High-Throughput Screening System Evaluation of Andrographis paniculata (Burm.f.) Extracts and Their Fractions against Mosquito Vectors
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Andrographis paniculata Crude Extract
2.2. Mosquitoes
2.3. Mosquito Rearing
2.4. Netting Strip Impregnation
2.5. High-Throughput Screening System (HITSS)
2.5.1. Contact Irritancy Test
2.5.2. Spatial Repellency Assay
2.5.3. Toxicity
2.6. Data Analysis
2.7. HPLC-MS Analysis
3. Results
3.1. Isolating and Fractionating Crude Extract
3.2. High Throughput Screening System
3.2.1. Contact Irritancy
| Repellent | Concentration (%, w/v) | R (N) | Number Escaping (Mean ± SE per Replicate) | Percent Escaping (Mean ± SE) | p-Value b | |
|---|---|---|---|---|---|---|
| Treated | Control a | |||||
| Crude extract | 1 | 6 (60) | 3.00 ± 0.26 | 0.50 ± 0.22 | 26.11 ± 3.11 | 0.0022 |
| 2.5 | 6 (60) | 4.00 ± 0.86 | 36.48 ± 9.75 | 0.0087 | ||
| 5 | 6 (60) | 4.67± 0.33 | 43.70 ± 3.87 | 0.0022 | ||
| F1 | 1 | 6 (60) | 0.83 ± 0.31 | 0.17 ± 0.17 | 6.48 ± 4.36 | 0.1970 |
| 2.5 | 6 (60) | 1.17 ± 0.17 | 10.00 ± 2.58 | 0.0130 | ||
| 5 | 6 (60) | 1.00 ± 0.26 | 8.33 ± 3.07 | 0.0671 | ||
| F2 | 1 | 6 (60) | 0.83 ± 0.17 | 0.33 ± 0.21 | 5.00 ± 2.23 | 0.2424 |
| 2.5 | 6 (60) | 1.33 ± 0.21 | 10.00 ± 3.65 | 0.0325 | ||
| 5 | 6 (60) | 1.17 ± 0.31 | 8.15 ± 4.92 | 0.1234 | ||
| F3 | 1 | 6 (60) | 1.50 ± 0.22 | 0.33 ± 0.21 | 11.85 ± 3.06 | 0.0216 |
| 2.5 | 6 (60) | 3.00 ± 0.52 | 27.78 ± 4.62 | 0.0065 | ||
| 5 | 6 (60) | 3.33 ± 0.21 | 30.9 ± 2.35 | 0.0022 | ||
| F4 | 1 | 6 (59) | 0.67 ± 0.21 | 0.67 ± 0.21 | 1.67 ± 3.32 | 1.0000 |
| 2.5 | 6 (60) | 1.33 ± 0.33 | 7.04 ± 3.41 | 0.2381 | ||
| 5 | 6 (60) | 1.50 ± 0.22 | 8.70 ± 3.12 | 0.0758 | ||
| F5 | 1 | 6 (60) | 1.00 ± 0.25 | 0.00 ± 0.00 | 8.33 ± 3.07 | 0.0152 |
| 2.5 | 6 (60) | 1.83 ± 0.60 | 17.22 ± 5.26 | 0.0152 | ||
