Life Table Study of Liriomyza trifolii and Its Contribution to Thermotolerance: Responding to Long-Term Selection Pressure for Abamectin Resistance
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insects
2.2. Determination of Physiological Parameters
2.3. Evaluation of Heat Knockdown Behavior
2.4. Measurement of Thermal Preference
2.5. Temperature Treatments and Expression of LtHsps
2.6. Statistical Analysis
3. Results
3.1. Basic Life History Statistics for L. trifolii
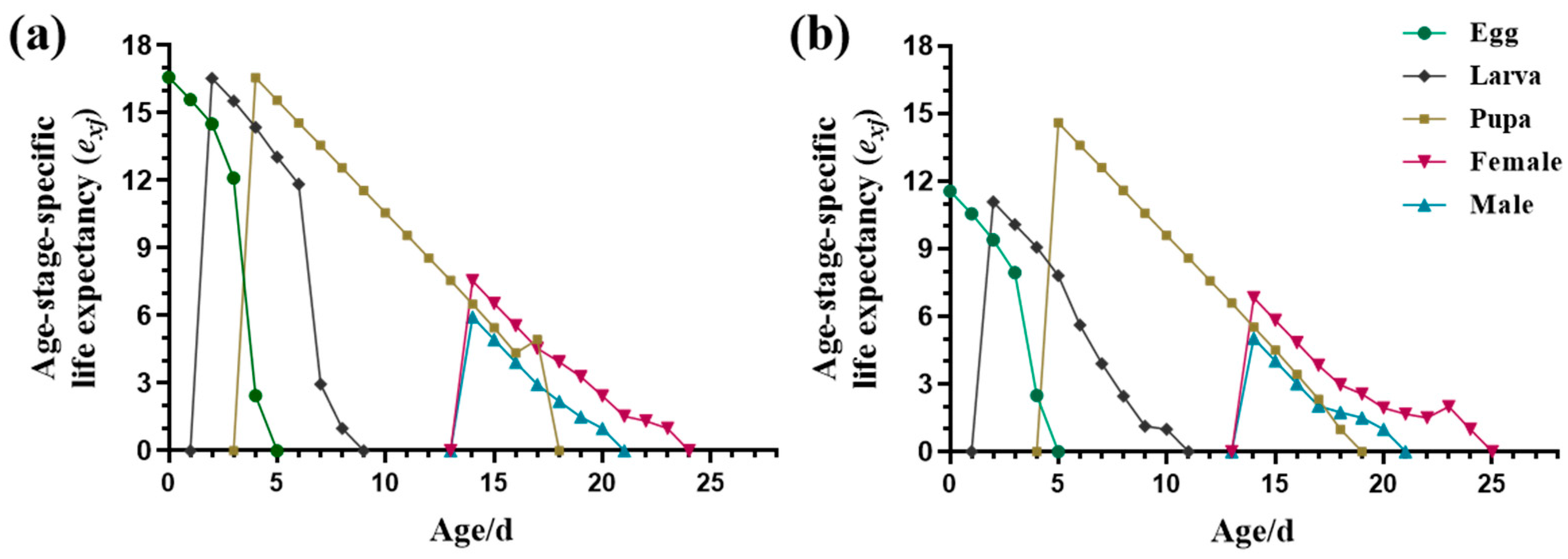
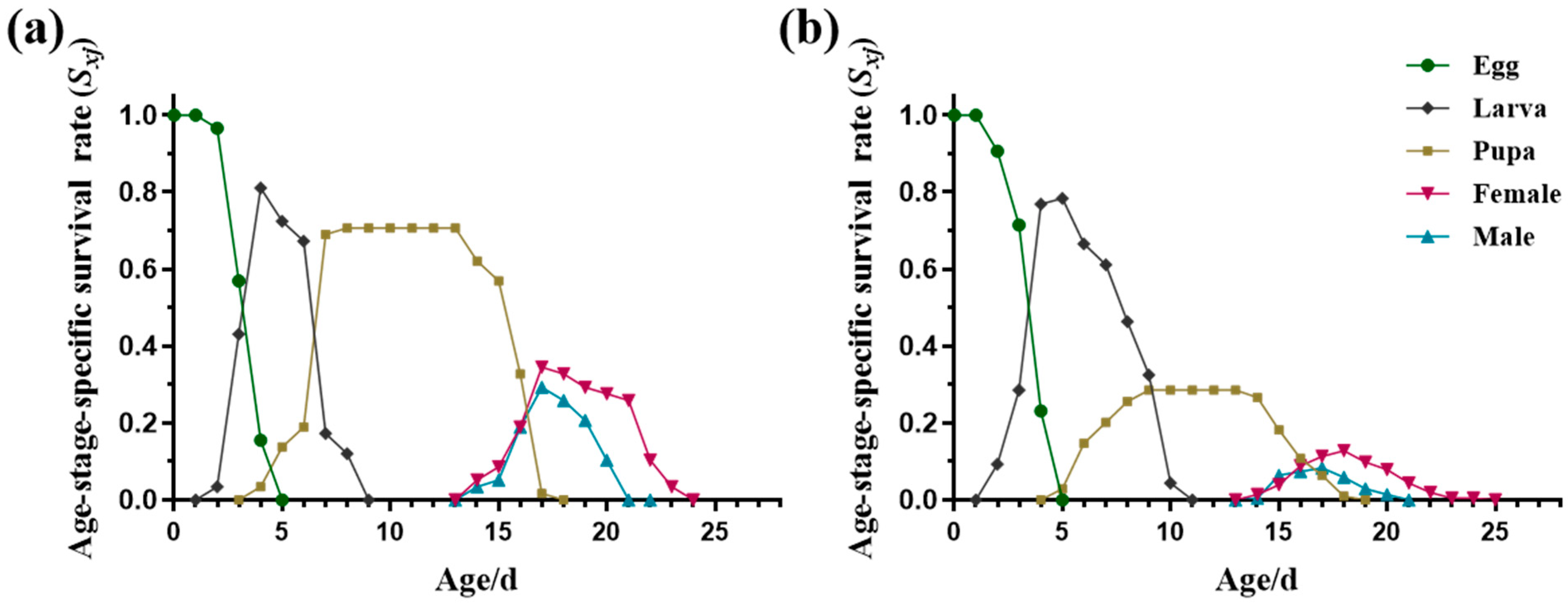
3.1.1. Durations of Different Stages and Age-Stage Life Expectancy (exj)
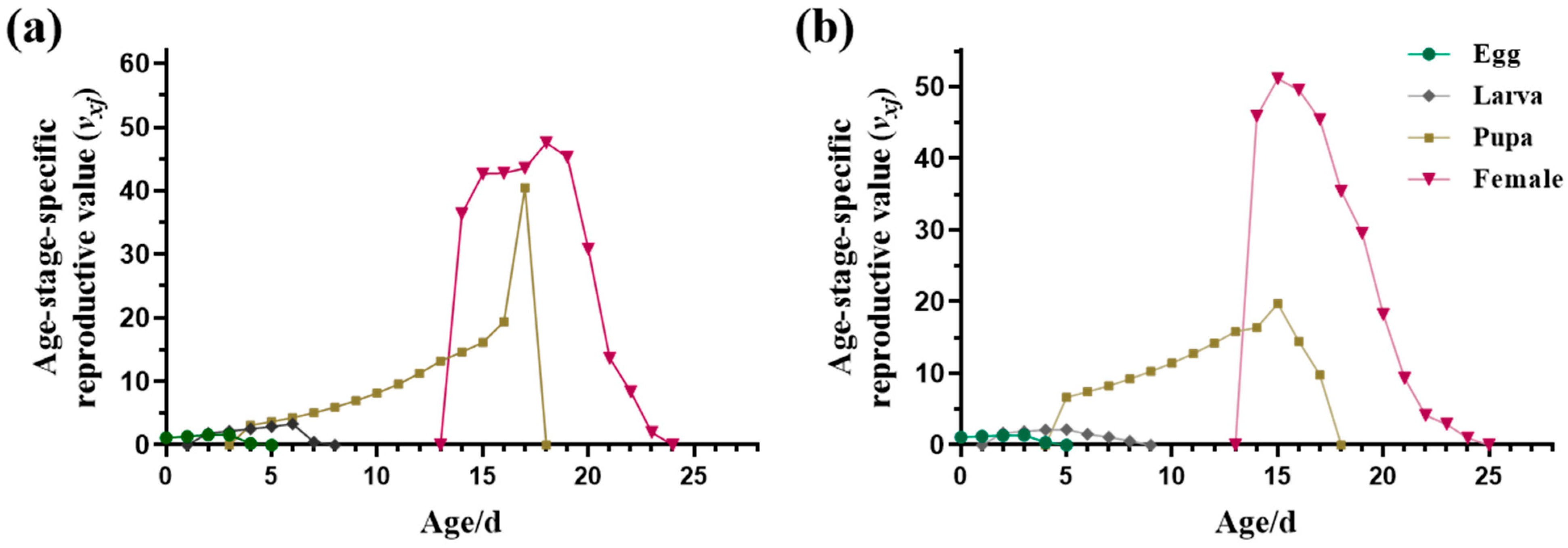
3.1.2. Fecundity and Life Table Parameters
3.1.3. Size of Pupae in Different Strains
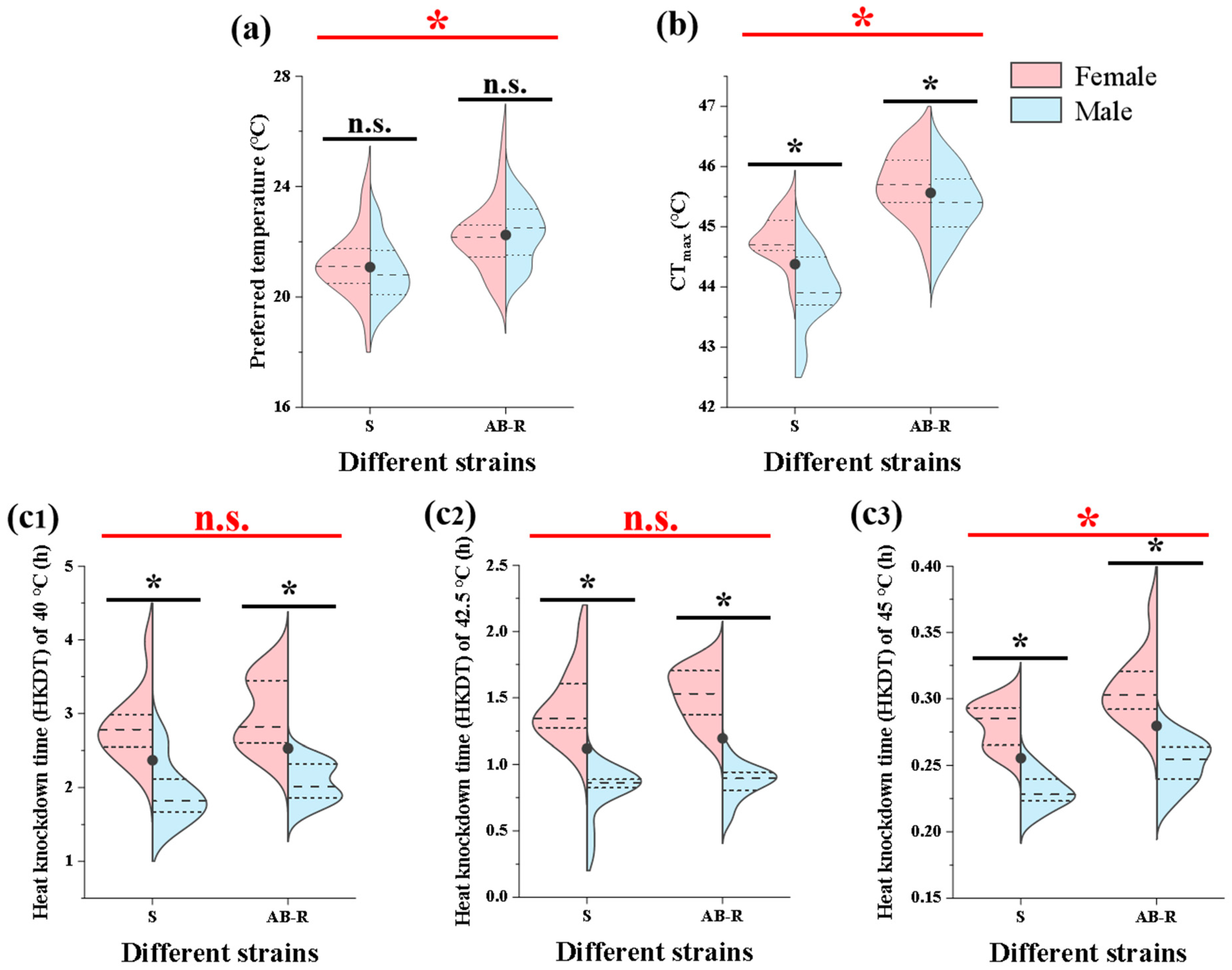
3.2. Thermal Preference and Tolerance of L. trifolii
3.3. Effect of Thermal Stress on L. trifolii Pupae and Adults
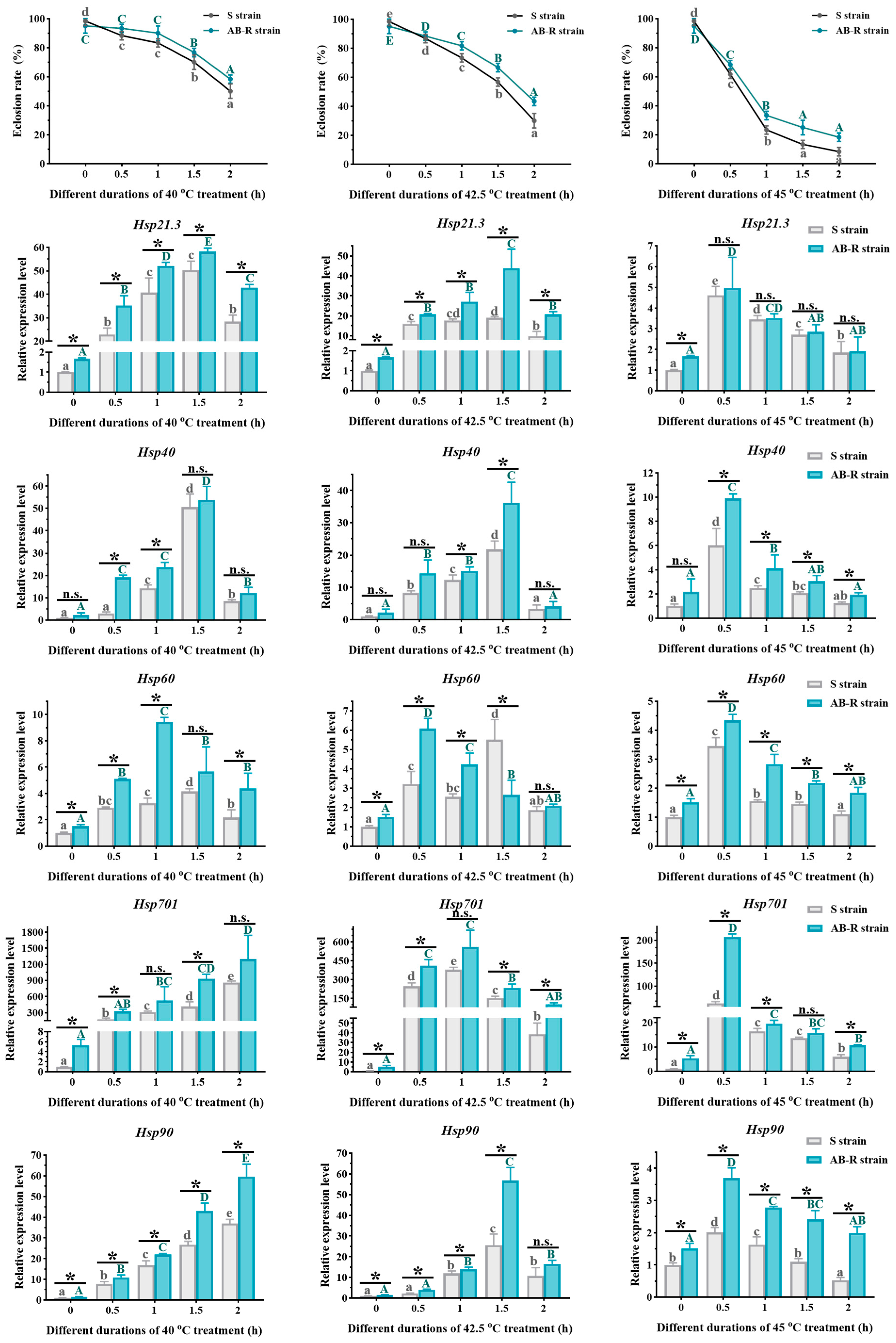
3.3.1. Eclosion Rates of L. trifolii Pupae Exposed to Thermal Stress
3.3.2. Survival Rates of L. trifolii Adults Exposed to Thermal Stress
3.3.3. Expression of LtHsps in Pupae and Adults Exposed to Thermal Stress
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Kang, L. Ecology and Sustainable Control of Serpentine Leafminers; Science Press: Beijing, China, 1996; pp. 86–90. [Google Scholar]
- Spencer, K.A. Agromyzidae (Diptera) of Economic Importance; Pitman Press: London, UK, 1973. [Google Scholar]
- Motteoni, J.A.; Broadbent, A.B. Wounds caused by Liriomyza trifolii (Diptera: Agromyzidae) as sites for infection of Chrysanthemum by Pseudomonas cichorii. Can. J. Plant Pathol. 1988, 10, 47–52. [Google Scholar] [CrossRef]
- Johnson, M.W.; Welter, S.C.; Toscano, N.C.; Tingi, P.; Trumble, J.T. Reduction of tomato leaflet photosynthesis rates by mining activity of Liriomyza sativae (Diptera: Agromyzidae). J. Econ. Entomol. 1983, 76, 1061–1063. [Google Scholar]
