Wolbachia Infection through Hybridization to Enhance an Incompatible Insect Technique-Based Suppression of Aedes albopictus in Eastern Spain
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ae. albopictus Lines and Their Maintenance
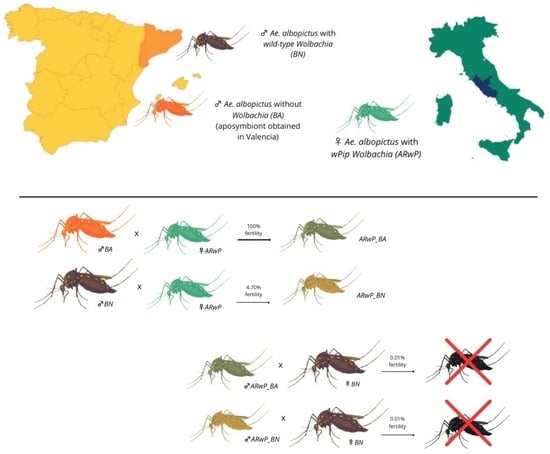
2.2. Hybridization Protocol
2.3. Monitoring of the Establishment and Maternal Transmission of the Infection
2.4. Sequencing of the COI Gene in Hybrid Populations
2.5. Determination of Fitness Parameters and Induced CI Levels of the Hybrid Lines
2.6. Data Analysis
3. Results
3.1. Establishment and Maternal Transmission of the Infection
3.2. Comparison of the COI Gene Sequences between Ae. albopictus Lines
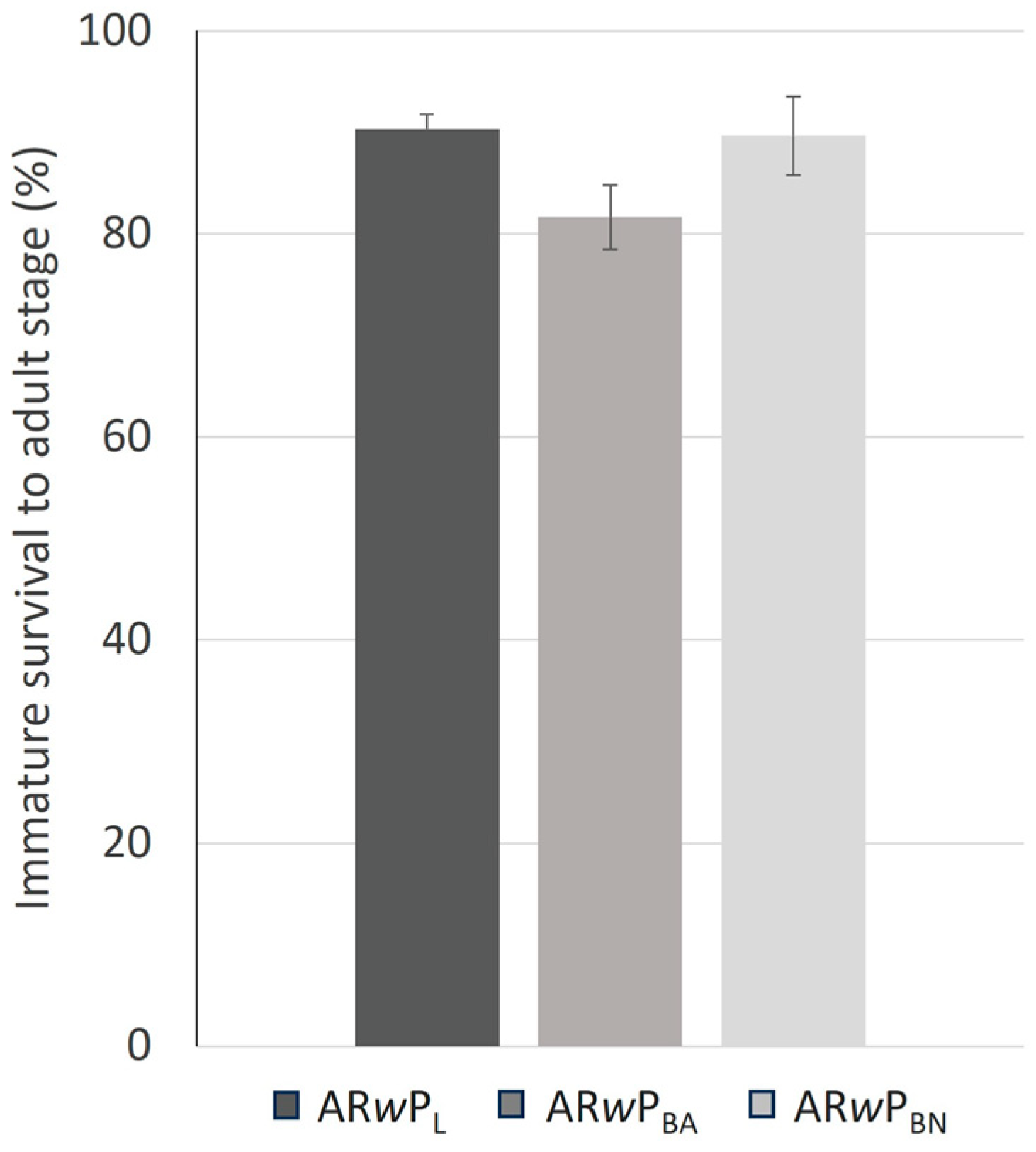
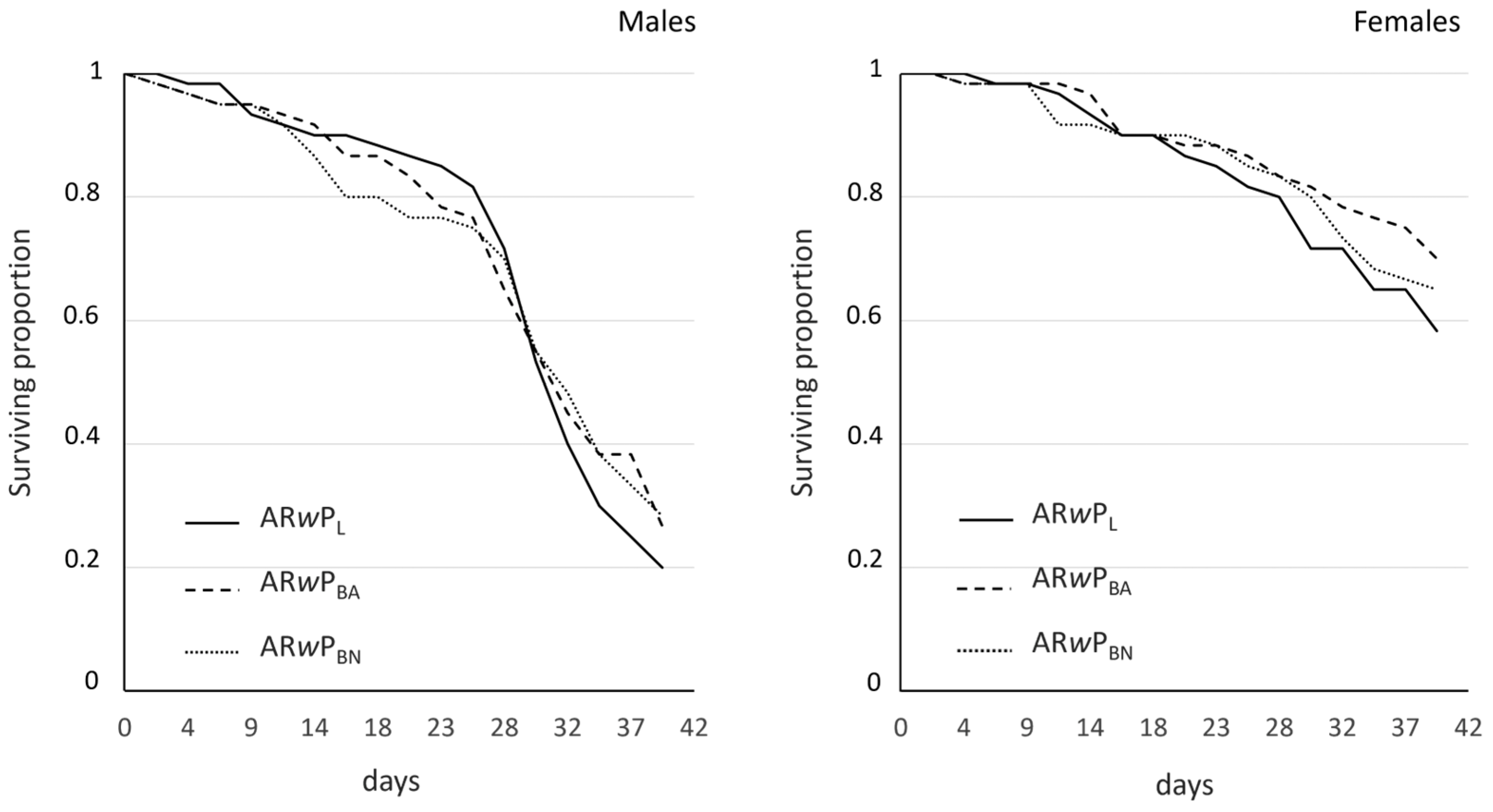