| 5 | 6 (60) | 2.17 ± 0.17 | 21.67 ± 1.67 | 0.0022 | ||
| Repellent | Concentration (%, w/v) | R (N) | Number Escaping (Mean ± SE per Replicate) | Percent Escaping (Mean ± SE) | p-Value b | |
|---|---|---|---|---|---|---|
| Treated | Control a | |||||
| Crude extract | 1 | 6 (60) | 1.17 ± 1.67 | 0.17 ± 0.17 | 10.19 ± 0.19 | 0.0130 |
| 2.5 | 6 (60) | 1.67 ± 0.33 | 15.37 ± 2.42 | 0.0087 | ||
| 5 | 6 (60) | 1.33 ± 0.21 | 11.85 ± 1.63 | 0.0108 | ||
| F1 | 1 | 6 (60) | 0.17 ± 0.17 | 0.00 ± 0.00 | 1.67 ± 1.67 | 1.0000 |
| 2.5 | 6 (59) | 0.50 ± 0.22 | 6.67 ± 3.33 | 0.1818 | ||
| 5 | 6 (60) | 0.67 ± 0.33 | 6.67 ± 3.33 | 0.1818 | ||
| F2 | 1 | 6 (60) | 0.33 ± 0.21 | 0.17 ± 0.17 | 1.48 ± 3.21 | 1.0000 |
| 2.5 | 6 (60) | 0.67 ± 0.21 | 5.00 ± 2.24 | 0.2424 | ||
| 5 | 6 (60) | 0.67 ± 0.21 | 5.00 ± 2.24 | 0.2424 | ||
| F3 | 1 | 6 (60) | 1.00 ± 0.26 | 0.33 ± 0.21 | 6.48 ± 4.36 | 0.1775 |
| 2.5 | 6 (59) | 1.33 ± 0.21 | 13.33 ± 4.21 | 0.0325 | ||
| 5 | 6 (60) | 1.50 ± 0.22 | 11.67 ± 4.01 | 0.0216 | ||
| F4 | 1 | 6 (59) | 0.17 ± 0.17 | 0.17 ± 0.17 | 1.48 ± 3.21 | 1.0000 |
| 2.5 | 6 (60) | 0.50 ± 0.22 | 3.15 ± 3.48 | 0.5455 | ||
| 5 | 6 (60) | 0.50 ± 0.22 | 3.15 ± 3.48 | 0.5455 | ||
| F5 | 1 | 6 (60) | 0.83 ± 0.31 | 0.33 ± 0.21 | 5.00 ± 3.42 | 0.4048 |
| 2.5 | 6 (59) | 1.50 ± 0.34 | 13.52 ± 5.56 | 0.0325 | ||
| 5 | 6 (60) | 1.67 ± 0.33 | 13.70 ± 3.50 | 0.0216 | ||
| Repellent | Concentration (%, w/v) | R (N) | Number Escaping (Mean ± SE per Replicate) | Percent Escaping (Mean ± SE) | p-Value b | |
|---|---|---|---|---|---|---|
| Treated | Control a | |||||
| Crude extract | 1 | 6 (60) | 1.00 ± 0.26 | 0.17 ± 0.17 | 8.52 ± 1.71 | 0.0671 |
| 2.5 | 6 (60) | 1.33 ± 0.22 | 11.67 ± 3.07 | 0.0108 | ||
| 5 | 6 (60) | 1.67 ± 0.33 | 15.00 ± 4.28 | 0.0087 | ||
| F1 | 1 | 6 (60) | 0.50 ± 0.22 | 0.17 ± 0.17 | 3.33 ± 2.11 | 0.5455 |