- Chandler, L.D.; Gilstrap, F.E. Seasonal fluctuation and age structure of Liriomyza trifolii (Diptera: Agromyzidae) larval populations on bell peppers. J. Econ. Entomol. 1987, 80, 102–106. [Google Scholar] [CrossRef]
- Parrella, M.P.; Jones, V.P.; Youngman, R.R.; Lebeck, L.M. Effect of leaf mining and leaf stippling of Liriomyza spp. on photosynthetic rates of chrysanthemum. Ann. Entomol. Soc. A 1985, 78, 90–93. [Google Scholar] [CrossRef]
- Zitter, T.A.; Tsai, J.H. Transmission of three potyviruses by the leafminer Liriomyza sativa (Diptera: Agromzidae). Plant Dis. Rep. 1977, 61, 1025–1029. [Google Scholar]
- Reitz, S.R.; Gao, Y.L.; Lei, Z.R. Insecticide use and the ecology of invasive Liriomyza leafminer management. In Insecticides—Development of Safer and More Effective Technologies; Trdan, S., Ed.; InTech: Rijeka, Croatia, 2013; pp. 233–253. [Google Scholar]
- Wei, Q.B.; Lei, Z.R.; Nauen, R.; Cai, D.C.; Gao, Y.L. Abamectin resistance in strains of vegetable leafminer, Liriomyza sativae (Diptera: Agromyzidae) is linked to elevated glutathione S-transferase activity. Insect Sci. 2015, 22, 243–250. [Google Scholar]
- Tang, Q.Y.; Zhang, C.X. Data Processing System (DPS) software with experimental design, statistical analysis and data mining developed for use in entomological research. Insect Sci. 2013, 20, 254–260. [Google Scholar] [CrossRef]
- Wang, L.Y.; Fan, R.F.; Yang, D.B.; Zhang, D.; Wang, L. Puerarin reverses cadmium-induced lysosomal dysfunction in primary rat proximal tubular cells via inhibiting Nrf2 pathway. Biochem. Pharmacol. 2019, 162, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Zhou, J.; Zhou, Z.; Zhu, Q. Abamectin induces apoptosis and autophagy by inhibiting reactive oxygen species-mediated PI3K/AKT signaling in MGC803 cells. J. Biochem. Mol. Toxicol. 2019, 33, e22336. [Google Scholar] [CrossRef]