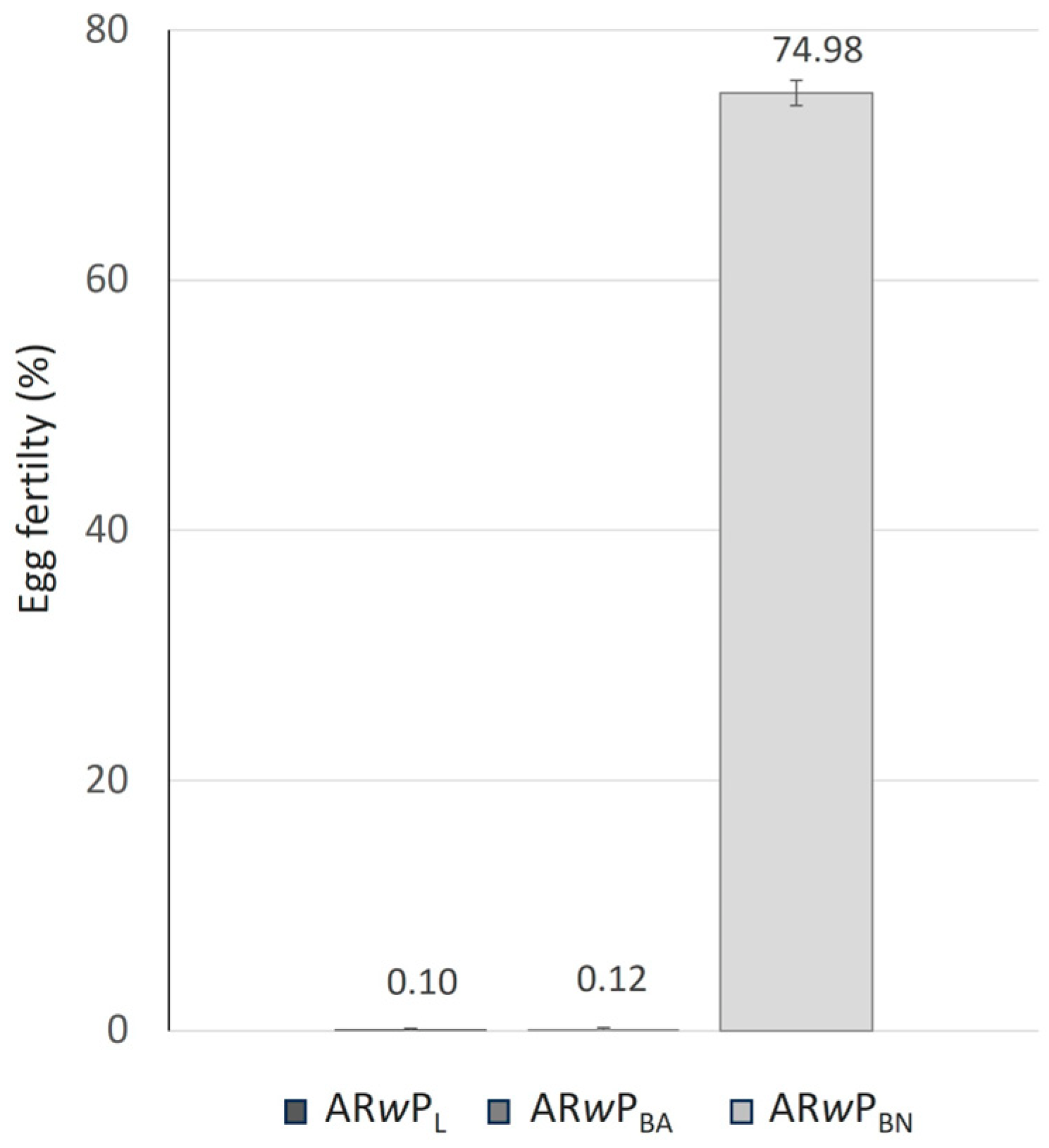
3.3. Fitness and CI Levels of Hybrid Lines Compared to ARwP
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO (World Health Organization). Global Vector Control Response 2017–2030; Licence: CC BY-NC-SA 3.0 IGO; World Health Organization: Geneva, Switzerland, 2017; Available online: http://www.jstor.org/stable/resrep35629 (accessed on 1 March 2024).