| 2.5 | 6 (60) | 0.50 ± 0.22 | 3.33 ± 2.11 | 0.5455 | ||
| 5 | 6 (60) | 0.83 ± 0.31 | 6.67 ± 3.33 | 0.1970 | ||
| F2 | 1 | 6 (60) | 0.50 ± 0.34 | 0.17 ± 0.17 | 3.15 ± 4.33 | 0.7273 |
| 2.5 | 6 (60) | 0.67 ± 0.21 | 5.00 ± 2.24 | 0.2424 | ||
| 5 | 6 (60) | 0.83 ± 0.17 | 6.67 ± 2.11 | 0.0801 | ||
| F3 | 1 | 6 (60) | 0.83 ± 0.31 | 0.50 ± 0.22 | 3.52 ± 2.23 | 0.6753 |
| 2.5 | 6 (60) | 1.17 ± 0.31 | 6.67 ± 4.40 | 0.2316 | ||
| 5 | 6 (60) | 1.50 ± 0.22 | 10.37 ± 2.59 | 0.0433 | ||
| F4 | 1 | 6 (60) | 0.17 ± 0.17 | 0.17 ± 0.17 | −0.19 ± 2.73 | 1.0000 |
| 2.5 | 6 (60) | 0.17 ± 0.17 | −0.19 ± 2.73 | 1.0000 | ||
| 5 | 6 (60) | 0.67 ± 0.21 | 5.00 ± 2.24 | 0.2424 | ||
| F5 | 1 | 6 (60) | 0.67 ± 0.21 | 0.33 ± 0.21 | 3.33 ± 2.10 | 0.5671 |
| 2.5 | 6 (59) | 1.33 ± 0.33 | 12.04 ± 1.61 | 0.0801 | ||
| 5 | 6 (60) | 1.50 ± 0.34 | 11.67 ± 4.77 | 0.0325 | ||
3.2.2. Spatial Repellency
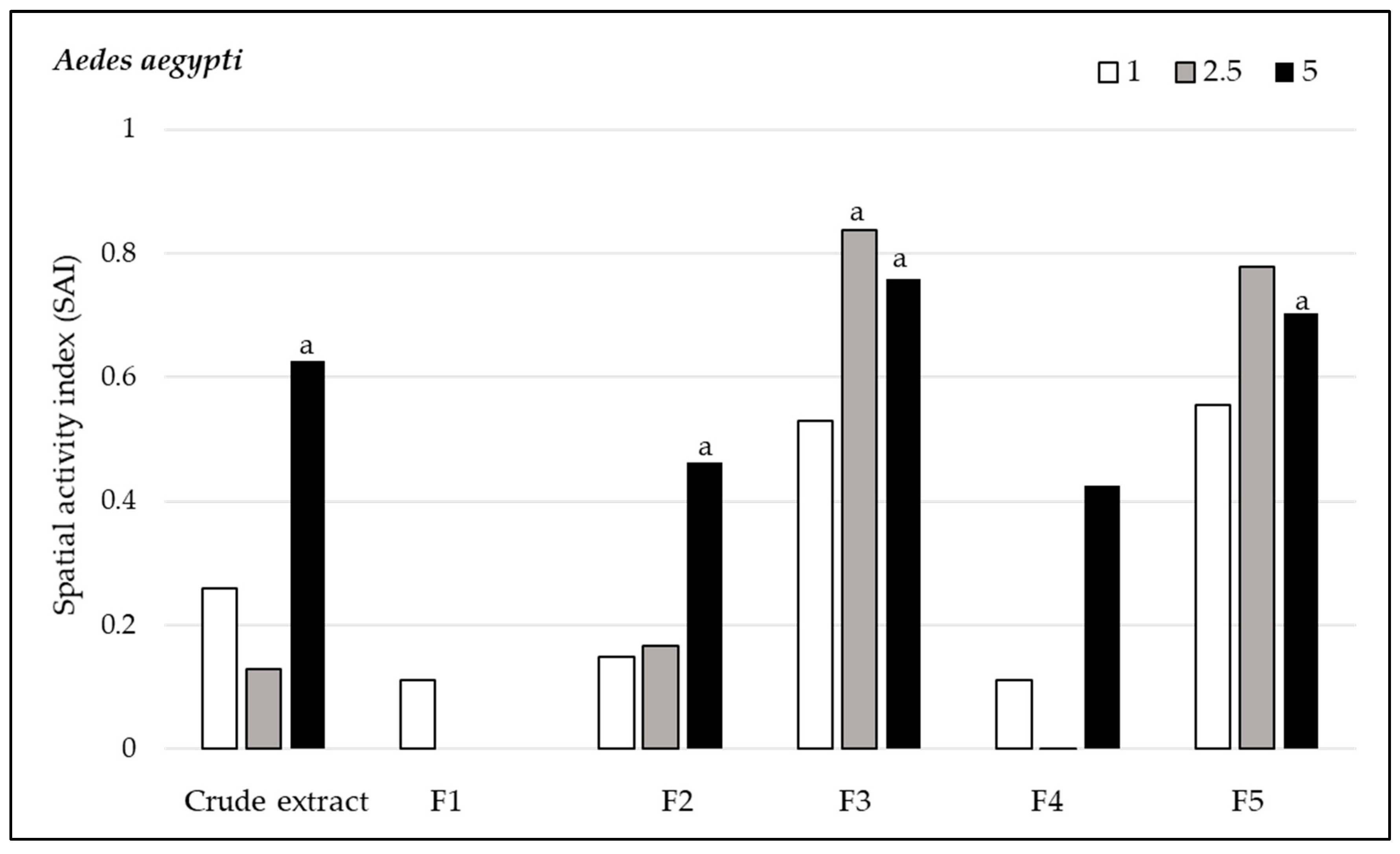
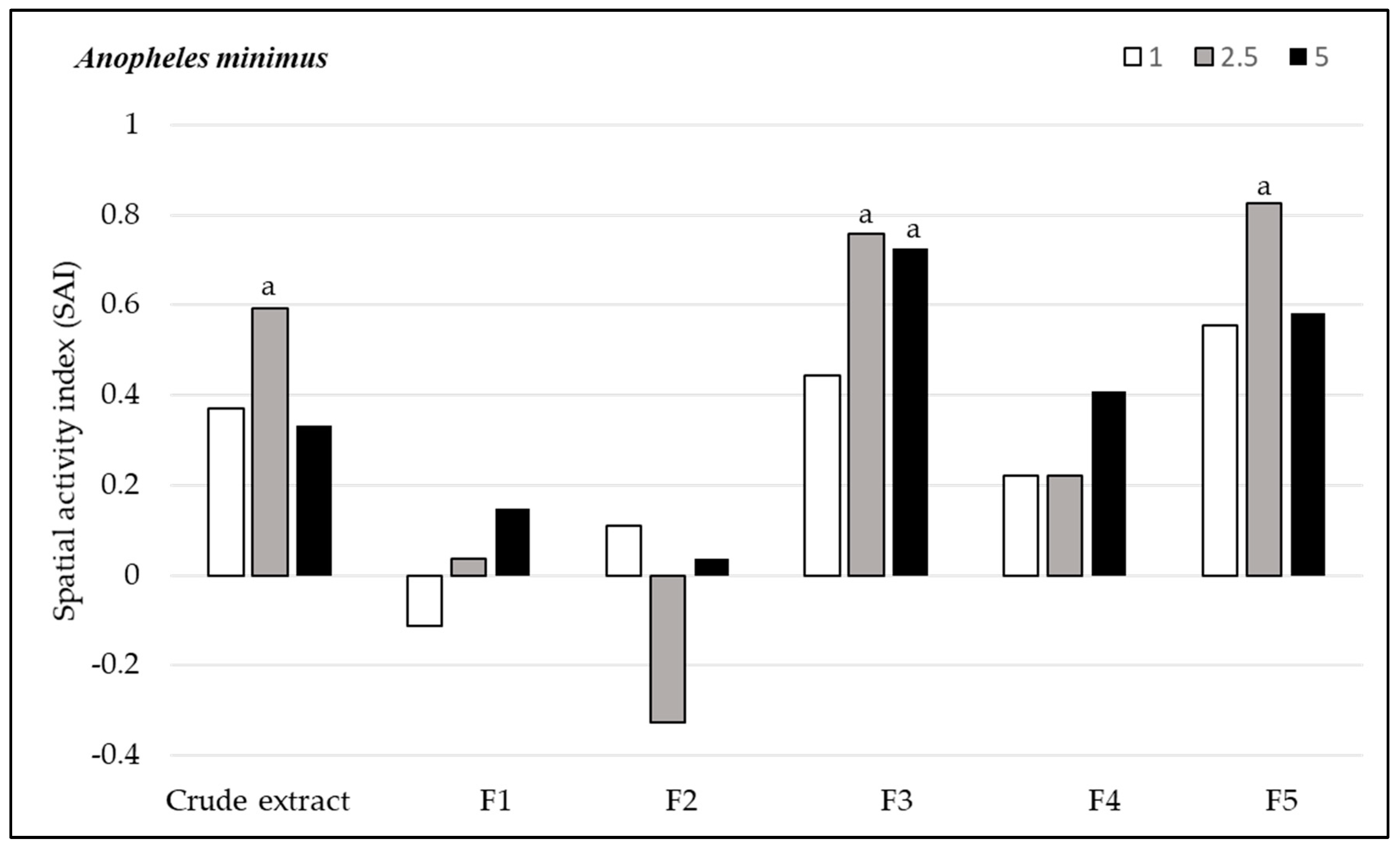
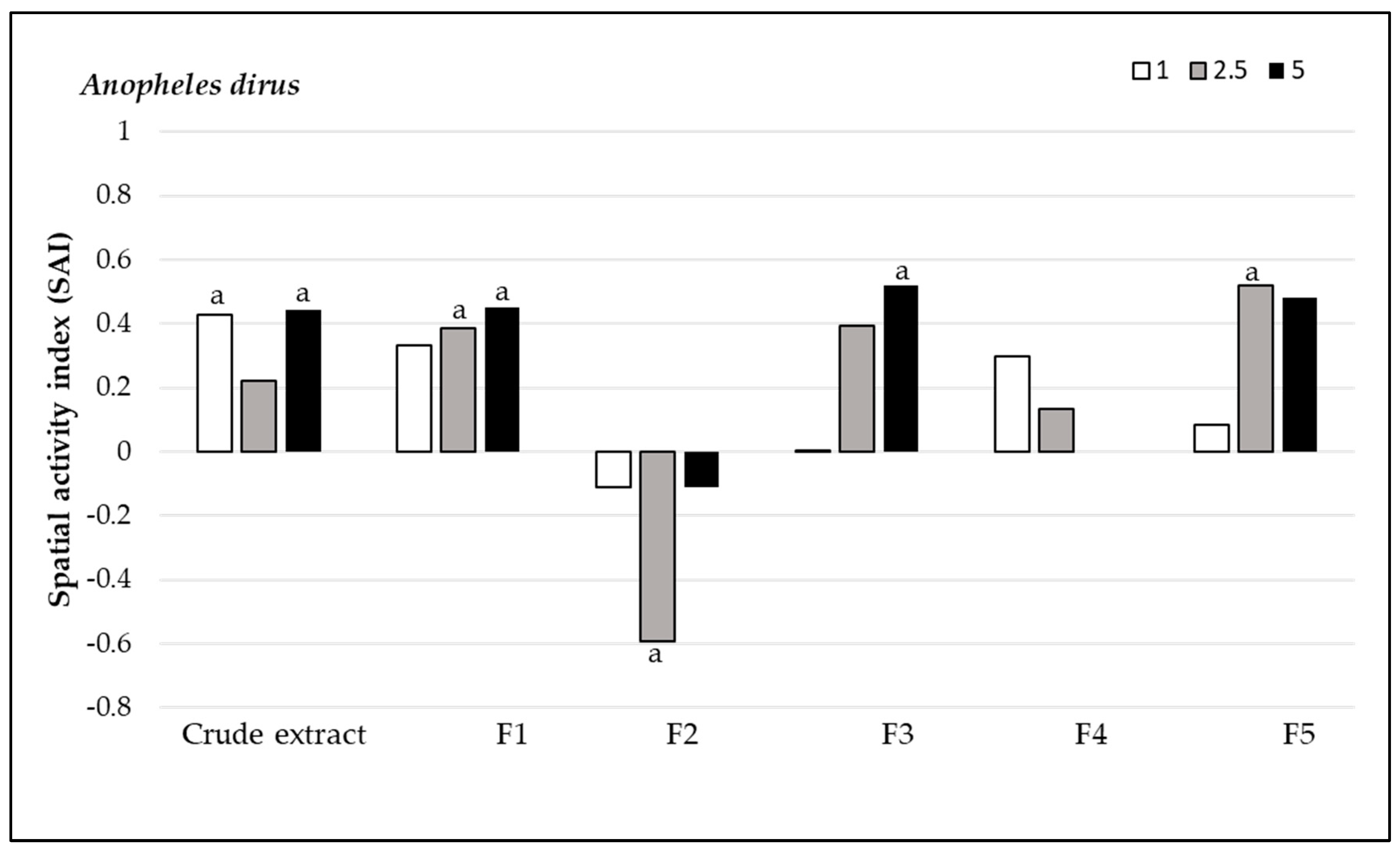
3.3. Toxicity Assay
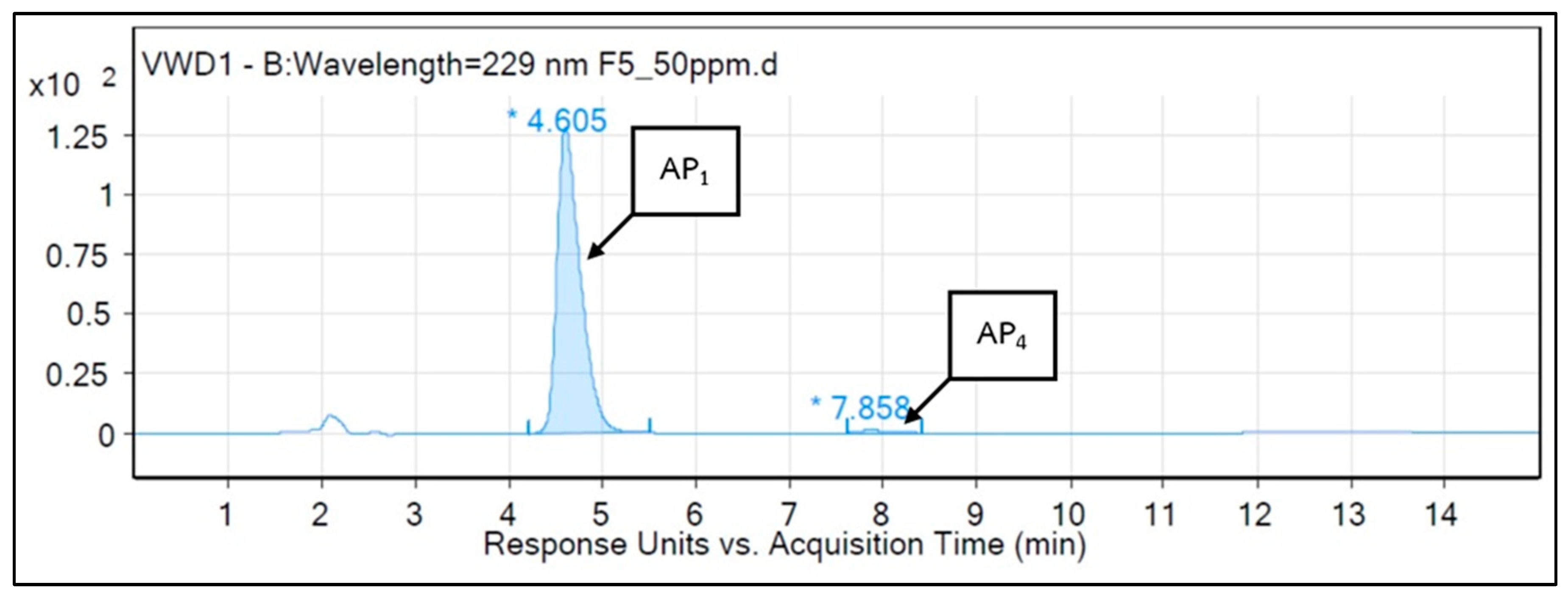
3.4. HPLC-MS Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. A Global Brief on Vector-Borne Diseases; World Health Organization: Geneva, Switzerland, 2014; Available online: https://iris.who.int/handle/10665/111008 (accessed on 17 March 2024).
- World Health Organization. Global Report on Neglected Tropical Diseases; World Health Organization: Geneva, Switzerland, 2023; Licence: CC BY-NC-SA 3.0 IGO; Available online: https://www.who.int/teams/control-of-neglected-tropical-diseases/global-report-on-neglected-tropical-diseases-2023 (accessed on 23 March 2023).
- Carrington, L.B.; Simmons, C.P. Human to mosquito transmission of dengue viruses. Front. Immunol. 2014, 5, 290. [Google Scholar] [CrossRef] [PubMed]
