The Use of Novel Alginate Capsules in a Monitoring System for Drosophila suzukii in a Cherry Orchard in the Region of La Araucanía, Chile
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect Collection
2.2. Monitoring Traps for SWD
2.3. Development of Baits for SWD
2.4. Y-Tube Olfactometric Bioassays
2.5. Encapsulation Process Development
2.6. Release Rate of the Selected Encapsulation
2.7. Field Trial with Traps and Encapsulated Bait
2.8. Statistical Analysis
3. Results
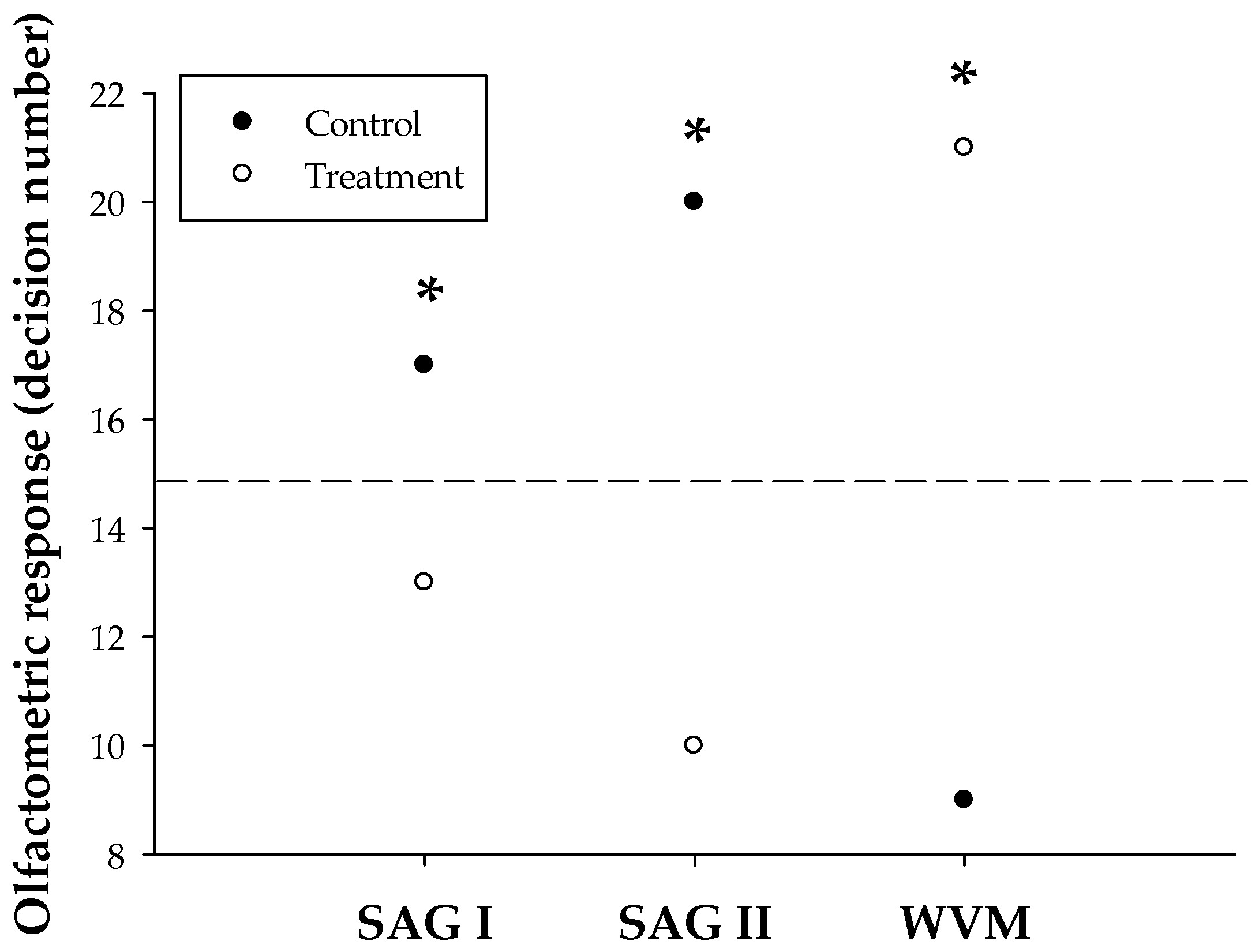
3.1. Olfactometric Bioassays
3.2. Release Rate
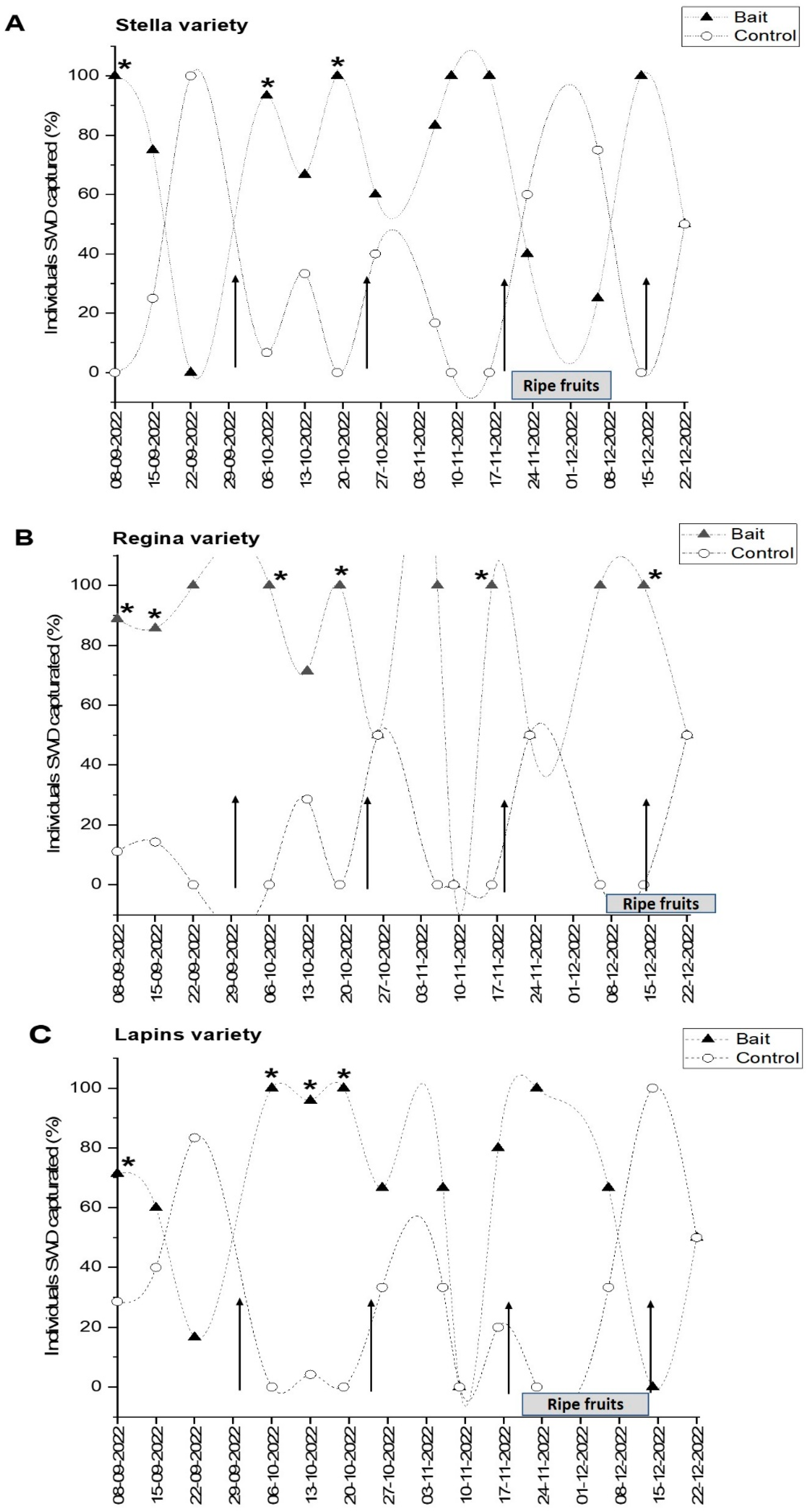
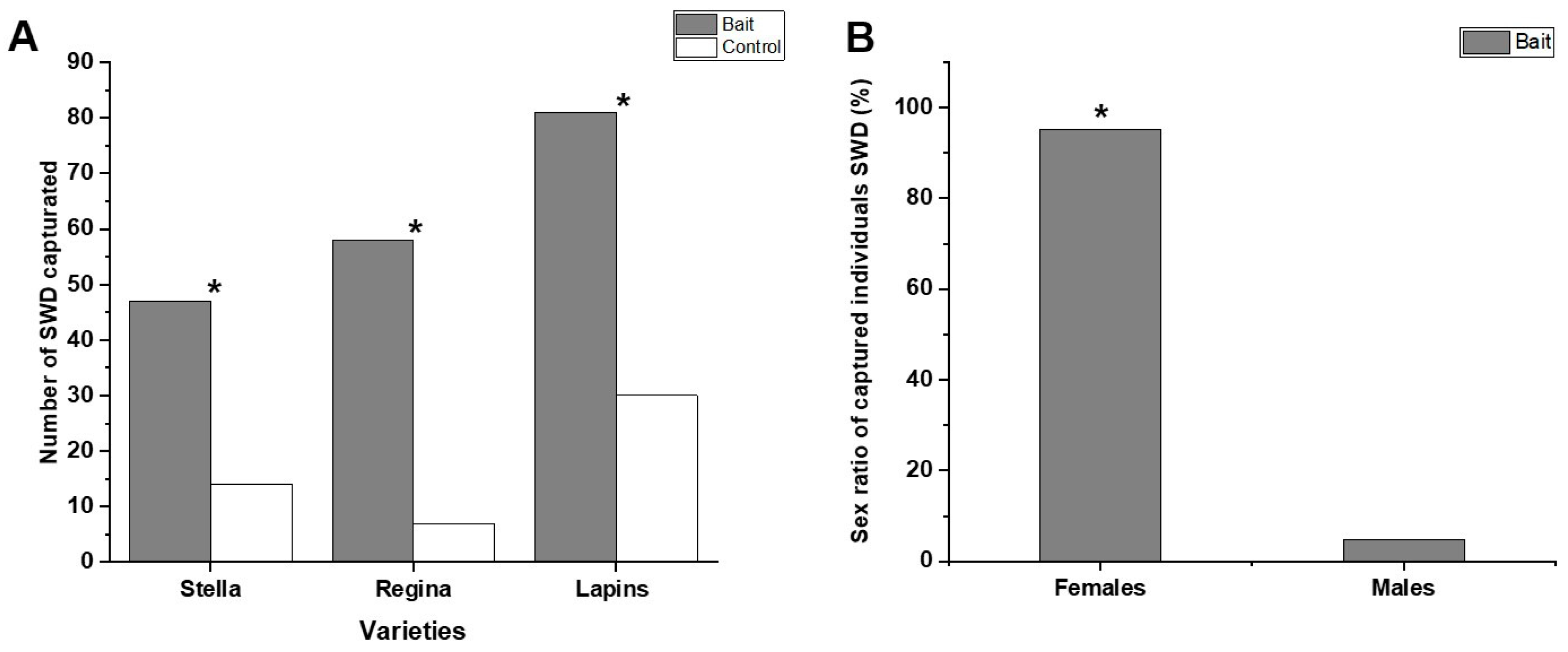
3.3. Field Trials
4. Discussion
4.1. Olfactometric Bioassays
4.2. Release Rate
4.3. Field Trial
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kanzawa, T. Studies on Drosophila suzukii Mats. Rev. Appl. Entomol. 1939, 29, 622. [Google Scholar]