- Tozan, Y.; Branch, O.L.H.; Rocklöv, J. Vector-borne diseases in a changing climate and world. In Climate Change and Global Public Health; Pinkerton, K.E., Rom, W.N., Eds.; Respiratory Medicine; Humana: Cham, Switzerland; Totowa, NJ, USA, 2021; pp. 253–271. [Google Scholar]
- Kraemer, M.U.G.; Reiner, R.C.; Brady, O.J.; Messina, J.P.; Gilbert, M.; Pigott, D.M.; Yi, D.; Johnson, K.; Earl, L.; Marczak, L.B.; et al. Past and future spread of the arbovirus vectors Aedes aegypti and Aedes albopictus. Nat. Microbiol. 2019, 4, 854–863. [Google Scholar] [CrossRef] [PubMed]
- Roiz, D.; Wilson, A.L.; Scott, T.W.; Fonseca, D.M.; Jourdain, F.; Müller, P.; Velayudhan, R.; Corbel, V. Integrated Aedes management for the control of Aedes-borne diseases. PLoS Negl. Trop. Dis. 2018, 12, e0006845, Erratum in PLoS Negl. Trop. Dis. 2022, 16, e0010310. [Google Scholar] [CrossRef] [PubMed]
- WHO (World Health Organization). Global Vector Control Response: Progress in Planning and Implementation; Licence: CC BY-NC-SA 3.0 IGO; World Health Organization: Geneva, Switzerland, 2020; Available online: https://www.who.int/publications/i/item/9789240007987 (accessed on 1 March 2024).
- Ogunlade, S.T.; Meehan, M.T.; Adekunle, A.I.; Rojas, D.P.; Adegboye, O.A.; McBryde, E.S. A Review: Aedes-borne arboviral infections, controls and Wolbachia-based strategies. Vaccines 2021, 9, 32. [Google Scholar] [CrossRef] [PubMed]
- Mulderij-Jansen, V.; Pundir, P.; Grillet, M.E.; Lakiang, T.; Gerstenbluth, I.; Duits, A.; Tami, A.; Bailey, A. Effectiveness of Aedes-borne infectious disease control in Latin America and the Caribbean region: A scoping review. PLoS ONE 2022, 17, e0277038. [Google Scholar] [CrossRef] [PubMed]
- Chala, B.; Hamde, F. Emerging and re-emerging vector-borne infectious diseases and the challenges for control: A review. Front. Public Health 2021, 9, 715759. [Google Scholar] [CrossRef]
- Sherpa, S.; Blum, M.G.; Després, L. Cold adaptation in the Asian tiger mosquito’s native range precedes its invasion success in temperate regions. Evolution 2019, 73, 1793–1808. [Google Scholar] [CrossRef] [PubMed]
- Hemingway, J.; Hawkes, N.J.; McCarroll, L.; Ranson, H. The molecular basis of insecticide resistance in mosquitoes. Insect Biochem. Mol. Biol. 2004, 34, 653–665. [Google Scholar] [CrossRef] [PubMed]
- Liu, N. Insecticide resistance in mosquitoes: Impact, mechanisms, and research directions. Annu. Rev. Entomol. 2015, 60, 537–559. [Google Scholar] [CrossRef]
- Wilke, A.B.B.; Vasquez, C.; Carvajal, A.; Medina, J.; Chase, C.; Cardenas, G.; Mutebi, J.P.; Petrie, W.D.; Beier, J.C. Proliferation of Aedes aegypti in urban environments mediated by the availability of key aquatic habitats. Sci. Rep. 2020, 10, 12925. [Google Scholar] [CrossRef]
- Yakob, L.; Funk, S.; Camacho, A.; Brady, O.; Edmunds, W.J. Aedes aegypti control through modernized, integrated vector management. PLoS Curr. 2017, 9, currents.outbreaks.45deb8e03a438c4d088afb4fafae8747. [Google Scholar] [CrossRef]
- Dusfour, I.; Vontas, J.; David, J.P.; Weetman, D.; Fonseca, D.M.; Corbel, V.; Raghavendra, K.; Coulibaly, M.B.; Martins, A.J.; Kasai, S.; et al. Management of insecticide resistance in the major Aedes vectors of arboviruses: Advances and challenges. PLoS Negl. Trop. Dis. 2019, 13, e0007615. [Google Scholar] [CrossRef] [PubMed]
- Marston, H.D.; Lurie, N.; Borio, L.L.; Fauci, A.S. Considerations for developing a Zika virus vaccine. N. Engl. J. Med. 2016, 375, 1209–1212. [Google Scholar] [CrossRef] [PubMed]
