Identification and Functional Insights of Knickkopf Genes in the Larval Cuticle of Leptinotarsa decemlineata
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Insect
2.2. Identification of LdKnk-Family Genes
2.3. Bioinformatics and Phylogenetic Analysis
2.4. Expression Analysis of LdKnk-Family Genes
2.5. Preparation of dsRNA Using Bacterial Expression
2.6. Dietary dsRNA Bioassays
2.7. Transmission Electron Microscopy (TEM) Analysis
2.8. Data Analysis
3. Results
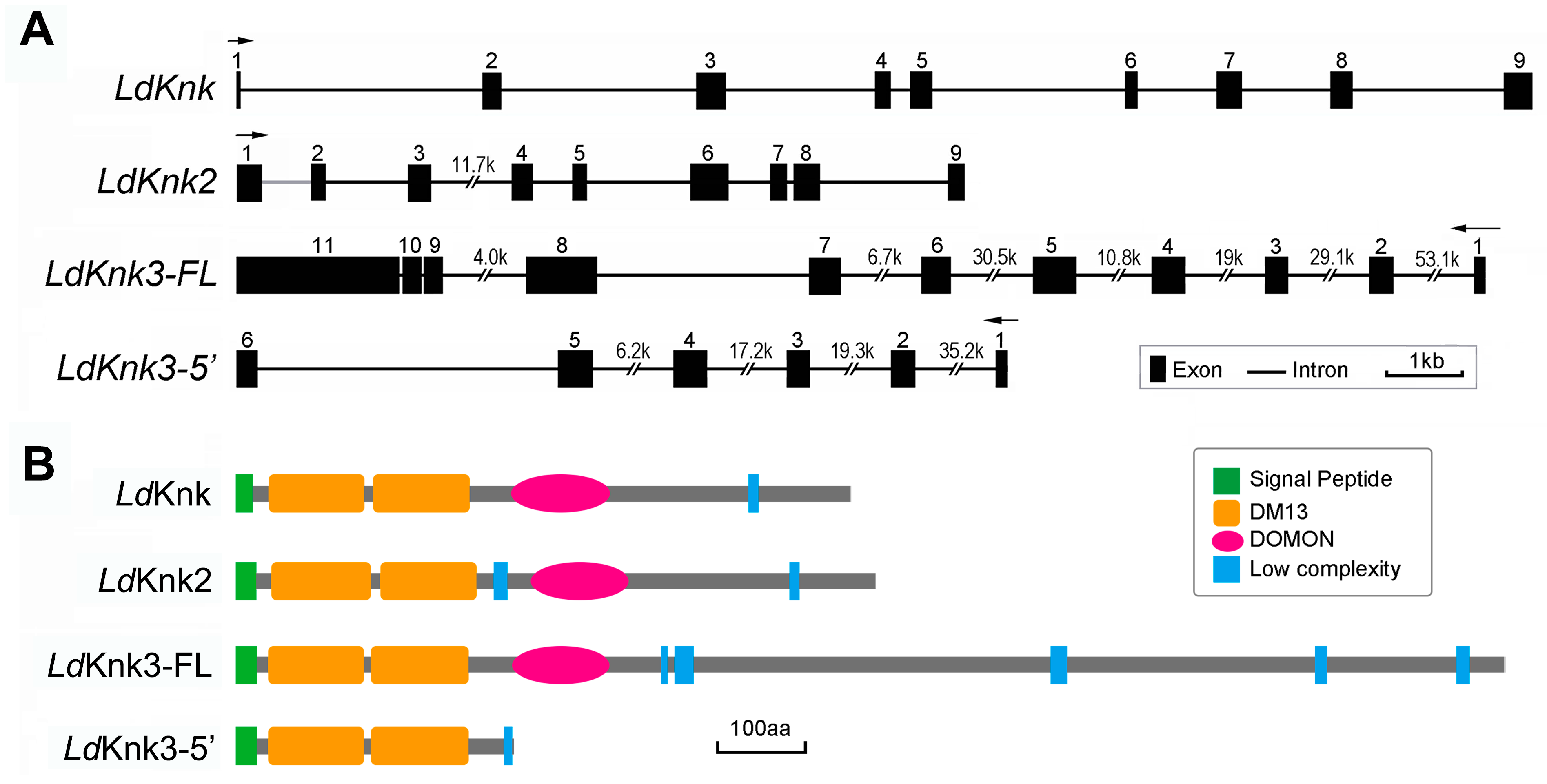
3.1. Identification and Characterization of LdKnk-Family Genes in L.decemlineata
3.2. Spatio-Temporal Expression Patterns of LdKnk-Family Genes
3.3. Effects of Silencing LdKnk Genes on Larval Development and Epidermis Structure
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Moussian, B. Recent advances in understanding mechanisms of insect cuticle differentiation. Insect Biochem. Mol. Biol. 2010, 40, 363–375. [Google Scholar] [CrossRef]
- Ren, Y.; Li, Y.; Ju, Y.; Zhang, W.; Wang, Y. Insect cuticle and insecticide development. Arch. Insect Biochem. Physiol. 2023, 114, e22057. [Google Scholar] [CrossRef] [PubMed]
- Qu, M.; Guo, X.; Tian, S.; Yang, Q.; Kim, M.; Mun, S.; Noh, M.Y.; Kramer, K.J.; Muthukrishnan, S.; Arakane, Y. AA15 lytic polysaccharide monooxygenase is required for efficient chitinous cuticle turnover during insect molting. Commun. Biol. 2022, 5, 518. [Google Scholar] [CrossRef] [PubMed]
- Riddiford, L.M. A Life’s Journey Through Insect Metamorphosis. Annu. Rev. Entomol. 2020, 65, 1–16. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, H.; Liu, X.; Li, H.; Lan, Q.; Wu, H.; Wang, Y.; Zhang, J.; Zhao, X. Nuclear Receptor FTZ-F1 Controls Locust Molt by Regulating the Molting Process of Locusta migratoria. Insects 2024, 15, 237. [Google Scholar] [CrossRef]
- Yang, W.J.; Xu, K.K.; Yan, Y.; Li, C.; Jin, D.C. Role of Chitin Deacetylase 1 in the Molting and Metamorphosis of the Cigarette Beetle Lasioderma serricorne. Int. J. Mol. Sci. 2020, 21, 2449. [Google Scholar] [CrossRef] [PubMed]
- Chaudhari, S.S.; Noh, M.Y.; Moussian, B.; Specht, C.A.; Kramer, K.J.; Beeman, R.W.; Arakane, Y.; Muthukrishnan, S. Knickkopf and retroactive proteins are required for formation of laminar serosal procuticle during embryonic development of Tribolium castaneum. Insect Biochem. Mol. Biol. 2015, 60, 1–6. [Google Scholar] [CrossRef]
- Tonning, A.; Helms, S.; Schwarz, H.; Uv, A.E.; Moussian, B. Hormonal regulation of mummy is needed for apical extracellular matrix formation and epithelial morphogenesis in Drosophila. Development 2006, 133, 331–341. [Google Scholar] [CrossRef]
- Moussian, B.; Tang, E.; Tonning, A.; Helms, S.; Schwarz, H.; Nusslein-Volhard, C.; Uv, A.E. Drosophila Knickkopf and Retroactive are needed for epithelial tube growth and cuticle differentiation through their specific requirement for chitin filament organization. Development 2006, 133, 163–171. [Google Scholar] [CrossRef]