- Subala, S.P.; Zubero, E.E.; Alatorre-Jimenez, M.A.; Shivakumar, M.S. Pre-treatment with melatonin decreases abamectin induced toxicity in a nocturnal insect Spodoptera litura (Lepidoptera: Noctuidae). Environ. Toxicol. Phar. 2018, 56, 76–85. [Google Scholar] [CrossRef]
- Gao, Y.L.; Reitz, S.R.; Wei, Q.B.; Yu, W.Y.; Lei, Z.R. Insecticide–mediated apparent displacement between two invasive species of leafminer fly. PLoS ONE 2012, 7, e36622. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kang, L.; Chen, B.; Wei, J.N.; Liu, T.X. Roles of thermal adaptation and chemical ecology in Liriomyza distribution and control. Annu. Rev. Entomol. 2009, 54, 127–145. [Google Scholar] [CrossRef] [PubMed]
- Xiang, J.C.; Lei, Z.R.; Wang, H.H. Interspecific competition among three invasive Liriomyza species. Acta Ecol. Sin. 2012, 32, 1616–1622. [Google Scholar] [CrossRef]
- Chang, Y.W.; Zhang, X.X.; Lu, M.X.; Gong, W.R.; Du, Y.Z. Transcriptome analysis of Liriomyza trifolii (Diptera: Agromyzidae) in response to temperature stress. Comp. Biochem. Phys. D 2020, 34, 100677. [Google Scholar] [CrossRef] [PubMed]
- Damme, R.V.; Bauwens, D.; Verheyen, R.F. The thermal dependence of feeding behaviour, food consumption and gut-passage time in the lizard Lacerta vivipara Jacquin. Funct. Ecol. 1991, 5, 507–517. [Google Scholar] [CrossRef]
- Du, W.G.; Yan, S.J.; Ji, X. Selected body temperature, thermal tolerance and thermal dependence of food assimilation and locomotor performance in adult blue-tailed skinks Eumeces elegans. J. Thermal. Biol. 2000, 25, 197–202. [Google Scholar] [CrossRef]