- Garcia, A.; Diego, L.; Judith, B. New approaches to chikungunya virus vaccine development. Recent Pat. Inflamm. Allergy Drug Discov. 2015, 9, 31–37. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Alphey, L. Genetic control of mosquitoes. Annu. Rev. Entomol. 2014, 59, 205–224. [Google Scholar] [CrossRef]
- Wang, G.H.; Gamez, S.; Raban, R.R.; Marshall, J.M.; Alphey, L.; Li, M.; Rasgon, J.L.; Akbari, O.S. Combating mosquito-borne diseases using genetic control technologies. Nat. Commun. 2021, 12, 4388. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Luo, J.; Wang, Y.; Gurav, A.S.; Li, M.; Akbari, O.S.; Montell, C. Suppression of female fertility in Aedes aegypti with a CRISPR-targeted male-sterile mutation. Proc. Natl. Acad. Sci. USA 2021, 118, e2105075118. [Google Scholar] [CrossRef] [PubMed]
- Harris, A.; Nimmo, D.; McKemey, A.; Kelly, N.; Scaife, S.; Donnelly, C.A.; Beech, C.; Petrie, W.D.; Alphey, L. Field performance of engineered male mosquitoes. Nat. Biotechnol. 2011, 29, 1034–1037. [Google Scholar] [CrossRef]
- Phuc, H.K.; Andreasen, M.H.; Burton, R.S.; Vass, C.; Epton, M.J.; Pape, G.; Fu, G.; Condon, K.C.; Scaife, S.; Donnelly, C.A.; et al. Late-acting dominant lethal genetic systems and mosquito control. BMC Biol. 2007, 5, 11. [Google Scholar] [CrossRef]
- Calvitti, M.; Moretti, R.; Skidmore, A.R.; Dobson, S.L. Wolbachia strain wPip yields a pattern of cytoplasmic incompatibility enhancing a Wolbachia-based suppression strategy against the disease vector Aedes albopictus. Parasite Vectors 2012, 5, 254. [Google Scholar] [CrossRef]
- Axford, J.K.; Ross, P.A.; Yeap, H.L.; Callahan, A.G.; Hoffmann, A.A. Fitness of wAlbB Wolbachia infection in Aedes aegypti: Parameter estimates in an outcrossed background and potential for population invasion. Am. J. Trop. Med. Hyg. 2016, 94, 507–516. [Google Scholar] [CrossRef]
- Pagendam, D.E.; Trewin, B.J.; Snoad, N.; Ritchie, S.A.; Hoffmann, A.A.; Staunton, K.M.; Paton, C.; Beebe, N. Modelling the Wolbachia incompatible insect technique: Strategies for effective mosquito population elimination. BMC Biol. 2020, 18, 161. [Google Scholar] [CrossRef] [PubMed]
- Liew, C.; Soh, L.T.; Chen, I.; Ng, L.C. Public sentiments towards the use of Wolbachia-Aedes technology in Singapore. BMC Public Health 2021, 21, 1417. [Google Scholar] [CrossRef] [PubMed]
- Zug, R.; Hammerstein, P. Still a host of hosts for Wolbachia: Analysis of recent data suggests that 40% of terrestrial arthropod species are infected. PLoS ONE 2012, 7, e38544. [Google Scholar] [CrossRef]
- Kaur, R.; Shropshire, J.D.; Cross, K.L.; Leigh, B.; Mansueto, A.J.; Stewart, V.; Bordenstein, S.R.; Bordenstein, S.R. Living in the endosymbiotic world of Wolbachia: A centennial review. Cell Host Microbe 2021, 29, 879–893. [Google Scholar] [CrossRef] [PubMed]
- Bourtzis, K.; Dobson, S.L.; Xi, Z.; Rasgon, J.L.; Calvitti, M.; Moreira, L.A.; Bossin, H.C.; Moretti, R.; Baton, L.A.; Hughes, G.L.; et al. Harnessing mosquito-Wolbachia symbiosis for vector and disease control. Acta Trop. 2014, 132, S150–S163. [Google Scholar] [CrossRef] [PubMed]
- Werren, J.H.; Baldo, L.; Clark, M.E. Wolbachia: Master manipulators of invertebrate biology. Nat. Rev. Microbiol. 2008, 6, 741–751. [Google Scholar] [CrossRef] [PubMed]
- Hochstrasser, M. Cytoplasmic incompatibility: A Wolbachia toxin-antidote mechanism comes into view. Curr. Biol. 2022, 32, R287–R289. [Google Scholar] [CrossRef]