- Zhu, K.Y.; Merzendorfer, H.; Zhang, W.; Zhang, J.; Muthukrishnan, S. Biosynthesis, Turnover, and Functions of Chitin in Insects. Annu. Rev. Entomol. 2016, 61, 177–196. [Google Scholar] [CrossRef]
- Noh, M.Y.; Muthukrishnan, S.; Kramer, K.J.; Arakane, Y. Cuticle formation and pigmentation in beetles. Curr. Opin. Insect Sci. 2016, 17, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kaya, M.; Sofi, K.; Sargin, I.; Mujtaba, M. Changes in physicochemical properties of chitin at developmental stages (larvae, pupa and adult) of Vespa crabro (wasp). Carbohydr. Polym. 2016, 145, 64–70. [Google Scholar] [CrossRef]
- Mun, S.; Noh, M.Y.; Geisbrecht, E.R.; Kramer, K.J.; Muthukrishnan, S.; Arakane, Y. Chitin deacetylases are necessary for insect femur muscle attachment and mobility. Proc. Natl. Acad. Sci. USA 2022, 119, e2120853119. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.F.; Mu, L.L.; Chen, X.; Guo, W.C.; Li, G.Q. RNA interference of chitin synthase genes inhibits chitin biosynthesis and affects larval performance in Leptinotarsa decemlineata (Say). Int. J. Biol. Sci. 2016, 12, 1319–1331. [Google Scholar] [CrossRef]
- Shi, J.F.; Fu, J.; Mu, L.L.; Guo, W.C.; Li, G.Q. Two Leptinotarsa uridine diphosphate N-acetylglucosamine pyrophosphorylases are specialized for chitin synthesis in larval epidermal cuticle and midgut peritrophic matrix. Insect Biochem. Mol. Biol. 2016, 68, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Noh, M.Y.; Muthukrishnan, S.; Kramer, K.J.; Arakane, Y. A chitinase with two catalytic domains is required for organization of the cuticular extracellular matrix of a beetle. PLoS Genet. 2018, 14, e1007307. [Google Scholar] [CrossRef]
- Noh, M.Y.; Kramer, K.J.; Muthukrishnan, S.; Kanost, M.R.; Beeman, R.W.; Arakane, Y. Two major cuticular proteins are required for assembly of horizontal laminae and vertical pore canals in rigid cuticle of Tribolium castaneum. Insect Biochem. Mol. Biol. 2014, 53, 22–29. [Google Scholar] [CrossRef]
- Noh, M.Y.; Muthukrishnan, S.; Kramer, K.J.; Arakane, Y. Tribolium castaneum RR-1 cuticular protein TcCPR4 is required for formation of pore canals in rigid cuticle. PLoS Genet. 2015, 11, e1004963. [Google Scholar] [CrossRef]
- Mun, S.; Noh, M.Y.; Dittmer, N.T.; Muthukrishnan, S.; Kramer, K.J.; Kanost, M.R.; Arakane, Y. Cuticular protein with a low complexity sequence becomes cross-linked during insect cuticle sclerotization and is required for the adult molt. Sci. Rep. 2015, 5, 10484. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Yi, L.; Lu, Z. Silencing of Chitin-Binding Protein with PYPV-Rich Domain Impairs Cuticle and Wing Development in the Asian Citrus Psyllid, Diaphorina citri. Insects 2022, 13, 353. [Google Scholar] [CrossRef]