- Wang, Y.C.; Chang, Y.W.; Gong, W.R.; Hu, J.; Du, Y.Z. The development of abamectin resistance in Liriomyza trifolii and its contribution to thermotolerance. Pest Manag. Sci. 2024, 80, 2053–2060. [Google Scholar] [CrossRef] [PubMed]
- Damos, P.; Savopoulou-Soultani, M. Temperature-driven models for insect development and vital thermal requirements. Psyche J. Entomol. 2012, 123405, 1–13. [Google Scholar] [CrossRef]
- Kaunisto, S.; Ferguson, L.V.; Sinclair, B.J. Can we predict the effects of multiple stressors on insects in a changing climate? Curr. Opin. Insect Sci. 2016, 17, 55–61. [Google Scholar] [CrossRef]
- Janssens, L.; Verberk, W.; Stoks, R. A widespread morphological antipredator mechanism reduces the sensitivity to pesticides and increases the susceptibility to warming. Sci. Total Environ. 2018, 626, 1230–1235. [Google Scholar] [CrossRef]
- Dinh Van, K.; Janssens, L.; Debecker, S.; De Jonge, M.; Lambret, P.; Nilsson-Örtman, V.; Bervoets, L.; Stoks, R. Susceptibility to a metal under global warming is shaped by thermal adaptation along a latitudinal gradient. Glob. Chang. Biol. 2013, 19, 2625–2633. [Google Scholar] [CrossRef]
- Hooper, M.J.; Ankley, G.T.; Cristol, D.A.; Maryoung, L.A.; Noyes, P.D.; Pinkerton, K.E. Interactions between chemical and climate stressors: A role for mechanistic toxicology in assessing climate change risks. Environ. Toxicol. Chem. 2013, 32, 32–48. [Google Scholar] [CrossRef]
- De Beeck, L.O.; Verheyen, J.; Olsen, K.; Stoks, R. Negative effects of pesticides under global warming can be counteracted by a higher degradation rate and thermal adaptation. J. Appl. Ecol. 2017, 54, 1847–1855. [Google Scholar] [CrossRef]
- Huang, L.H.; Chen, B.; Kang, L. Impact of mild temperature hardening on thermotolerance, fecundity, and Hsp gene expression in Liriomyza huidobrensis. J. Insect Physiol. 2008, 53, 1199–1205. [Google Scholar] [CrossRef]
