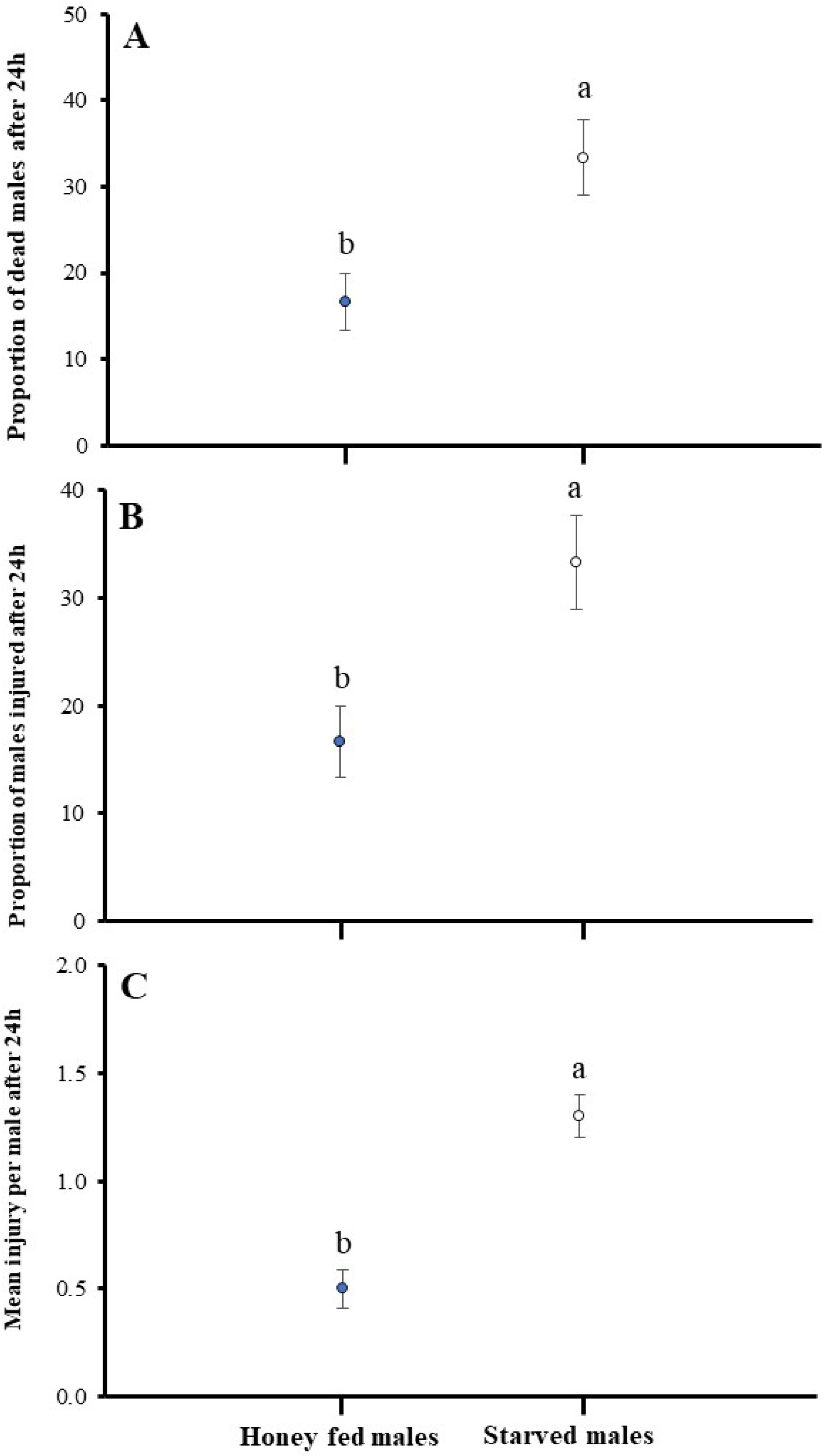
2.5. Aggression as a Function of Male Condition (Fed vs. Unfed)
2.6. Male–Male Aggression as a Function of Female Mating Status and Density
2.7. Statistical Analysis
3. Results
3.1. Impact of Competitor Density on Male–Male Combat
3.2. Impact of Related Males on Male–Male Combat
3.3. Impact of Body Size on Male–Male Combat
3.4. Impact of Starved and Honey-Fed Males on Male–Male Combat
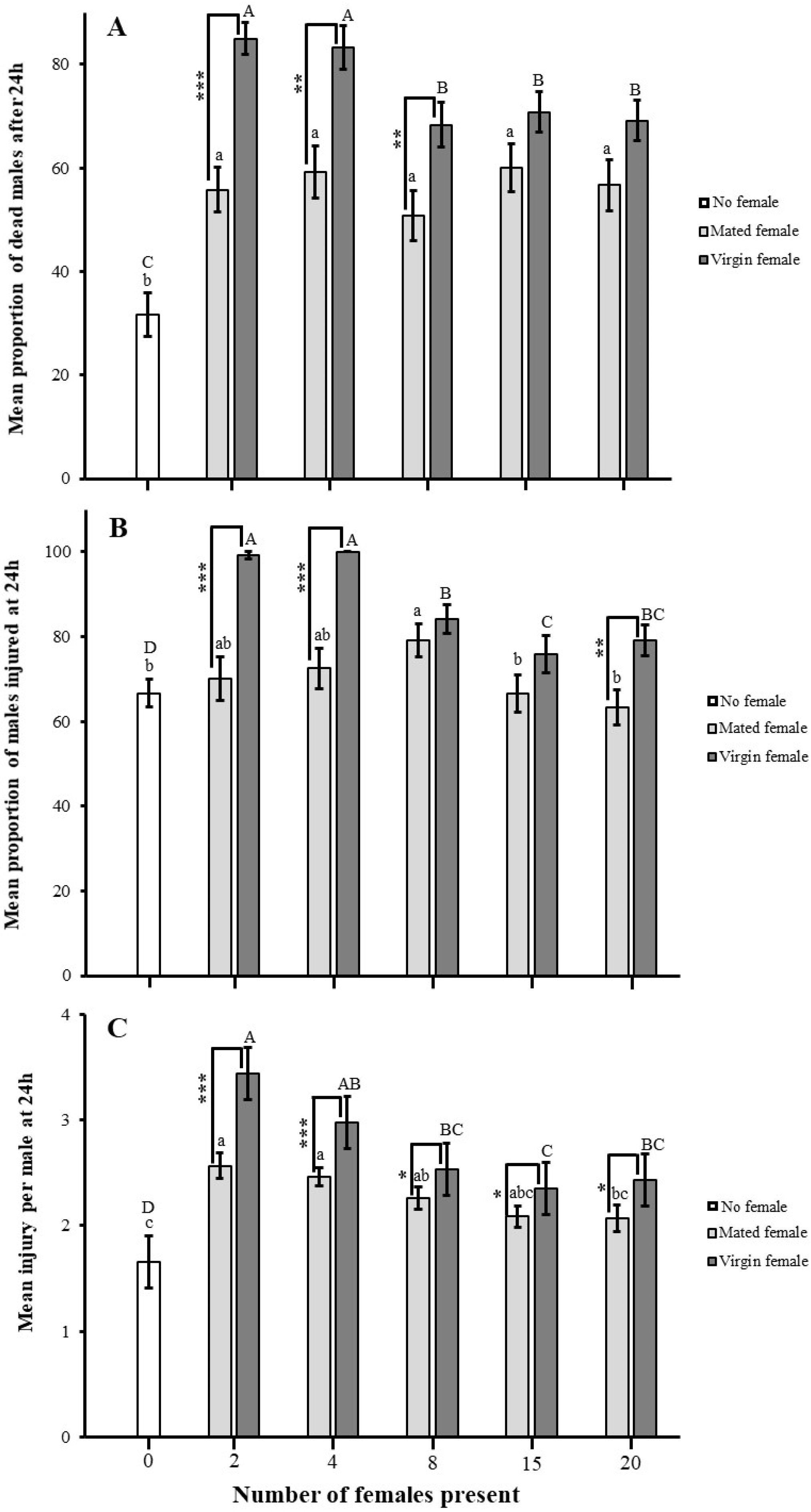
3.5. Impact of Female Presence on Male–Male Combat
4. Discussion
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hamilton, W.D. Wingless and Fighting Males in Fig Wasps and Other Insects. In Reproductive Competition, Mate Choice and Sexual Selection in Insects; Blum, M., Blum, N., Eds.; Academic Press: Cambridge, MA, USA, 1979; pp. 167–220. [Google Scholar]
- Greeff, J.M.; Ferguson, J.W.H. Mating Ecology of the Nonpollinating Fig Wasps of Ficus ingens. Anim. Behav. 1999, 57, 215–222. [Google Scholar] [CrossRef][Green Version]
- Matthews, R.W. Courtship in Parasitic Wasps; Price, P.W., Ed.; Plenum Press: New York, NY, USA, 1982. [Google Scholar]
- Kapranas, A.; Zenner, A.N.R.L.; Mangan, R.; Griffin, C.T. Objective and Subjective Components of Resource Value in Lethal Fights between Male Entomopathogenic Nematodes. Anim. Behav. 2020, 164, 149–154. [Google Scholar] [CrossRef]
- Cremer, S.; Suefuji, M.; Schrempf, A.; Heinze, J. The Dynamics of Male-Male Competition in Cardiocondyla obscurior Ants. BMC Ecol. 2012, 12, 7. [Google Scholar] [CrossRef] [PubMed]
- Moore, J.C.; Obbard, D.J.; Reuter, C.; West, S.A.; Cook, J.M. Fighting Strategies in Two Species of Fig Wasp. Anim. Behav. 2008, 76, 315–322. [Google Scholar] [CrossRef]
- Murray, M. The Closed Environment of the Fig Receptacle and Its Influence on Male Conflict in the Old World Fig Wasp, Philotrypesis pilosa. Anim. Behav. 1987, 35, 488–506. [Google Scholar] [CrossRef]
- Innocent, T.M.; West, S.A.; Sanderson, J.L.; Hyrkkanen, N.; Reece, S.E. Lethal Combat over Limited Resources: Testing the Importance of Competitors and Kin. Behav. Ecol. 2011, 22, 923–931. [Google Scholar] [CrossRef]
- Enquist, M.; Leimar, O. Evolution of Fighting Behaviour: The Effect of Variation in Resource Value. J. Theor. Biol. 1987, 127, 187–205. [Google Scholar] [CrossRef]