- Kanzawa, T. Studies on Drosophila suzukii Mats. J. Plant Prot. 1936, 23, 66–70. [Google Scholar]
- Dos Santos, L.A.; Mendes, M.F.; Krüger, A.P.; Blauth, M.L.; Gottschalk, M.S.; Garcia, F.R. Global potential distribution of Drosophila suzukii (Diptera, Drosophilidae). PLoS ONE 2017, 12, e0174318. [Google Scholar] [CrossRef] [PubMed]
- Hauser, M. A historic account of the invasion of Drosophila suzukii (Matsumura) (Diptera: Drosophilidae) in the continental United States, with remarks on their identification. Pest Manag. Sci. 2011, 67, 1352–1357. [Google Scholar] [CrossRef]
- SENASICA. Mosca Del Vinagre de Alas Manchadas (Drosophila suzukii Matsumura); Sader: Mexico City, México, 2013; 21p. [Google Scholar]
- Deprá, M.; Poppe, J.L.; Schmitz, H.J.; De Toni, D.C.; Valente, V.L.S. The first records of the invasive pest Drosophila suzukii in the South American continent. J. Pest Sci. 2014, 87, 379–383. [Google Scholar] [CrossRef]
- González, G.; Mary, A.L.; Goñi, B. Drosophila suzukii (Matsumura) found in Uruguay. Drosoph. Inf. Serv. 2015, 98, 103–107. [Google Scholar]
- Santadino, M.V.; Riquelme, M.B.; Ansa, M.A.; Bruno, M.; Di Silvestro, G.; Lunazzi, E.G. Primer registro de Drosophila suzukii (Diptera: Drosophilidae) asociado al cultivo de arándanos (Vaccinium spp.) de Argentina. Rev. Soc. Entomol. Arg. 2015, 74, 183–185. [Google Scholar]
- Bizama, G. Invasión de Drosophila suzukii (Matsumura) en Chile: Utilizando los modelos de distribución de especies como herramienta de bioseguridad. Rev. Chil. Entomol. 2020, 46, 61–71. [Google Scholar] [CrossRef]
- European and Mediterranean Plant Protection Organization (EPPO). Drosophila suzukii Does Not Occur in Chile. 2016. Available online: https://gd.eppo.int/reporting/article-5875 (accessed on 12 December 2024).