- Zhang, L.J.; Wang, K.F.; Jing, Y.P.; Zhuang, H.M.; Wu, G. Identification of heat shock protein genes hsp70s and hsc70 and their associated mRNA expression under heat stress in insecticide–resistant and susceptible diamondback moth, Plutella xylostella (Lepidoptera: Plutellidae). Euro. J. Entomol. 2015, 112, 215–226. [Google Scholar] [CrossRef]
- Noyes, P.D.; McElwee, M.K.; Miller, H.D.; Clark, B.W.; Van Tiem, L.A.; Walcott, K.C.; Erwin, K.N.; Levin, E.D. The toxicology of climate change: Environmental contaminants in a warming world. Environ. Int. 2009, 35, 971–986. [Google Scholar] [CrossRef]
- Chen, B.; Kang, L. Implication of pupal cold tolerance for the northern over-wintering range limit of the leafminer Liriomyza sativae (Diptera: Agromyzidae) in China. Appl. Entomol. Zool. 2005, 40, 437–446. [Google Scholar] [CrossRef][Green Version]
- Huey, R.B.; Crill, W.D.; Kingsolver, J.G.; Weber, K.E. A method for rapid measurement of heat or cold resistance of small insects. Funct. Ecol. 1992, 6, 489. [Google Scholar] [CrossRef]
- Folk, D.G.; Hoekstra, L.A.; Gilchrist, G.W. Critical thermal maxima in knockdown-selected Drosophila: Are thermal endpoints correlated? J. Exp. Biol. 2007, 210, 2649–2656. [Google Scholar] [CrossRef] [PubMed]
- Nyamukondiwa, C.; Terblanche, J.S. Thermal tolerance in adult Mediterranean and Natal fruit flies (Ceratitis capitata and Ceratitis rosa): Effects of age, gender and feeding status. J. Therm. Biol. 2009, 34, 406–414. [Google Scholar] [CrossRef]
- Mpofu, P.; Cuthbert, R.N.; Machekano, H.; Nyamukondiwa, C. Transgenerational responses to heat and fasting acclimation in the Angoumois grain moth. J. Stored Prod. Res. 2022, 97, 101979. [Google Scholar] [CrossRef]
- Light, P.; Dawson, W.R.; Shoemaker, V.H.; Main, A.R. Observations on the thermal relations of western Australian lizards. Copeia 1996, 1, 97–110. [Google Scholar] [CrossRef]