- Churcher, T.S.; Sinden, R.E.; Edwards, N.J.; Poulton, I.D.; Rampling, T.W.; Brock, P.M.; Griffin, J.T.; Upton, L.M.; Zakutansky, S.E.; Sala, K.A.; et al. Probability of transmission of malaria from mosquito to human is regulated by mosquito parasite density in naïve and vaccinated Hosts. PLoS Pathog. 2017, 13, e1006108. [Google Scholar] [CrossRef] [PubMed]
- Ahebwa, A.; Hii, J.; Neoh, K.-B.; Chareonviriyaphap, T. Aedes aegypti and Aedes albopictus (Diptera: Culicidae) ecology, biology, behaviour, and implications on arbovirus transmission in Thailand: Review. One Health. 2023, 16, 100555. [Google Scholar] [CrossRef] [PubMed]
- DVBD. Division of Vector-Borne Diseases. Thailand [Internet]. 2024. Available online: https://lookerstudio.google.com/u/0/reporting/dfa7d4e2-b7f5-48ed-b40a-54f1cd4cbdfb/page/cFWgC (accessed on 13 June 2024).
- Tainchum, K.; Ritthison, W.; Chuaycharoensuk, T.; Bangs, M.J.; Manguin, S.; Chareonviriyaphap, T. Diversity of Anopheles species and trophic behavior of putative malaria vectors in two malaria endemic areas of northwestern Thailand. J. Vector Ecol. 2014, 39, 424–436. [Google Scholar] [CrossRef] [PubMed]
- Tananchai, C.; Pattanakul, M.; Nararak, J.; Sinou, V.; Manguin, S.; Chareonviriyaphap, T. Diversity and biting patterns of Anopheles species in a malaria endemic area, Umphang Valley, Tak Province, western Thailand. Acta Trop. 2019, 190, 183–192. [Google Scholar] [CrossRef]
- Zhang, C.; Yang, R.; Wu, L.; Luo, C.; Yang, Y.; Deng, Y.; Wu, J.; Liu, Y.; Zhou, H. Survey of malaria vectors on the Cambodia, Thailand, and China-Laos Borders. Malar. J. 2022, 21, 399. [Google Scholar] [CrossRef]
- Bisanzio, D.; Sudathip, P.; Kitchakarn, S.; Kanjanasuwan, J.; Gopinath, D.; Pinyajeerapat, N.; Sintasath, D.; Shah, J.A. Malaria Stratification Mapping in Thailand to Support Prevention of Reestablishment. Am. J. Trop. Med. Hyg. 2024, 110, 79. [Google Scholar] [CrossRef]