- SAG Resolución Exenta Nº3672: Medidas Fitosanitarias de Emergencia Provisionales Para la Plaga Drosófila de Alas Manchadas-Drosophila suzukii (Matsumura) Diptera: Drosophilidae. División Protección Agrícola y Forestal, Ministerio de Agricultura de Chile/Departamento de Sanidad Vegetal. 2017. Available online: http://www.diariooficial.interior.gob.cl/publicaciones/2017/06/20/41788/01/1229986.pdf (accessed on 20 January 2024).
- Walsh, D.B.; Bolda, M.P.; Goodhue, R.E.; Dreves, A.J.; Lee, J.C.; Bruck, D.J.; Walton, V.M.; O’Neal, S.D.; Zalom, F.G. Drosophila suzukii (Diptera: Drosophilidae): Invasive pest of ripening soft fruit expanding its geographic range and damage potential. J. Integr. Pest Manag. 2011, 1, G1–G7. [Google Scholar] [CrossRef]
- Rota-Stabelli, O.; Blaxter, M.; Anfora, G. Quick Guide: Drosophila suzukii. Curr. Biol. 2013, 23, R8–R9. [Google Scholar] [CrossRef]
- Bolda, M.; Goodhue, R.; Zalom, F. 2010. Spotted wing drosophila: Potential economic impact of a newly established pest. Agric. Resour. Econ. 2010, 13, 5–8. [Google Scholar]
- Yeh, D.; Drummond, F.; Gómez, M.; Fan, X. The Economic Impacts and Management of Spotted Wing Drosophila (Drosophila suzukii): The Case of Wild Blueberries in Maine. J. Econ. Entomol. 2020, 113, 1262–1269. [Google Scholar] [CrossRef] [PubMed]
- Thistlewood, H.; Gill, P.; Beers, E.; Shearer, P.; Walsh, D.; Rozema, B.; Acheampong, S.; Castagnoli, S.; Yee, W.; Smytheman, P.; et al. Spatial Analysis of Seasonal Dynamics and Overwintering of Drosophila suzukii (Diptera: Drosophilidae) in the Okanagan-Columbia Basin, 2010–2014. Environ. Entomol. 2018, 47, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Cini, A.; Ioriatti, C.; Anfora, A. A review of the invasion of Drosophila suzukii in Europe and a draft research agenda for integrated pest management. Bull. Insectol. 2012, 65, 149–160. [Google Scholar]
- Jaffe, B.D.; Avanesyan, A.; Bal, H.K.; Feng, Y.; Grant, J.; Grieshop, M.J.; Lee, J.C.; Liburd, O.E.; Rhodes, E.; Rodriguez-Saona, C.; et al. Multistate comparison of attractants and the impact of fruit development stage on trapping Drosophila suzukii (Diptera: Drosophilidae) in raspberry and blueberry. Environ. Entomol. 2018, 47, 935–945. [Google Scholar] [CrossRef]
- Lee, J.C.; Bruck, D.J.; Curry, H.; Edwards, D.L.; Haviland, D.R.; Van Steenwyk, R.A.; Yorgey, B.M. The susceptibility of small fruits and cherries to the spotted wing drosophila, Drosophila suzukii. Pest Manag. Sci. 2011, 67, 1358–1367. [Google Scholar] [CrossRef]
- Lee, J.C.; Shearer, P.W.; Barrantes, L.D.; Beers, E.H.; Burrack, H.J.; Dalton, D.T.; Dreves, A.J.; Gut, L.J.; Hamby, K.A.; Haviland, D.R.; et al. Trap Designs for Monitoring Drosophila suzukii (Diptera: Drosophilidae). Environ. Entomol. 2013, 42, 1348–1355. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.C.; Burrack, H.J.; Barrantes, L.D.; Beers, E.H.; Dreves, A.J.; Hamby, K.A.; Haviland, D.R.; Isaacs, R.; Richardson, T.A.; Shearer, P.W.; et al. Evaluation of monitoring traps for Drosophila suzukii (Diptera: Drosophilidae) in North America. J. Econ. Entomol. 2012, 105, 1350–1357. [Google Scholar] [CrossRef]
- Kleiber, J.R.; Unelius, C.R.; Lee, J.C.; Suckling, D.M.; Qian, M.C.; Bruck, D.J. Attractiveness of fermentation and related products to spotted wing Drosophila (Diptera: Drosophilidae). Environ. Entomol. 2014, 43, 439–447. [Google Scholar] [CrossRef] [PubMed]
- Burrak, H.J.; Asplen, M.; Bahder, L.; Collins, J.; Drummond, F.A.; Guédot, C.; Issacs, R.; Johnson, D.; Blanton, A.; Lee, J.C.; et al. Multistate Comparison of Attractants for Monitoring Drosophila suzukii (Diptera: Drosophilidae) in Blueberries and Caneberries. Environ. Entomol. 2015, 44, 704–712. [Google Scholar] [CrossRef] [PubMed]
- Groupe de Travall Baies. Drosophila suzukii: Strategie 2015 pour les petits fruits. In Agroscope Fiche Technique N° 20; Agroscope: Conthey, Switzerland, 2015. [Google Scholar]