- Georgiev, A.V.; Klimczuk, A.C.E.; Traficonte, D.M.; Maestripieri, D. When Violence Pays: A Cost-Benefit Analysis of Aggressive Behavior in Animals and Humans. Evol. Psychol. 2013, 11, 678–699. [Google Scholar] [CrossRef]
- Maynard Smith, J.; Price, G.R. The Logic of Animal Conflict. Nature 1973, 246, 15–18. [Google Scholar] [CrossRef]
- Mi, Q.Q.; Zhang, J.P.; Ali, M.Y.; Zhong, Y.Z.; Mills, N.J.; Li, D.S.; Lei, Y.M.; Zhang, F. Reproductive Attributes and Functional Response of Anastatus japonicus on Eggs of Antheraea pernyi, a Factitious Host. Pest Manag. Sci. 2022, 78, 4679–4688. [Google Scholar] [CrossRef]
- Li, D.-S.; Liao, C.; Zhang, B.-X.; Song, Z.-W. Biological Control of Insect Pests in Litchi Orchards in China. Biol. Control 2014, 68, 23–36. [Google Scholar] [CrossRef]
- Hou, Z. Application of Anastatus Sp. against Halyomorpha halys. For. Pest Dis. 2009, 28, 43. [Google Scholar]
- Stahl, J.M.; Babendreier, D.; Marazzi, C.; Caruso, S.; Costi, E.; Maistrello, L.; Haye, T. Can Anastatus bifasciatus Be Used for Augmentative Biological Control of the Brown Marmorated Stink Bug in Fruit Orchards? Insects 2019, 10, 108. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Gibson, G.A.P.; Tang, L.; Xiang, J. Review of the Species of Anastatus (Hymenoptera: Eupelmidae) Known from China, with Description of Two New Species with Brachypterous Females. Zootaxa 2020, 4767, 351–401. [Google Scholar] [CrossRef]
- Wilson, F. Adult Reproductive Behaviour in Asolcus basalis (Hymenoptera: Scelionidae). Aust. J. Zool. 1961, 9, 739. [Google Scholar] [CrossRef]
- Van Den Assem, J.; Gijswijt, M.J.; Nübel, B.K. Observations on Courtship-and Mating Strategies in a Few Species of Parasitic Wasps (Chalcidoidea). Neth. J. Zool. 2008, 30, 208–227. [Google Scholar] [CrossRef]
- Godfray, H.C.J. Parasitoids: Behavioral and Evolutionary Ecology; Princeton University Press: Princeton, NJ, USA, 1994; Volume 67. [Google Scholar]
- Kuramitsu, K.; Yooboon, T.; Tomatsuri, M.; Yamada, H.; Yokoi, T. First Come, First Served: Precopulatory Mate-Guarding Behavior and Male–Male Contests by a Hymenopteran Saproxylic Parasitoid. Sci. Nat. 2019, 106, 203. [Google Scholar] [CrossRef]