- Dillon, M.E.; Liu, R.S.; Wang, G.; Huey, R.B. Disentangling thermal preference and the thermal dependence of movement in ectotherms. J. Therm. Biol. 2012, 37, 631–639. [Google Scholar] [CrossRef]
- Addeo, N.F.; Li, C.; Rusch, T.W.; Dickerson, A.J.; Tarone, A.M.; Bovera, F.; Tomberlin, J.K. Impact of age, size, and sex on adult black soldier fly [Hermetia illucens L. (Diptera: Stratiomyidae)] thermal preference. J. Insects Food Feed. 2022, 8, 129–139. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmitgen, T.D. Analysis of relative gene expression data using real-time quantification PCR and the 2(-Delta Delta CT) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Chang, Y.W.; Chen, J.Y.; Lu, M.X.; Gao, Y.; Tian, Z.H.; Gong, W.R.; Dong, C.S.; Du, Y.Z. Cloning and expression of genes encoding heat shock proteins in Liriomyza trifolii and comparison with two congener leafminer species. PLoS ONE 2017, 12, e0181355. [Google Scholar] [CrossRef]
- Chi, H. Life-table analysis incorporating both sexes and variable development rates among individuals. Environ. Entomol. 1988, 17, 26–34. [Google Scholar] [CrossRef]
- Chi, H.; Liu, H. Two new methods for the study of insect population ecology. Bull. Inst. Zool. Acad. Sin. 1985, 24, 225–240. [Google Scholar]
- Chi, H.; You, M.; Atlihan, R.; Smith, C.L.; Kavousi, A.; Özgökçe, M.S.; Güncan, A.; Tuan, S.J.; Fu, J.W.; Xu, Y.Y.; et al. Age-Stage, two-sex life table: An introduction to theory, data analysis, and application. Entomol. Gen. 2020, 40, 103–124. [Google Scholar] [CrossRef]
- Chi, H. TWOSEX-MSChart: A Computer Program for the Age Stage, Two-Sex Life Table Analysis. National Chung Hsing University, Taichung, Taiwan. Available online: http://140.120.197.173/Ecology/prod02.htm (accessed on 30 March 2024).