- Muthukrishnan, S.; Merzendorfer, H.; Arakane, Y.; Yang, Q. Chitin Organizing and Modifying Enzymes and Proteins Involved In Remodeling of the Insect Cuticle. Adv. Exp. Med. Biol. 2019, 1142, 83–114. [Google Scholar] [CrossRef]
- Yu, R.R.; Duan, J.Q.; Zhao, X.M.; Abbas, M.; Zhang, Y.P.; Shi, X.K.; Chen, N.; Zhang, J.Z. Knickkopf (LmKnk) is required for chitin organization in the foregut of Locusta migratoria. Insect Sci. 2024. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Zhang, X.; Zuo, Y.; Liu, W.; Zhang, J.; Moussian, B. Timed Knickkopf function is essential for wing cuticle formation in Drosophila melanogaster. Insect Biochem. Mol. Biol. 2017, 89, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Devine, W.P.; Lubarsky, B.; Shaw, K.; Luschnig, S.; Messina, L.; Krasnow, M.A. Requirement for chitin biosynthesis in epithelial tube morphogenesis. Proc. Natl. Acad. Sci. USA 2005, 102, 17014–17019. [Google Scholar] [CrossRef] [PubMed]
- Shaik, K.S.; Wang, Y.; Aravind, L.; Moussian, B. The Knickkopf DOMON domain is essential for cuticle differentiation in Drosophila melanogaster. Arch. Insect Biochem. Physiol. 2014, 86, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Pesch, Y.Y.; Riedel, D.; Behr, M. Obstructor A organizes matrix assembly at the apical cell surface to promote enzymatic cuticle maturation in Drosophila. J. Biol. Chem. 2015, 290, 10071–10082. [Google Scholar] [CrossRef]
- Petkau, G.; Wingen, C.; Jussen, L.C.; Radtke, T.; Behr, M. Obstructor-A is required for epithelial extracellular matrix dynamics, exoskeleton function, and tubulogenesis. J. Biol. Chem. 2012, 287, 21396–21405. [Google Scholar] [CrossRef]
- Chaudhari, S.S.; Moussian, B.; Specht, C.A.; Arakane, Y.; Kramer, K.J.; Beeman, R.W.; Muthukrishnan, S. Functional specialization among members of Knickkopf family of proteins in insect cuticle organization. PLoS Genet. 2014, 10, e1004537. [Google Scholar] [CrossRef] [PubMed]
- Chaudhari, S.S.; Arakane, Y.; Specht, C.A.; Moussian, B.; Kramer, K.J.; Muthukrishnan, S.; Beeman, R.W. Retroactive maintains cuticle integrity by promoting the trafficking of Knickkopf into the procuticle of Tribolium castaneum. PLoS Genet. 2013, 9, e1003268. [Google Scholar] [CrossRef] [PubMed]
- Chaudhari, S.S.; Arakane, Y.; Specht, C.A.; Moussian, B.; Boyle, D.L.; Park, Y.; Kramer, K.J.; Beeman, R.W.; Muthukrishnan, S. Knickkopf protein protects and organizes chitin in the newly synthesized insect exoskeleton. Proc. Natl. Acad. Sci. USA 2011, 108, 17028–17033. [Google Scholar] [CrossRef]