- West, S.A.; Murray, M.G.; Machado, C.A.; Griffin, A.S.; Herre, E.A. Testing Hamilton’s Rule with Competition between Relatives. Nature 2001, 409, 510–513. [Google Scholar] [CrossRef]
- Ali, M.Y.; Liu, Y.D.; Li, F.Q.; Hou, M.L.; Zhang, J.P.; Zhang, F. Molecular Identification of the Brown Marmorated Stink Bug’s Egg Parasitoids by Species—Specific PCR Collected from Beijing, China. CABI Agric. Biosci. 2023, 4, 41. [Google Scholar] [CrossRef]
- Reinhold, K. Influence of Male Relatedness on Lethal Combat in Fig Wasps: A Theoretical Analysis. Proc. R. Soc. Lond. Ser. B Biol. Sci. 2003, 270, 1171–1175. [Google Scholar] [CrossRef]
- Enquist, M.; Leimar, O. The Evolution of Fatal Fighting. Anim. Behav. 1990, 39, 1–9. [Google Scholar] [CrossRef]
- Liu, P.C.; Wei, J.R.; Tian, S.; Hao, D.J. Male-Male Lethal Combat in the Quasi-Gregarious Parasitoid Anastatus disparis (Hymenoptera: Eupelmidae). Sci. Rep. 2017, 7, 11875. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, W.D. The Genetical Evolution of Social Behaviour. I. J. Theor. Biol. 1964, 7, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Marris, G.C.; Hubbard, S.F.; Scrimgeour, C. The Perception of Genetic Similarity by the Solitary Parthenogenetic Parasitoid Venturia canescens, and Its Effects on the Occurrence of Superparasitism. Entomol. Exp. Appl. 1996, 78, 167–174. [Google Scholar] [CrossRef]
- Reece, S.E.; Shuker, D.M.; Pen, I.; Duncan, A.B.; Choudhary, A.; Batchelor, C.M.; West, S.A. Kin Discrimination and Sex Ratios in a Parasitoid Wasp. J. Evol. Biol. 2004, 17, 208–216. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Tao, Y.M.; Yin, S.Y.; Zhang, W.J.; Ding, Y.D.; Hu, H.Y.; Liu, P.C. Early Social Experience, Rather than Kinship Affecting Aggression in an Egg Parasitoid Wasp, Anastatus disparis (Hymenoptera: Eupelmidae), That Exhibits Extreme Male–Male Combat Behaviour. Ecol. Entomol. 2022, 47, 801–809. [Google Scholar] [CrossRef]
- Clutton-Brock, T.H.; Huchard, E. Social Competition and Selection in Males and Females. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2013, 368, 20130074. [Google Scholar] [CrossRef]
- Yin, S.-Y.; Tao, Y.-M.; Liu, P.-C. Effect of Male–Female Relatedness on Aggression and Inbreeding in an Egg Parasitoid Wasp. Entomol. Sci. 2023, 26, e12541. [Google Scholar] [CrossRef]