- Chareonviriyaphap, T.; Bangs, M.J.; Suwonkerd, W.; Kongmee, M.; Corbel, V.; Ngoen-Klan, R. Review of insecticide resistance and behavioral avoidance of vectors of human diseases in Thailand. Parasites Vectors 2013, 6, 280. [Google Scholar] [CrossRef]
- Swale, D.R.; Bloomquist, J.R. Is DEET a dangerous neurotoxicant? Pest Manag. Sci. 2014, 75, 2068–2070. [Google Scholar] [CrossRef]
- Sudakin, D.L.; Trevathan, W.R. DEET: A review and update of safety and risk in the general population. J. Toxicol. Clin. Toxicol. 2003, 41, 831–839. [Google Scholar] [CrossRef]
- Stanczyk, N.M.; Brookfield, J.F.; Field, L.M.; Logan, J.G. Aedes aegypti mosquitoes exhibit decreased repellency by DEET following previous exposure. PLoS ONE 2013, 8, e54438. [Google Scholar] [CrossRef] [PubMed]
- Maia, M.F.; Moore, S.J. Plant-based insect repellents: A review of their efficacy, development, and testing. Malar. J. 2011, 10 (Suppl. 1), S11. [Google Scholar] [CrossRef] [PubMed]
- Rehman, J.U.; Ali, A.; Khan, I.A. Plant-based products: Use and development as repellents against mosquitoes: A review. Fitoterapia 2014, 95, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Diaz, J.H. Chemical and plant-based insect repellents: Efficacy, safety, and toxicity. Wilderness Environ. Med. 2016, 27, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Moore, S.J.; Lenglet, A.; Hill, N. Plant-based insect repellents. In Insect Repellents: Principles Methods, and Use; CRC Press: Boca Raton, FL, USA, 2006. [Google Scholar]
- World Health Organisation. Guidelines for Efficacy Testing of Spatial Repellents; World Health Organization: Geneva, Switzerland, 2013; ISBN 978 92 4 150502 4. Available online: https://www.who.int/publications/i/item/9789241505024 (accessed on 22 January 2022).