- Kirkpatrick, D.M.; McGhee, P.S.; Gut, L.J.; Miller, J.R. Improving monitoring tools for spotted wing drosophila, Drosophila suzukii. Entomol. Exp. Appl. 2017, 164, 87–93. [Google Scholar] [CrossRef]
- Clymans, R.; Van Kerckvoorde, V.; Bangels, E.; Akkermans, W.; Alhmedi, A.; De Clercq, P.; Beliën, T.; Bylemans, D. Olfactory preference of Drosophila suzukii shifts between fruit and fermentation cues over the season: Effects of physiological status. Insects 2019, 10, 200. [Google Scholar] [CrossRef]
- Del Fava, E.; Ioriatti, C.; Melegaro, A. Cost–benefit analysis of controlling the spotted wing drosophila (Drosophila suzukii (Matsumura)) spread and infestation of soft fruits in Trentino, Northern Italy. Pest Manag. Sci. 2017, 73, 2318–2327. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations/World Health Organization (FAO/WHO). FAO/WHO Expert Meeting on the Application of Nanotechnologies in the Food and Agriculture Sectors: Potential Food Safety Implications; Meeting Report; FAO/WHO: Rome, Italy, 2010; 130p.
- Mutis, A.; Parra, L.; Manosalva, L.; Palma, R.; Candia, O.; Lizama, M.; Pardo, F.; Perich, F.; Quiroz, A. Electroantennographic and behavioral responses of adults of raspberry weevil Aegorhinus superciliosus (Coleoptera: Curculionidae) to odors released from conspecific females. Environ. Entomol. 2010, 39, 1276–1282. [Google Scholar] [CrossRef]
- Khot, L.R.; Sankaran, S.; Maja, J.M.; Ehsani, R.; Schuster, E.W. Applications of nanomaterials in agricultural production and crop protection: A review. Crop Prot. 2012, 35, 64–70. [Google Scholar] [CrossRef]
- Ghormade, V.; Deshpande, M.V.; Paknikar, K.M. Perspectives for nano-biotechnology enabled protection and nutrition of plants. Biotechnol. Adv. 2011, 29, 792–803. [Google Scholar] [CrossRef] [PubMed]
- Brignone, S.G.; Ravetti, S.; Palma, S.D. Microencapsulación/recubrimiento de sistemas particulados de uso farmacéutico. Pharm. Technol. Ed. Sudam. 2020, 165, 34–37. [Google Scholar]
- Ríos-Aguirre, S.; Gil-Garzón, M.A. Microencapsulación por secado por aspersión de compuestos bioactivos en diversas matrices: Una revisión. TecnoLógicas 2021, 24, 206–223. [Google Scholar] [CrossRef]
- De Oliveira, J.; Ramos Campos, E.; Bakshi, M.; Abhilash, P.C.; Fernandes Fraceto, L. Application of nanotechnology for the encapsulation of botanical insecticides for sustainable agriculture: Prospects and promises. Biotechnol. Adv. 2014, 32, 1550–1561. [Google Scholar] [CrossRef] [PubMed]
- Koschier, E.; De Kogel, W.; Visser, J. Assessing the attractiveness of volatile plant compounds to western flower thrips Frankliniella occidentalis. J. Chem. Ecol. 2000, 26, 2643–2655. [Google Scholar] [CrossRef]
- Renkema, J.M.; Buitenhuis, R.; Hallett, R.H. Optimizing Trap Design and Trapping Protocols for Drosophila suzukii (Diptera: Drosophilidae). J. Econ. Entomol. 2014, 107, 2107–2118. [Google Scholar] [CrossRef] [PubMed]
- Lasa, R.; Tadeo, E.; Toledo-Hernández, R.A.; Carmona, L.; Lima, I.; Williams, T. Improved capture of Drosophila suzukii by a trap baited with two attractants in the same device. PLoS ONE 2017, 12, e0188350. [Google Scholar] [CrossRef]
- Carroll, J. Spotted Wing Drosophila (SWD) Monitoring Traps; Program Based on Methods Tested by Steven Alm, Dept. of Plant Sciences and Entomology, University of Rhode Island, Richard Cowles, Connecticut Agricultural Experiment Station and Greg Loeb, Dept. of Entomology, Cornell University; EEUU. 2016. 4p. Available online: https://www.google.com/url?sa=t&source=web&rct=j&opi=89978449&url=https://cpb-us-e1.wpmucdn.com/blogs.cornell.edu/dist/0/7265/files/2017/01/SWDTraps_CornellFruit-1vwi4oo.pdf&ved=2ahUKEwjfz_-Mqr-KAxUB4jQHHZUnN4EQFnoECBoQAQ&usg=AOvVaw3nE2rk2VV_RkxMwh-_F3O5 (accessed on 20 September 2020).