- Beebe, N.W.; Pagendam, D.; Trewin, B.J.; Boomer, A.; Bradford, M.; Ford, A.; Liddington, C.; Bondarenco, A.; De Barro, P.J.; Gilchrist, J.; et al. Releasing incompatible males drives strong suppression across populations of wild and Wolbachia-carrying Aedes aegypti in Australia. Proc. Natl. Acad. Sci. USA 2021, 118, e2106828118. [Google Scholar] [CrossRef]
- Calvitti, M.; Moretti, R.; Lampazzi, E.; Bellini, R.; Dobson, S.L. Characterization of a new Aedes albopictus (Diptera: Culicidae)-Wolbachia pipientis (Rickettsiales: Rickettsiaceae) symbiotic association generated by artificial transfer of the wPip strain from Culex pipiens (Diptera: Culicidae). J. Med. Entomol. 2010, 47, 179–187. [Google Scholar] [CrossRef]
- Blagrove, M.S.; Arias-Goeta, C.; Failloux, A.B.; Sinkins, S.P. Wolbachia strain wMel induces cytoplasmic incompatibility and blocks dengue transmission in Aedes albopictus. Proc. Natl. Acad. Sci. USA 2012, 109, 255–260. [Google Scholar] [CrossRef]
- Moretti, R.; Yen, P.-S.; Houé, V.; Lampazzi, E.; Desiderio, A.; Failloux, A.-B.; Calvitti, M. Combining Wolbachia-induced sterility and virus protection to fight Aedes albopictus-borne viruses. PLoS Negl. Trop. Dis. 2018, 12, e0006626. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Q.; She, L.; Yuan, H.; Luo, Y.; Wang, R.; Mao, W.; Wang, W.; She, Y.; Wang, C.; Shi, M.; et al. A standalone incompatible insect technique enables mosquito suppression in the urban subtropics. Commun. Biol. 2022, 5, 1419. [Google Scholar] [CrossRef] [PubMed]
- Mains, J.; Brelsfoard, C.; Rose, R.I.; Dobson, S.L. Female Adult Aedes albopictus Suppression by Wolbachia-Infected Male Mosquitoes. Sci. Rep. 2016, 6, 33846. [Google Scholar] [CrossRef] [PubMed]
- Crawford, J.E.; Clarke, D.W.; Criswell, V.; Desnoyer, M.; Cornel, D.; Deegan, B.; Gong, K.; Hopkins, K.C.; Howell, P.; Hyde, J.S.; et al. Efficient production of male Wolbachia-infected Aedes aegypti mosquitoes enables large-scale suppression of wild populations. Nat. Biotechnol. 2020, 38, 482–492. [Google Scholar] [CrossRef] [PubMed]
- Caputo, B.; Moretti, R.; Virgillito, C.; Manica, M.; Lampazzi, E.; Lombardi, G.; Serini, P.; Pichler, V.; Beebe, N.W.; della Torre, A.; et al. A bacterium against the tiger: Further evidence of the potential of non-inundative releases of males with manipulated Wolbachia infection in reducing fertility of Aedes albopictus field populations in Italy. Pest Manag. Sci. 2023, 79, 3167–3176. [Google Scholar] [CrossRef] [PubMed]
- Caputo, B.; Moretti, R.; Manica, M.; Serini, P.; Lampazzi, E.; Bonanni, M.; Fabbri, G.; Pichler, V.; Della Torre, A.; Calvitti, M. A bacterium against the tiger: Preliminary evidence of fertility reduction after release of Aedes albopictus males with manipulated Wolbachia infection in an Italian urban area. Pest Manag. Sci. 2020, 76, 1324–1332. [Google Scholar] [CrossRef]
- Moretti, R.; Marzo, G.A.; Lampazzi, E.; Calvitti, M. Cytoplasmic incompatibility management to support Incompatible Insect Technique against Aedes albopictus. Parasites Vectors 2018, 11 (Suppl. S2), 649. [Google Scholar] [CrossRef]
- Ong, J.; Ho, S.H.; Soh, S.X.H.; Wong, Y.; Ng, Y.; Vasquez, K.; Lai, Y.L.; Setoh, Y.X.; Chong, C.S.; Lee, V.; et al. Assessing the efficacy of male Wolbachia-infected mosquito deployments to reduce dengue incidence in Singapore: Study protocol for a cluster-randomized controlled trial. Trials 2022, 23, 1023. [Google Scholar] [CrossRef]