- Amir-Maafi, M.; Chi, H.; Chen, Z.Z.; Xu, Y.Y. Innovative bootstrapmatch technique for life table set up. Entomol. Gen. 2022, 42, 597–609. [Google Scholar] [CrossRef]
- Meyer, J.S.; Ingersoll, C.G.; McDonald, L.L.; Boyce, M.S. Estimating uncertainty in population growth rates: Jackknife vs. bootstrap techniques. Ecology 1986, 67, 1156–1166. [Google Scholar] [CrossRef]
- Crowley, P.H. Resampling methods for computation-intensive data analysis in ecology and evolution. Annu. Rev. Ecol. Syst. 1992, 23, 405–447. [Google Scholar] [CrossRef]
- Efron, B.; Tibshirani, R.J. An Introduction to the Bootstrap; Chapman & Hall: New York, NY, USA, 1993. [Google Scholar]
- Huang, Y.B.; Chi, H. Assessing the application of the jackknife and bootstrap techniques to the estimation of the variability of the net reproductive rate and gross reproductive rate: A case study in Bactrocera cucurbitae (Coquillett) (Diptera: Tephritidae). J. Agric. Fore. 2012, 61, 37–45. [Google Scholar]
- Wei, M.F.; Chi, H.; Guo, Y.F.; Li, X.W.; Zhao, L.L.; Ma, R.Y. Demography of Cacopsylla chinensis (Hemiptera: Psyllidae) reared on four cultivars of Pyrus bretschneideri and P. communis (Rosales: Rosaceae) pears with estimations of confidence intervals of specific life table statistics. J. Econ. Entomol. 2020, 113, 2343–2353. [Google Scholar] [CrossRef]
- Brandstätter, E. Confidence intervals as an alternative to significance testing. Methods Psychol. Res. Online 1999, 4, 33–46. [Google Scholar]
- Hesterberg, T.; Moore, D.S.; Monaghan, S.; Clipson, A.; Epstein, R. Bootstrap methods and permutation tests. In The Practice of Business Statistics, 2nd ed.; Moore, D.S., McCabe, G.P., Duckworth, W.M., Sclove, S.L., Eds.; W. H. Freeman and Company: New York, NY, USA, 2005. [Google Scholar]
- Smucker, M.D.; Allan, J.; Carterette, B. A comparison of statistical significance tests for information retrieval evaluation. In Proceedings of the Sixteenth ACM Conference on Information and Knowledge Management, CIKM 2007, Lisbon, Portugal, 6–10 November 2007; pp. 623–632. [Google Scholar]
- Chang, C.; Huang, C.Y.; Dai, S.M.; Atlihan, R.; Chi, H. Genetically engineered ricin suppresses Bactrocera dorsalis (Diptera: Tephritidae) based on demographic analysis of group-reared life table. J. Econ. Entomol. 2016, 109, 987–992. [Google Scholar] [CrossRef]
- Chi, H.; Su, H.Y. Age-stage, two-sex life tables of Aphidius gifuensis (Ashmead) (Hymenoptera: Braconidae) and its host Myzus persicae (Sulzer) (Homoptera: Aphididae) with mathematical proof of the relationship between female fecundity and the net reproductive rate. Environ. Entomol. 2006, 35, 10–21. [Google Scholar] [CrossRef]
- Tuan, S.J.; Lee, C.C.; Chi, H. Population and damage projection of Spodoptera litura (F.) on peanuts (Arachis hypogaea L.) under different conditions using the age-stage, two-sex life table. Pest Manag. Sci. 2014, 70, 805–813. [Google Scholar] [CrossRef]
- Amer, K.; Saavedra-Rodriguez, K.; Black, W.C., IV; Gray, E.M. Effect of selection for pyrethroid resistance on abiotic stress tolerance in Aedes aegypti from Merida, Yucatan, Mexico. Insects 2021, 12, 124. [Google Scholar] [CrossRef]
- Li, Y.T.; Zhang, Y.L.; Liu, X.D.; Guo, H.F. Does resistance to buprofezin improve heat and cold tolerance of Laodelphax striatellus (Hemiptera: Delphacidae)? Environ. Entomol. 2017, 46, 988–994. [Google Scholar]
- Miller, G.A.; Clissold, F.J.; Mayntz, D.; Simpson, S.J. Speed over efficiency: Locusts select body temperatures that favour growth rate over efficient nutrient utilization. Proc. R. Soc. B 2009, 276, 3581–3589. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.S. The Biological Characteristics and Control of Liriomyza. World Trop. Agric. Inf. 1999, 2, 1–3. [Google Scholar]