- Grieco, J.P.; Achee, N.L.; Sardelis, M.R.; Chauhan, K.R.; Roberts, D.R. A novel high-throughput screening system to evaluate the behavioral response of adult mosquitoes to chemicals. J. Am. Mosq. Control Assoc. 2005, 21, 404–411. [Google Scholar] [CrossRef]
- Sathantriphop, S.; Achee, N.L.; Sanguanpong, U.; Chareonviriyaphap, T. The effects of plant essential oils on escape response and mortality rate of Aedes aegypti and Anopheles minimus. J. Vector Ecol. 2015, 40, 318–326. [Google Scholar] [CrossRef]
- Boonyuan, W.; Ahebwa, A.; Nararak, J.; Sathantriphop, S.; Chareonviriyaphap, T. Enhanced excito-repellency of binary mixtures of plant-based mosquito repellents against Culex quinquefasciatus say (Diptera: Culicidae), a night biting mosquito species. J. Med. Entomol. 2022, 59, 891–902. [Google Scholar] [CrossRef]
- Ahebwa, A.; Mongkol, R.; Sawangsri, P.; Kanjanamaneesathian, M. Vapour-phase efficacy of selected essential oils individually and in combination against Aspergillus flavus, A. niger, Fusarium proliferatum, and Curvularia lunata. N. Z. Plant Prot. 2020, 73, 40–48. [Google Scholar] [CrossRef]
- Thakur, A.K.; Chatterjee, S.S.; Kumar, V. Adaptogenic potential of andrographolide: An active principle of the king of bitters (Andrographis paniculata). J. Tradit. Complement. Med. 2015, 5, 42–50. [Google Scholar] [CrossRef]
- Kumar, S.; Singh, B.; Bajpai, V. Andrographis paniculata (Burm.f.) Nees: Traditional uses, phytochemistry, pharmacological properties and quality control/quality assurance. J. Ethnopharmacol. 2021, 275, 114054. [Google Scholar] [CrossRef]
- Xu, Y. Adaptive Immune Response-Modifying and Antimicrobial Properties of Andrographis paniculata and Andrographolide. Doctoral Dissertation, University of Southern Queensland, Toowoomba, Australia, 2009. [Google Scholar]
- Subramanian, R.; Zaini Asmawi, M.; Sadikun, A. A bitter plant with a sweet future? A comprehensive review of an oriental medicinal plant: Andrographis paniculata. Phytochem. Rev. 2011, 11, 39–75. [Google Scholar] [CrossRef]
- Misra, P.; Pal, N.L.; Guru, P.Y.; Katiyar, J.C.; Srivastava, V.; Tandon, J.S. Antimalarial activity of Andrographis paniculata (Kalmegh) against Plasmodium berghei NK 65 in Mastomys natalensis. Int. J. Pharmacogn 1992, 30, 263–274. [Google Scholar] [CrossRef]
- Akbar, S. Andrographis paniculata: A review of pharmacological activities and clinical effects. Altern. Med. Rev. 2011, 16, 66–77. [Google Scholar] [PubMed]
- Hossain, M.S.; Urbi, Z.; Sule, A.; Hafizur Rahman, K.M. Andrographis paniculata (Burm.f.) Wall. ex Nees: A review of ethnobotany, phytochemistry, and pharmacology. Sci. World J. 2014, 2014, 274905. [Google Scholar] [CrossRef] [PubMed]
- Elango, G.; Rahuman, A.A.; Zahir, A.A.; Kamaraj, C.; Bagavan, A.; Rajakumar, G.; Jayaseelan, C.; Santhoshkumar, T.; Marimuthu, S. Evaluation of repellent properties of botanical extracts against Culex tritaeniorhynchus Giles (Diptera: Culicidae). Parasitol. Res. 2010, 107, 577–584. [Google Scholar] [CrossRef]
- Elango, G.; Rahuman, A.A.; Bagavan, A.; Kamaraj, C.; Zahir, A.A.; Rajakumar, G.; Marimuthu, S.; Santhoshkumar, T. Efficacy of botanical extracts against Japanese encephalitis vector, Culex tritaeniorhynchus. Parasitol. Res. 2010, 106, 481–492. [Google Scholar] [CrossRef]
- Govindarajan, M.; Sivakumar, R. Mosquito adulticidal and repellent activities of botanical extracts against malarial vector, Anopheles stephensi Liston (Diptera: Culicidae). Asian Pac. J. Trop. Med. 2011, 4, 941–947. [Google Scholar] [CrossRef]
- Elango, G.; Rahuman, A.A.; Kamaraj, C.; Bagavan, A.; Zahir, A.A. Efficacy of medicinal plant extracts against malarial vector, Anopheles subpictus Grassi. Parasitol. Res. 2011, 108, 1437–1445. [Google Scholar] [CrossRef]
- Panneerselvam, C.; Murugan, K. Adulticidal, repellent, and ovicidal properties of indigenous plant extracts against the malarial vector, Anopheles stephensi (Diptera: Culicidae). Parasitol. Res. 2013, 112, 679–692. [Google Scholar] [CrossRef]