- Manejo Cultural de Drosophila suzukii; SAG Ficha Técnica N°1; Subdepartamento Moscas de la Fruta/Departamento de Sanidad Vegetal/División Protección Agrícola y Forestal/SAG/Ministerio de Agricultura de Chile: Santiago, Chile. 2017. 4p. Available online: https://www.google.com/url?sa=t&source=web&rct=j&opi=89978449&url=https://www.sag.gob.cl/sites/default/files/ficha_drosophila_n1.pdf&ved=2ahUKEwiYi8OhvL-KAxUioK8BHVqFCLMQFnoECB0QAQ&usg=AOvVaw0a46DohqqdHzdJP5Id2iQj (accessed on 15 December 2022).
- Wollmann, J.; Schlesener, D.C.H.; Vieira, J.G.A.; Bernardi, D.; Garcia, M.S.; Garcia, F.R.M. Evaluation of Food Baits to Capture Drosophila suzukii in the Southern of Brazil. An. Acad. Bras. Ciênc. 2019, 91, e20180375. [Google Scholar] [CrossRef] [PubMed]
- Espinoza, J.; Chacón-Fuentes, M.; Quiroz, A.; Bardehle, L.; Escobar-Bahamondes, P.; Ungerfeld, E. Antifeedant Effects and Repellent Activity of Loline Alkaloids from Endophyte-Infected Tall Fescue against Horn Flies, Haematobia irritans (Diptera: Muscidae). Molecules 2021, 26, 817. [Google Scholar] [CrossRef]
- Riyajan, S.-A.; Sakdapipanich, J. Encapsulated neem extract containing Azadiractin-A within hydrolyzed poly(vinyl acetate) for controlling its release and photodegradation stability. Chem. Eng. J. 2009, 152, 591–597. [Google Scholar] [CrossRef]
- Hamby, K.A.; Kwok, R.S.; Zalom, F.G.; Chiu, J.C. Integrating circadian activity and gene expression profiles to predict chronotoxicity of Drosophila suzukii response to insecticides. PLoS ONE 2013, 8, e68472. [Google Scholar] [CrossRef]
- Red Agrometeorológica de INIA. Estación Maquehue UFRO, Freire. 2022. Available online: https://agrometeorologia.cl/ (accessed on 7 January 2024).
- Fincheira, P.; Jofré, I.; Tortella, G.; Medina, C.; Quiroz, A.; Seabra, A.B.; Nascimento, H.M.; Diez, M.C.; Rubilar, O. The Prospection of Plant Response to 2-Ketones Released from Nanostructured Lipid Carriers. J. Soil Sci. Plant Nutr. 2021, 21, 1474–1483. [Google Scholar] [CrossRef]
- Fincheira, P.; Rubilar, O.; Tortella, G.; Medina, C.; Seabra, A.B.; Nascimento, M.; Diez, M.C.; Quiroz, A. Formulation of a Controlled-Release Carrier for 2-ketones Based on Solid Lipid Nanoparticles to Increase Seedling Growth in Lactuca sativa and Solanum lycopersicum. J. Soil Sci. Plant Nutr. 2021, 21, 3002–3015. [Google Scholar] [CrossRef]
- Spies, J.; Liburd, O. Comparison of attractants, insecticides, and mass trapping for managing Drosophila suzukii (Diptera: Drosophilidae) in blueberries. Fla. Entomol. 2019, 102, 315–321. [Google Scholar] [CrossRef]
- Detección de Insectos Adultos de Drosophila suzukii por Medio del uso de Trampas; SAG Ficha Técnica N°2; Subdepartamento Moscas de la Fruta/Departamento de Sanidad Vegetal/División Protección Agrícola y Forestal/SAG/Ministerio de Agricultura de Chile: Santiago, Chile. 2017. 12p. Available online: https://www.google.com/url?sa=t&source=web&rct=j&opi=89978449&url=https://www.sag.gob.cl/sites/default/files/ficha_drosophila_n2.pdf&ved=2ahUKEwjf3YHKvb-KAxXlhq8BHbVEFp0QFnoECB0QAQ&usg=AOvVaw0JwEQemqpBHedOgv9q0Ma5 (accessed on 17 December 2022).