- Yu, R.R.; Zhang, R.; Liu, W.M.; Zhao, X.M.; Zhu, K.Y.; Moussian, B.; Zhang, J.Z. The DOMON domain protein LmKnk contributes to correct chitin content, pore canal formation and lipid deposition in the cuticle of Locusta migratoria during moulting. Insect Mol. Biol. 2022, 31, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Zhao, X.; Liu, X.; Zhang, X.; Yu, R.; Ma, E.; Moussian, B.; Zhu, K.; Zhang, J. Effect of RNAi-mediated silencing of two Knickkopf family genes (LmKnk2 and LmKnk3) on cuticle formation and insecticide susceptibility in Locusta migratoria. Pest Manag. Sci. 2020, 76, 2907–2917. [Google Scholar] [CrossRef] [PubMed]
- Pelissie, B.; Chen, Y.H.; Cohen, Z.P.; Crossley, M.S.; Hawthorne, D.J.; Izzo, V.; Schoville, S.D. Genome Resequencing Reveals Rapid, Repeated Evolution in the Colorado Potato Beetle. Mol. Biol. Evol. 2022, 39, msac016. [Google Scholar] [CrossRef]
- Ma, M.Q.; He, W.W.; Xu, S.J.; Xu, L.T.; Zhang, J. RNA interference in Colorado potato beetle (Leptinotarsa decemlineata): A potential strategy for pest control. J. Integr. Agric. 2020, 19, 428–437. [Google Scholar] [CrossRef]
- Szendrei, Z.; Grafius, E.; Byrne, A.; Ziegler, A. Resistance to neonicotinoid insecticides in field populations of the Colorado potato beetle (Coleoptera: Chrysomelidae). Pest Manag. Sci. 2012, 68, 941–946. [Google Scholar] [CrossRef]
- Scott, I.M.; Tolman, J.H.; MacArthur, D.C. Insecticide resistance and cross-resistance development in Colorado potato beetle Leptinotarsa decemlineata Say (Coleoptera: Chrysomelidae) populations in Canada 2008–2011. Pest Manag. Sci. 2015, 71, 712–721. [Google Scholar] [CrossRef] [PubMed]
- Kadoic Balasko, M.; Mikac, K.M.; Bazok, R.; Lemic, D. Modern Techniques in Colorado Potato Beetle (Leptinotarsa decemlineata Say) Control and Resistance Management: History Review and Future Perspectives. Insects 2020, 11, 581. [Google Scholar] [CrossRef]
- Shi, J.F.; Cheng, M.H.; Zhou, W.; Zeng, M.Z.; Chen, Y.; Yang, J.X.; Wu, H.; Ye, Q.H.; Tang, H.; Zhang, Q.; et al. Crucial roles of specialized chitinases in elytral and hindwing cuticles construction in Leptinotarsa decemlineata. Pest Manag. Sci. 2024, 80, 4437–4449. [Google Scholar] [CrossRef]
- Shi, X.Q.; Guo, W.C.; Wan, P.J.; Zhou, L.T.; Ren, X.L.; Ahmat, T.; Fu, K.Y.; Li, G.Q. Validation of reference genes for expression analysis by quantitative real-time PCR in Leptinotarsa decemlineata (Say). BMC Res. Notes 2013, 6, 93. [Google Scholar] [CrossRef] [PubMed]
- Bustin, S.A.; Benes, V.; Garson, J.A.; Hellemans, J.; Huggett, J.; Kubista, M.; Mueller, R.; Nolan, T.; Pfaffl, M.W.; Shipley, G.L.; et al. The MIQE guidelines: Minimum information for publication of quantitative real-time PCR experiments. Clin. Chem. 2009, 55, 611–622. [Google Scholar] [CrossRef]
- Chen, Y.; Tang, H.; Zhou, W.; Li, C.; Chen, Y.N.; Zhang, Q.; Fu, K.Y.; Guo, W.C.; Shi, J.F. Identification of chitinase genes and roles in the larval-pupal transition of Leptinotarsa decemlineata. Pest Manag. Sci. 2024, 80, 282–295. [Google Scholar] [CrossRef] [PubMed]