- McClure, M.; Whistlecraft, J.; McNeil, J.N. Courtship behavior in relation to the female sex pheromone in the parasitoid, Aphidius ervi (Hymenoptera: Braconidae). J. Chem. Ecol. 2007, 33, 1946–1959. [Google Scholar] [CrossRef]
- Kingan, T.G.; Bodnar, W.M.; Raina, A.K.; Shabanowitz, J.; Hunt, D.F. The Loss of Female Sex Pheromone after Mating in the Corn Earworm Moth Helicoverpa zea: Identification of a Male Pheromonostatic Peptide. Proc. Natl. Acad. Sci. USA 1995, 92, 5082–5086. [Google Scholar] [CrossRef]
- Estrada, C.; Schulz, S.; Yildizhan, S.; Gilbert, L.E. Sexual Selection Drives the Evolution of Antiaphrodisiac Pheromones in Butterflies. Evolution 2011, 65, 2843–2854. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.C.; Hao, D.J. Effect of Variation in Objective Resource Value on Extreme Male Combat in a Quasi-Gregarious Species, Anastatus disparis. BMC Ecol. 2019, 19, 21. [Google Scholar] [CrossRef] [PubMed]
- Clutton-Brock, T.H. Reproductive Success: Studies of Individual Variation in Contrasting Breeding Systems; University of Chicago Press: Chicago, IL, USA, 1988; ISBN 0226110583. [Google Scholar]
- Visser, M.E. The Importance of Being Large: The Relationship between Size and Fitness in Females of the Parasitoid Aphaereta minuta (Hymenoptera: Braconidae). J. Anim. Ecol. 1994, 63, 963–978. [Google Scholar] [CrossRef]
- Sagarra, L.A.; Vincent, C.; Stewart, R.K. Body Size as an Indicator of Parasitoid Quality in Male and Female Anagyrus kamali (Hymenoptera: Encyrtidae). Bull. Entomol. Res. 2001, 91, 363–368. [Google Scholar] [CrossRef]
- Petersen, G.; Hardy, I.A.N.C.W. The Importance of Being Larger: Parasitoid Intruder–Owner Contests and Their Implications for Clutch Size. Anim. Behav. 1996, 51, 1363–1373. [Google Scholar] [CrossRef]
- Grafen, A. The Logic of Divisively Asymmetric Contests: Respect for Ownership and the Desperado Effect. Anim. Behav. 1987, 35, 462–467. [Google Scholar] [CrossRef]
- Monnin, T.; Peeters, C. Dominance Hierarchy and Reproductive Conflicts among Subordinates in a Monogynous Queenless Ant. Behav. Ecol. 1999, 10, 323–332. [Google Scholar] [CrossRef]