- Martín-Park, A.; Che-Mendoza, A.; Contreras-Perera, Y.; Pérez-Carrillo, S.; Puerta-Guardo, H.; Villegas-Chim, J.; Guillermo-May, G.; Medina-Barreiro, A.; Delfín-González, H.; Méndez-Vales, R.; et al. Pilot trial using mass field-releases of sterile males produced with the incompatible and sterile insect techniques as part of integrated Aedes aegypti control in Mexico. PLoS Negl. Trop. Dis. 2022, 16, e0010324. [Google Scholar] [CrossRef]
- Zheng, X.; Zhang, D.; Li, Y.; Yang, C.; Wu, Y.; Liang, X.; Liang, Y.; Pan, X.; Hu, L.; Sun, Q.; et al. Incompatible and sterile insect techniques combined eliminate mosquitoes. Nature 2019, 572, 56–61. [Google Scholar] [CrossRef]
- Hughes, G.L.; Rasgon, J.L. Transinfection: A method to investigate Wolbachia-host interactions and control arthropod-borne disease. Insect Mol. Biol. 2014, 23, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Calvitti, M.; Marini, F.; Desiderio, A.; Puggioli, A.; Moretti, R. Wolbachia density and cytoplasmic incompatibility in Aedes albopictus: Concerns with using artificial Wolbachia infection as a vector suppression tool. PLoS ONE 2015, 10, e0121813. [Google Scholar] [CrossRef]
- Balestrino, F.; Puggioli, A.; Carrieri, M.; Bouyer, J.; Bellini, R. Quality control methods for Aedes albopictus sterile male production. PLoS Negl. Trop. Dis. 2017, 11, e0005881. [Google Scholar] [CrossRef] [PubMed]
- Calkins, C.O.; Parker, A.G. Sterile insect quality. In Sterile Insect Technique; Dyck, V.A., Hendrichs, J., Robinson, A., Eds.; Springer: Dordrecht, The Netherlands, 2005; pp. 269–296. [Google Scholar]
- Puggioli, A.; Calvitti, M.; Moretti, R.; Bellini, R. wPip Wolbachia contribution to Aedes albopictus SIT performance: Advantages under intensive rearing. Acta Trop. 2016, 11, 649. [Google Scholar]
- Liang, X.; Tan, C.H.; Sun, Q.; Zhang, M.; Wong, P.S.J.; Li, M.I.; Mak, K.W.; Martín-Park, A.; Contreras-Perera, Y.; Puerta-Guardo, H.; et al. Wolbachia wAlbB remains stable in Aedes aegypti over 15 years but exhibits genetic background-dependent variation in virus blocking. PNAS Nexus 2022, 1, pgac203. [Google Scholar] [CrossRef] [PubMed]
- Correa, C.C.; Ballard, J.W.O. Wolbachia gonadal density in female and male Drosophila vary with laboratory adaptation and respond differently to physiological and environmental challenges. J. Invertebr. Pathol. 2012, 111, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Benedict, M.Q.; Knols, B.G.; Bossin, H.C.; Howell, P.I.; Mialhe, E.; Caceres, C.; Robinson, A.S. Colonisation and mass rearing: Learning from others. Malar. J. 2009, 8 (Suppl. S2), S4. [Google Scholar] [CrossRef]
- Garcia, G.A.; Sylvestre, G.; Aguiar, R.; da Costa, G.B.; Martins, A.J.; Lima, J.B.P.; Petersen, M.T.; Lourenço-de-Oliveira, R.; Shadbolt, M.F.; Rašić, G.; et al. Matching the genetics of released and local Aedes aegypti populations is critical to assure Wolbachia invasion. PLoS Negl. Trop. Dis. 2019, 13, e0007023. [Google Scholar] [CrossRef]
- Aranda, C.; Eritja, R.; Roiz, D. First record and establishment of the mosquito Aedes albopictus in Spain. Med. Vet. Entomol. 2006, 20, 150–152. [Google Scholar] [CrossRef]
- Mendoza García, M.; Laburu Dañobeitia, A. Annual Entomological Surveillance Report; Ministry of Health: Madrid, Spain, 2024; p. 74. Available online: http://www.mscbs.es/ciudadanos/saludAmbLaboral/agenBiologicos/pdfs/INFORME_ENTOMOLOGICO_2022_VII.pdf (accessed on 1 March 2024).