- Schoville, S.D.; Chen, Y.H.; Andersson, M.N.; Benoit, J.B.; Bhandari, A.; Bowsher, J.H.; Brevik, K.; Cappelle, K.; Chen, M.M.; Childers, A.K.; et al. A model species for agricultural pest genomics: The genome of the Colorado potato beetle, Leptinotarsa decemlineata (Coleoptera: Chrysomelidae). Sci. Rep. 2018, 8, 1931. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Zhang, C.; Zhang, M.; Zhou, H.; Zuo, Z.; Ding, X.; Zhang, R.; Li, F.; Gao, Y. Chromosome-level genome assembly of the Colorado potato beetle, Leptinotarsa decemlineata. Sci. Data 2023, 10, 36. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Congiu, L.; Lindstrom, L.; Piiroinen, S.; Vidotto, M.; Grapputo, A. Sequencing, De Novo assembly and annotation of the Colorado Potato Beetle, Leptinotarsa decemlineata, Transcriptome. PLoS ONE 2014, 9, e86012. [Google Scholar] [CrossRef]
- De Giorgio, E.; Giannios, P.; Espinas, M.L.; Llimargas, M. A dynamic interplay between chitin synthase and the proteins Expansion/Rebuf reveals that chitin polymerisation and translocation are uncoupled in Drosophila. PLoS Biol. 2023, 21, e3001978. [Google Scholar] [CrossRef]





| Gene Name | Accession Number | cDNA (bp) | Coding Region | 5′-UTR (bp) | 3′-UTR (bp) | aa | pl | Mw(kDa) |
|---|---|---|---|---|---|---|---|---|
| LdKnk | PP962378 | 2118 | 61–2088 | 60 | 30 | 675 | 6.35 | 76.03 |
| LdKnk2 | PP962379 | 2142 | 1–2109 | 0 | 33 | 702 | 5.09 | 78.90 |
| LdKnk3-FL | PP962380 | 5100 | 49–4236 | 48 | 864 | 1395 | 7.41 | 156.48 |
| LdKnk3-5′ | PP962381 | 1042 | 61–975 | 60 | 67 | 304 | 7.65 | 33.81 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, M.-Z.; Zhou, W.; Wen, S.-S.; Wu, H.; Zhang, Q.; Fu, K.-Y.; Guo, W.-C.; Shi, J.-F. Identification and Functional Insights of Knickkopf Genes in the Larval Cuticle of Leptinotarsa decemlineata. Insects 2024, 15, 623. https://doi.org/10.3390/insects15080623
Zeng M-Z, Zhou W, Wen S-S, Wu H, Zhang Q, Fu K-Y, Guo W-C, Shi J-F. Identification and Functional Insights of Knickkopf Genes in the Larval Cuticle of Leptinotarsa decemlineata. Insects. 2024; 15(8):623. https://doi.org/10.3390/insects15080623
Chicago/Turabian StyleZeng, Mu-Zi, Wei Zhou, Shan-Shan Wen, Hao Wu, Qing Zhang, Kai-Yun Fu, Wen-Chao Guo, and Ji-Feng Shi. 2024. "Identification and Functional Insights of Knickkopf Genes in the Larval Cuticle of Leptinotarsa decemlineata" Insects 15, no. 8: 623. https://doi.org/10.3390/insects15080623
APA StyleZeng, M.-Z., Zhou, W., Wen, S.-S., Wu, H., Zhang, Q., Fu, K.-Y., Guo, W.-C., & Shi, J.-F. (2024). Identification and Functional Insights of Knickkopf Genes in the Larval Cuticle of Leptinotarsa decemlineata. Insects, 15(8), 623. https://doi.org/10.3390/insects15080623