- Elango, G.; Rahuman, A.A.; Bagavan, A.; Kamaraj, C.; Zahir, A.A.; Venkatesan, C. Laboratory study on larvicidal activity of indigenous plant extracts against Anopheles subpictus and Culex tritaeniorhynchus. Parasitol. Res. 2009, 104, 1381–1388. [Google Scholar]
- Kuppusamy, C.; Murugan, K. Effects of Andrographis paniculata Nees on growth, development, and reproduction of malarial vector Anopheles stephensi Liston (Diptera: Culicidae). Trop. Biomed. 2010, 27, 509–516. [Google Scholar] [PubMed]
- Kotewong, R.; Duangkaew, P.; Srisook, E.; Sarapusit, S.; Rongnoparut, P. Structure-function relationships of inhibition of mosquito cytochrome P450 enzymes by flavonoids of Andrographis paniculata. Parasitol. Res. 2014, 113, 3381–3392. [Google Scholar] [CrossRef] [PubMed]
- Pholphana, N.; Panomvana, D.; Rangkadilok, N.; Suriyo, T.; Ungtrakul, T.; Pongpun, W.; Thaeopattha, S.; Satayavivad, J. A Simple and Sensitive LC-MS/MS Method for Determination of Four Major Active Diterpenoids from Andrographis paniculata in Human Plasma and Its Application to a Pilot Study. Planta Med. 2016, 82, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Panthawong, A.; Sukkanon, C.; Ngoen-Klan, R.; Hii, J.; Chareonviriyaphap, T. Forced egg laying method to establish F1 progeny from field populations and laboratory strains of Anopheles mosquitoes (Diptera: Culicidae) in Thailand. J. Med. Entomol. 2021, 58, 2107–2113. [Google Scholar] [CrossRef]
- Sukkanon, C.; Nararak, J.; Bangs, M.j.; Hii, J.; Chareonviriyaphap, T. Behavioral responses to transfluthrin by Aedes aegypti, Anopheles minimus, Anopheles harrisoni, and Anopheles dirus (Diptera: Culicidae). PLoS ONE 2020, 15, e0237353. [Google Scholar] [CrossRef]
- Ahebwa, A.; Hii, J.; Neoh, K.-B.; Leepasert, T.; Chareonviriyaphap, T. Effects of transfluthrin-treated jute and cotton clothing against resistant and susceptible Aedes aegypti (Diptera: Culicidae) in a semifield system. J. Med. Entomol. 2023, 12, 181–190. [Google Scholar] [CrossRef]
- Phasomkusolsil, S.; Tawong, J.; Monkanna, N.; Pantuwatana, K.; Damdangdee, N.; Khongtak, W.; Kertmanee, Y.; Evans, B.P.; Schuster, A.L. Maintenance of mosquito vectors: Effects of blood source on feeding, survival, fecundity, and egg hatching rates. J. Vector Ecol. 2013, 38, 38–45. [Google Scholar] [CrossRef]
- Nararak, J.; Giorgio, C.D.; Thanispong, K.; Sukkanon, C.; Sanguanpong, U.; Mahiou-Leddet, V.; Ollivier, E.; Chareonviriyaphap, T.; Manguin, S. Behavioral avoidance and biological safety of vetiver oil and its constituents against Aedes aegypti (L.), Aedes albopictus (Skuse) and Culex quinquefasciatus Say. Curr. Res. Insect Sci. 2022, 2, 100044. [Google Scholar] [CrossRef]
- Achee, N.L.; Sardelis, M.R.; Dusfour, L.; Chauhan, K.R.; Grieco, J.P. Characterization of spatial repellent, contact irritant, and toxicant chemical actions of standard vector control compounds1. J. Am. Mosq. Control. Assoc. 2009, 25, 156–167. [Google Scholar] [CrossRef]
- SAS Institute Inc. SAS Online Doc, Version 8; CD-ROM; SAS Institute Inc.: Cary, NC, USA, 1999. [Google Scholar]
- Seyoum, A.; Killeen, G.; Kabiru, E.; Knols, B.; Hassanali, A. Field efficacy of thermally expelled or live potted repellent plants against African malaria vectors in Western Kenya. Trop. Med. Int. Health 2003, 8, 1005–1011. [Google Scholar] [CrossRef]
- Nerio, L.S.; Olivero-Verbel, J.; Stashenko, E. Repellent activity of essential oils: A review. Bioresour. Technol. 2010, 101, 372–378. [Google Scholar] [CrossRef] [PubMed]
- George, D.R.; Finn, R.D.; Graham, K.M.; Sparagano, O.A. Present and future potential of plant-derived products to control arthropods of veterinary and medical significance. Parasites Vectors 2014, 7, 28. [Google Scholar] [CrossRef]
- Kalita, B.; Bora, S.; Sharma, A.K. Plant essential oils as mosquito repellent-a review. Int. J. Res. Dev. Pharm. Life Sci. 2013, 3, 741–747. [Google Scholar]