- Rojas, O.; Andrade, J.; Concha, C.; Astudillo, F. Estados de Desarrollo de Drosophila suzukii (Diptera: Drosophilidae) y Otras Especies del Género, Comunes en el sur de Chile; Ministerio de Agricultura/Servicio Agrícola y Ganadero/Primera edición: Osorno, Chile, 2019; 76p.
- Ilgin, P.; Ozay, H.; Ozay, O. A new dual stimuli responsive hydrogel: Modeling approaches for theprediction of drug loading and release profile. Eur. Polym. J. 2019, 113, 244–253. [Google Scholar] [CrossRef]
- Pourtalebi, L.; Ghazali, M.; Ashrafi, H.; Azadi, A. 2020. A comparison of models for the analysis of the kinetics of drug release fromPLGA-based nanoparticles. Heliyon 2020, 6, e03451. [Google Scholar] [CrossRef]
- Cha, D.H.; Adams, T.; Rogg, H.; Landolt, P.J. Identification and Field Evaluation of Fermentation Volatiles from Wine and Vinegar that Mediate Attraction of Spotted Wing Drosophila, Drosophila suzukii. J. Chem. Ecol. 2012, 38, 1419–1431. [Google Scholar] [CrossRef]
- Asplen, M.K.; Anfora, G.; Biondi, A.; Choi, D.S.; Chu, D.; Daane, K.M.; Gibert, P.; Gutierrez, A.P.; Hoelmer, K.A.; Hutchison, W.D.; et al. Invasion biology of spotted wing Drosophila (Drosophila suzukii): A global perspective and future priorities. J. Pest Sci. 2015, 88, 469–494. [Google Scholar] [CrossRef]
- Dzialo, M.C.; Park, R.; Steensels, J.; Lievens, B.; Verstrepen, K.J. Physiology, ecology and industrial applications of aroma formation in yeast. FEMS Microbiol. Rev. 2017, 41, S95–S128. [Google Scholar] [CrossRef]
- Tait, G.; Mermer, S.; Stockton, D.; Lee, J.; Avosani, S.; Abrieux, A.; Anfora, G.; Beers, E.; Biondi, A.; Burrack, H.; et al. Drosophila suzukii (Diptera: Drosophilidae): A Decade of Research Towards a Sustainable Integrated Pest Management Program. J. Econ. Entomol. 2021, 114, 1950–1974. [Google Scholar] [CrossRef]
- Liu, Y.; Cui, Z.; Shi, M.; Kenis, M.; Dong, W.; Zhang, F.; Zhang, J.; Xiao, C.; Chen, L. Antennal and Behavioral Responses of Drosophila suzukii to Volatiles from a Non-Crop Host, Osyris wightiana. Insects 2021, 12, 166. [Google Scholar] [CrossRef] [PubMed]
- Cloonan, K.R.; Abraham, J.; Angeli, S.; Syed, Z.; Rodríguez-Saona, C. Advances in the chemical ecology of the spotted wing Drosophila (Drosophila suzukii) and its applications. J. Chem. Ecol. 2018, 44, 922–939. [Google Scholar] [CrossRef]
- Raji, J.I.; Melo, N.; Castillo, J.S.; González, S.; Saldana, V.; Stensmyr, M.C.; DeGennaro, M. Aedes aegypti mosquitoes detect acidic volatiles found in human odor using the IR8a pathway. Curr. Biol. 2019, 29, 1253–1262. [Google Scholar] [CrossRef]
- Agnish, S.; Sharma, A.D.; Kaur, I. Nanoemulsions (O/W) containing Cymbopogon pendulus essential oil: Development, characterization, stability study, and evaluation of in vitro anti-bacterial, anti-inflammatory, anti-diabetic activities. Bionanoscience 2022, 12, 540–554. [Google Scholar] [CrossRef] [PubMed]
- Funami, T.; Fang, Y.; Noda, S.; Ishihara, S.; Nakauma, M.; Draget, K.; Nishinari, K.; Phillips, G. Rheological properties of sodium alginate in an aqueous system during gelation in relation to supermolecular structures and Ca2+ binding. Food Hydrocoll. 2009, 23, 1746–1755. [Google Scholar] [CrossRef]
- Tonina, L.; Grassi, A.; Caruso, S.; Mori, N.; Gottardello, A.; Anfora, G.; Giomi, F.; Vaccari, G.; Ioriatti, C. Comparison of attractants for monitoring Drosophila suzukii in sweet cherry orchards in Italy. J. Appl. Entomol. 2018, 142, 18–25. [Google Scholar] [CrossRef]