- Snart, C.J.P.; Kapranas, A.; Williams, H.; Barrett, D.A.; Hardy, I.C.W. Sustenance and Performance: Nutritional Reserves, Longevity, and Contest Outcomes of Fed and Starved Adult Parasitoid Wasps. Front. Ecol. Evol. 2018, 6, 12. [Google Scholar] [CrossRef]
- Tsai, Y.-J.J.; Barrows, E.M.; Weiss, M.R. Why Do Larger and Older Males Win Contests in the Parasitoid Wasp Nasonia vitripennis? Anim. Behav. 2014, 91, 151–159. [Google Scholar] [CrossRef]
- Liu, P.C.; Hao, D.J.; Hu, H.Y.; Wei, J.R.; Wu, F.; Shen, J.; Xu, S.J.; Xie, Q.Y. Effect of Winning Experience on Aggression Involving Dangerous Fighting Behavior in Anastatus disparis (Hymenoptera: Eupelmidae). J. Insect Sci. 2020, 20, 8. [Google Scholar] [CrossRef]

| Description of Injury * | Score for Each Injury |
|---|---|
| Loss of Part or Whole Antenna | 0.5 |
| Loss of Part or Whole Tarsi | 1.0 |
| Loss of Part or Whole Tibia (Plus Tarsi) | 2.0 |
| Loss of Part or Whole Femur (Plus Tibia and Tarsi) and Bruise | 3.0 |
| Loss of Part or Whole Coxa (Plus Femur, Tibia and Tarsi) | 4.0 |
| ~<Half Severed Abdomen or Head, No Evisceration | 5.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali, M.Y.; Avila, G.A.; Luo, Z.-Y.; Hassan, M.A.; Khan, K.A.; Zhang, J.-P.; Zhang, F. Lethal Male Combat of Anastatus japonicus (Hymenoptera: Eupelmidae), an Egg Parasitoid of Lepidopterous and Hemipterous Pests. Insects 2025, 16, 45. https://doi.org/10.3390/insects16010045
Ali MY, Avila GA, Luo Z-Y, Hassan MA, Khan KA, Zhang J-P, Zhang F. Lethal Male Combat of Anastatus japonicus (Hymenoptera: Eupelmidae), an Egg Parasitoid of Lepidopterous and Hemipterous Pests. Insects. 2025; 16(1):45. https://doi.org/10.3390/insects16010045
Chicago/Turabian StyleAli, Muhammad Yasir, Gonzalo A. Avila, Zheng-Yu Luo, Muhammad Asghar Hassan, Khalid Ali Khan, Jin-Ping Zhang, and Feng Zhang. 2025. "Lethal Male Combat of Anastatus japonicus (Hymenoptera: Eupelmidae), an Egg Parasitoid of Lepidopterous and Hemipterous Pests" Insects 16, no. 1: 45. https://doi.org/10.3390/insects16010045
APA StyleAli, M. Y., Avila, G. A., Luo, Z.-Y., Hassan, M. A., Khan, K. A., Zhang, J.-P., & Zhang, F. (2025). Lethal Male Combat of Anastatus japonicus (Hymenoptera: Eupelmidae), an Egg Parasitoid of Lepidopterous and Hemipterous Pests. Insects, 16(1), 45. https://doi.org/10.3390/insects16010045