- Feng, Y.T.; Wu, Q.J.; Wang, S.L.; Chang, X.L.; Xie, W.; Xu, B.Y.; Zhang, Y.J. Cross-resistance study and biochemical mechanisms of thiamethoxam resistance in b-biotype Bemisia tabaci (Hemiptera: Aleyrodidae). Pest Manag. Sci. 2010, 66, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.J.; Huang, L.F.; Li, L.F.; Chen, H.S.; Wang, F.Y.; Yang, L. Cloning and expression of the Bactrocera cucurbitae (Coquillett) heat shock protein 90 gene. Chin. J. Appl. Entomol. 2019, 56, 444–453. [Google Scholar]



| Developmental Stages | Strains | |||
|---|---|---|---|---|
| S | AB-R | p Value | ||
| Egg (d) | Total | 3.480 ± 0.086 | 3.588 ± 0.060 | 0.3062 |
| Female | 3.333 ± 0.125 | 3.482 ± 0.098 | 0.35522 | |
| Male | 3.412 ± 0.172 b | 3.842 ± 0.086 a | 0.02681 | |
| Larva (d) | Total | 3.098 ± 0.119 | 3.069 ± 0.140 | 0.87704 |
| Female | 3.191 ± 0.189 | 3.148 ± 0.197 | 0.87845 | |
| Male | 3.000 ± 0.171 | 2.684 ± 0.242 | 0.28939 | |
| Pupa (d) | Total | 9.632 ± 0.078 a | 9.283 ± 0.074 b | 0.00111 |
| Female | 9.619 ± 0.107 | 9.444 ± 0.111 | 0.26098 | |
| Male | 9.647 ± 0.118 a | 9.053 ± 0.053 b | 0.00006 | |
| Adult (d) | Total | 4.737 ± 0.186 a | 4.261 ± 0.144 b | 0.04344 |
| Female | 5.429 ± 0.243 a | 4.778 ± 0.163 b | 0.02657 | |
| Male | 3.882 ± 0.080 a | 3.526 ± 0.141 b | 0.02881 | |
| APOP (d) | 1.048 ± 0.108 | 0.889 ± 0.061 | 0.2086 | |
| TPOP (d) | 17.191 ± 0.296 | 16.963 ± 0.0.222 | 0.53915 | |
| Ovi-days | 4.381 ± 0.303 | 3.889 ± 0.187 | 0.16704 | |
| Total longevity | 20.842 ± 0.271 | 20.130 ± 0.246 | 0.05013 | |
| Population Parameters | Strains | ||
|---|---|---|---|
| S | AB-R | p Value | |
| r (day−1) | 0.160 ± 0.012 a | 0.108 ± 0.011 b | 0.00231 |
| λ (day−1) | 1.173 ± 0.013 a | 1.114 ± 0.012 b | 0.00209 |
| R0 (offspring/individual) | 23.483 ± 5.201 a | 7.769 ± 1.560 b | 0.00424 |
| T (days) | 19.771 ± 0.267 a | 19.019 ± 0.244 b | 0.03941 |
| Nf/N | 0.362 ± 0.063 a | 0.133 ± 0.024 b | 0.00077 |
| F (eggs per female) | 64.857 ± 8.944 | 58.407 ± 5.381 | 0.53589 |
| Developmental Stages | Strains | |||
|---|---|---|---|---|
| S | AB-R | p Value | ||
| Immature stage | Egg | 0.138 ± 0.045 | 0.187 ± 0.027 | 0.36342 |
| Larva | 0.155 ± 0.048 b | 0.527 ± 0.035 a | p < 0.00001 | |
| Pupa | 0.052 ± 0.029 | 0.059 ± 0.017 | 0.85398 | |
| Mature stage | Female | 0.362 ± 0.063 a | 0.133 ± 0.024 b | 0.00077 |
| Male | 0.239 ± 0.060 a | 0.094 ± 0.021 b | 0.00178 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Chang, Y.; Gong, W.; Du, Y. Life Table Study of Liriomyza trifolii and Its Contribution to Thermotolerance: Responding to Long-Term Selection Pressure for Abamectin Resistance. Insects 2024, 15, 462. https://doi.org/10.3390/insects15060462
Wang Y, Chang Y, Gong W, Du Y. Life Table Study of Liriomyza trifolii and Its Contribution to Thermotolerance: Responding to Long-Term Selection Pressure for Abamectin Resistance. Insects. 2024; 15(6):462. https://doi.org/10.3390/insects15060462
Chicago/Turabian StyleWang, Yucheng, Yawen Chang, Weirong Gong, and Yuzhou Du. 2024. "Life Table Study of Liriomyza trifolii and Its Contribution to Thermotolerance: Responding to Long-Term Selection Pressure for Abamectin Resistance" Insects 15, no. 6: 462. https://doi.org/10.3390/insects15060462
APA StyleWang, Y., Chang, Y., Gong, W., & Du, Y. (2024). Life Table Study of Liriomyza trifolii and Its Contribution to Thermotolerance: Responding to Long-Term Selection Pressure for Abamectin Resistance. Insects, 15(6), 462. https://doi.org/10.3390/insects15060462