- Swoboda-Bhattarai, K.A.; McPhie, D.R.; Burrack, H.J. Reproductive Status of Drosophila suzukii (Diptera: Drosophilidae) Females Influences Attraction to Fermentation-Based Baits and Ripe Fruits. J. Econ. Entomol. 2017, 110, 1648–1652. [Google Scholar] [CrossRef] [PubMed]
- Hamby, K.A.; Bellamy, D.E.; Chiu, J.C.; Lee, J.C.; Walton, V.M.; Wiman, N.G.; York, R.M.; Biondi, A. Biotic and abiotic factors impacting development, behavior, phenology, and reproductive biology of Drosophila suzukii. J. Pest Sci. 2016, 89, 605–619. [Google Scholar] [CrossRef]
- Wallingford, A.K.; Lee, J.C.; Loeb, G.M. The influence of temperature and photoperiod on the reproductive diapause and cold tolerance of spotted-wing Drosophila, Drosophila suzukii. Entomol. Exp. Appl. 2016, 159, 327–337. [Google Scholar] [CrossRef]
- Guédot, C.; Avanesyan, A.; Hietala-Henschell, K. Effect of temperature and humidity on the seasonal phenology of Drosophila suzukii (Diptera: Drosophilidae) in Wisconsin. Environ. Entomol. 2018, 47, 1365–1375. [Google Scholar] [CrossRef] [PubMed]
- Buzzetti, K. The Spotted Wing Drosophila in the South of the World: Chilean Case and Its First Productive Impacts. In Invasive Species—Introduction Pathways, Economic Impact, and Possible Management Options; IntechOpen: London, UK, 2020. [Google Scholar] [CrossRef]



| Bait | Ingredients |
|---|---|
| SAG I | Apple cider vinegar: 900 mL Ethyl alcohol 95°: 100 mL |
| SAG II | Sugar: 2 g Yeast: 0.325 g Wheat flour: 17.25 g Apple cider vinegar: 1 mL Water: 25 mL |
| WVM | Merlot wine: 600 mL Apple cider vinegar: 400 mL Sugar cane molasses: 20 g |
| Date | Temperature (°C) | Minimum Temperature (°C) | Maximum Temperature (°C) | Relative Humidity (%) | Accumulated Precipitation (mm) |
|---|---|---|---|---|---|
| Sep-2022 | 9 | 2.8 | 15.2 | 88.1 | 44 |
| Oct-2022 | 11.1 | 5.2 | 16.9 | 83.3 | 116.5 |
| Nov-2022 | 15.2 | 9 | 21.5 | 80.1 | 38.2 |
| Dec-2022 | 16.6 | 9.6 | 23.7 | 71.9 | 14 |
| Variety | WVM Bait (fly/ha) | Control (fly/ha) |
|---|---|---|
| Regina | 16,104 * | 1944 |
| Lapins | 22,491 * | 8330 |
| Stella | 13,050 * | 3887 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lizama, M.; Alves-Santos, F.M.; Navas-Gracia, L.M.; Martínez-Cisterna, D.; Medina, C.; Rebolledo, R.; Chacón-Fuentes, M.; Bardehle, L. The Use of Novel Alginate Capsules in a Monitoring System for Drosophila suzukii in a Cherry Orchard in the Region of La Araucanía, Chile. Insects 2025, 16, 13. https://doi.org/10.3390/insects16010013
Lizama M, Alves-Santos FM, Navas-Gracia LM, Martínez-Cisterna D, Medina C, Rebolledo R, Chacón-Fuentes M, Bardehle L. The Use of Novel Alginate Capsules in a Monitoring System for Drosophila suzukii in a Cherry Orchard in the Region of La Araucanía, Chile. Insects. 2025; 16(1):13. https://doi.org/10.3390/insects16010013
Chicago/Turabian StyleLizama, Marcelo, Fernando Manuel Alves-Santos, Luis Manuel Navas-Gracia, Daniel Martínez-Cisterna, Cristian Medina, Ramón Rebolledo, Manuel Chacón-Fuentes, and Leonardo Bardehle. 2025. "The Use of Novel Alginate Capsules in a Monitoring System for Drosophila suzukii in a Cherry Orchard in the Region of La Araucanía, Chile" Insects 16, no. 1: 13. https://doi.org/10.3390/insects16010013
APA StyleLizama, M., Alves-Santos, F. M., Navas-Gracia, L. M., Martínez-Cisterna, D., Medina, C., Rebolledo, R., Chacón-Fuentes, M., & Bardehle, L. (2025). The Use of Novel Alginate Capsules in a Monitoring System for Drosophila suzukii in a Cherry Orchard in the Region of La Araucanía, Chile. Insects, 16(1), 13. https://doi.org/10.3390/insects16010013