- Grison, C.; Carrasco, D.; Pelissier, F.; Moderc, A. Reflexion on bio-sourced mosquito repellents: Nature, activity, and preparation. Front. Ecol. Evol. 2020, 8, 8. [Google Scholar] [CrossRef]
- Prajapati, V.; Tripathi, A.K.; Aggarwal, K.K.; Khanuja, S.P. Insecticidal, repellent and oviposition-deterrent activity of selected essential oils against Anopheles stephensi, Aedes aegypti and Culex quinquefasciatus. Bioresour. Technol. 2005, 96, 1749–1757. [Google Scholar] [CrossRef] [PubMed]
- Vilvest, J.; Milton, M.C.J.; Yagoo, A. Andrographis paniculata leaf extracts: A natural mosquito control agent in combating Aedes aegypti and Culex quinquefasciatus. Int. J. Mosq. Res. 2023, 10, 1–6. [Google Scholar] [CrossRef]
- Vilvest, J.; Milton, M.C.J.; Yagoo, A. Crude extract and effective fractions from Andrographis paniculata for immature Aedes aegypti and Culex quinquefasciatus mosquito. Ecol. Front. 2024, 44, 396–402. [Google Scholar] [CrossRef]
- Govindarajan, M.; Sivakumar, R. Adulticidal and repellent properties of indigenous plant extracts against Culex quinquefasciatus and Aedes aegypti (Diptera: Culicidae). Parasitol. Res. 2012, 110, 1607–1620. [Google Scholar] [CrossRef]
- Tawatsin, A.; Wratten, S.D.; Scott, R.R.; Thavara, U.; Techadamrongsin, Y. Repellency of volatile oils from plants against three mosquito vectors. J. Vector Ecol. 2001, 26, 76–82. [Google Scholar]
- Sukkanon, C.; Karpkird, T.; Saeung, M.; Leepasert, T.; Panthawong, A.; Suwonkerd, W.; Bangs, M.J.; Chareonviriyaphap, T. Excito-repellency activity of Andrographis paniculata (Lamiales: Acanthaceae) against colonized mosquitoes. J. Med. Entomol. 2020, 57, 192–203. [Google Scholar] [CrossRef]
- Norris, E.J.; Coats, J.R. Current and future repellent technologies: The potential of spatial repellents and their place in mosquito-borne disease control. Int. J. Environ. Res. Public Health 2017, 14, 124. [Google Scholar] [CrossRef] [PubMed]
- Tisgratog, R.; Sukkanon, C.; Sugiharto, V.A.; Bangs, M.J.; Chareonviriyaphap, T. Time of test periods influence the behavioral responses of Anopheles minimus and Anopheles dirus (Diptera: Culicidae) to DEET. Insects 2021, 12, 867. [Google Scholar] [CrossRef] [PubMed]
- Chao, W.W.; Lin, B.F. Isolation and identification of bioactive compounds in Andrographis paniculata (Chuanxinlian). Chin. Med. 2010, 5, 17. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Zhang, B.; Xu, H. Synthesis of andrographolide-related esters as insecticidal and acaricidal agents. Bioorg. Med. Chem. Lett. 2018, 28, 360–364. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sirisopa, P.; Leepasert, T.; Karpkird, T.; Nararak, J.; Thanispong, K.; Ahebwa, A.; Chareonviriyaphap, T. High-Throughput Screening System Evaluation of Andrographis paniculata (Burm.f.) Extracts and Their Fractions against Mosquito Vectors. Insects 2024, 15, 712. https://doi.org/10.3390/insects15090712
Sirisopa P, Leepasert T, Karpkird T, Nararak J, Thanispong K, Ahebwa A, Chareonviriyaphap T. High-Throughput Screening System Evaluation of Andrographis paniculata (Burm.f.) Extracts and Their Fractions against Mosquito Vectors. Insects. 2024; 15(9):712. https://doi.org/10.3390/insects15090712
Chicago/Turabian StyleSirisopa, Patcharawan, Theerachart Leepasert, Thitinun Karpkird, Jirod Nararak, Kanutcharee Thanispong, Alex Ahebwa, and Theeraphap Chareonviriyaphap. 2024. "High-Throughput Screening System Evaluation of Andrographis paniculata (Burm.f.) Extracts and Their Fractions against Mosquito Vectors" Insects 15, no. 9: 712. https://doi.org/10.3390/insects15090712
APA StyleSirisopa, P., Leepasert, T., Karpkird, T., Nararak, J., Thanispong, K., Ahebwa, A., & Chareonviriyaphap, T. (2024). High-Throughput Screening System Evaluation of Andrographis paniculata (Burm.f.) Extracts and Their Fractions against Mosquito Vectors. Insects, 15(9), 712. https://doi.org/10.3390/insects15090712