- Bueno-Marí, R.; Domínguez-Santos, R.; Trelis, M.; Garrote-Sánchez, E.; Cholvi, M.; Quero de Lera, F.; Khoubbane, M.; Marcilla, A.; Gil, R. Wolbachia pipientis infections in populations of Aedes albopictus in the city of València (Spain): Implications for mosquito control. Rev. Esp. Salud Pública 2023, 97, e202303017. [Google Scholar]
- Dobson, S.L.; Rattanadechakul, W. A novel technique for removing Wolbachia infections from Aedes albopictus. J. Med. Entomol. 2001, 38, 844–849. [Google Scholar] [CrossRef] [PubMed]
- Xi, Z.; Dean, J.L.; Khoo, C.; Dobson, S.L. Generation of a novel Wolbachia infection in Aedes albopictus (Asian Tiger mosquito) via embryonic microinjection. Insect Biochem. Mol. Biol. 2005, 35, 903–910. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Rousset, F.; O’Neil, S. Phylogeny and PCR-based classification of Wolbachia strains using wsp gene sequences. Proc. Biol. Sci. 1998, 265, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Lucati, F.; Delacour, S.; Palmer, J.R.; Caner, J.; Oltra, A.; Paredes-Esquivel, C.; Mariani, S.; Escartin, S.; Roiz, D.; Collantes, F.; et al. Multiple invasions, Wolbachia and human-aided transport drive the genetic variability of Aedes albopictus in the Iberian Peninsula. Sci. Rep. 2022, 12, 20682. [Google Scholar] [CrossRef] [PubMed]
- Larkin, M.A.; Blackshields, G.; Brown, N.P.; Chenna, R.; McGettigan, P.A.; McWilliam, H.; Valentin, F.; Wallace, I.M.; Wilm, A.; Lopez, R.; et al. Clustal W and Clustal X version 2.0. Bioinformatics 2007, 23, 2947–2948. [Google Scholar] [CrossRef]
- Blanco-Sierra, L.; Mariani, S.; Escartin, S.; Eritja, R.; Palmer, J.R.B.; Bartumeus, F. Drivers of longevity of wild-caught Aedes albopictus populations. Parasites Vectors 2023, 16, 328. [Google Scholar] [CrossRef] [PubMed]
- Hughes, G.L.; Dodson, B.L.; Johnson, R.M.; Murdock, C.C.; Tsujimoto, H.; Suzuki, Y.; Patt, A.A.; Cui, L.; Nossa, C.W.; Barry, R.M.; et al. Native microbiome impedes vertical transmission of Wolbachia in Anopheles mosquitoes. Proc. Natl. Acad. Sci. USA 2014, 111, 12498–12503. [Google Scholar] [CrossRef]
- Moretti, R.; Marini, F.; Lampazzi, E.; Calvitti, M. On the suitability of Aedes vexans to Wolbachia-based control strategies. Entomol. Exp. Appl. 2021, 169, 772–778. [Google Scholar] [CrossRef]
- Hoffmann, A.A.; Clancy, D.J.; Duncan, J. Naturally-occurring Wolbachia infection in Drosophila simulans that does not cause cytoplasmic incompatibility. Heredity 1996, 76, 1–8. [Google Scholar] [CrossRef]
- Ross, P.A.; Axford, J.K.; Callahan, A.G.; Richardson, K.M.; Hoffmann, A.A. Persistent deleterious effects of a deleterious Wolbachia infection. PLoS Negl. Trop. Dis. 2020, 14, e0008204. [Google Scholar] [CrossRef]
- Islam, M.S.; Dobson, S.L. Wolbachia effects on Aedes albopictus (Diptera: Culicidae) immature survivorship and development. J. Med. Entomol. 2006, 43, 689–695. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, K.T.; Thomson, L.J.; Hoffmann, A.A. The effects of host age, host nuclear background and temperature on phenotypic effects of the virulent Wolbachia strain popcorn in Drosophila melanogaster. Genetics 2003, 164, 1027–1034. [Google Scholar] [CrossRef] [PubMed]
- Brelsfoard, C.L.; Dobson, S.L. Wolbachia effects on host fitness and the influence of male aging on cytoplasmic incompatibility in Aedes polynesiensis (Diptera: Culicidae). J. Med. Entomol. 2011, 48, 1008–1015. [Google Scholar] [CrossRef] [PubMed]
- Sarwar, M.S.; Jahan, N.; Ali, A.; Yousaf, H.K.; Munzoor, I. Establishment of Wolbachia infection in Aedes aegypti from Pakistan via embryonic microinjection and semi-field evaluation of general fitness of resultant mosquito population. Parasites Vectors 2022, 15, 191. [Google Scholar] [CrossRef]
- Porter, J.; Sullivan, W. The cellular lives of Wolbachia. Nat. Rev. Microbiol. 2023, 21, 750–766. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.Z.; Sun, J.T.; Wang, L.T.; Shu, X.H.; Guo, Y.; Keiichiro, M.; Zhu, Y.X.; Bing, X.L.; Hoffmann, A.A.; Hong, X.Y. Recent infection by Wolbachia alters microbial communities in wild Laodelphax striatellus populations. Microbiome 2020, 8, 104. [Google Scholar] [CrossRef]
- Simões, P.M.; Mialdea, G.; Reiss, D.; Sagot, M.F.; Charlat, S. Wolbachia detection: An assessment of standard PCR protocols. Mol. Ecol. Resour. 2011, 11, 567–572. [Google Scholar] [CrossRef] [PubMed]
- Inácio da Silva, L.M.; Dezordi, F.Z.; Paiva, M.H.S.; Wallau, G.L. Systematic review of Wolbachia symbiont detection in mosquitoes: An entangled topic about methodological power and true symbiosis. Pathogens 2021, 10, 39. [Google Scholar] [CrossRef]
- Correa, C.C.; Ballard, J.W.O. Wolbachia associations with insects: Winning or losing against a master manipulator. Front. Ecol. Evol. 2016, 3, 153. [Google Scholar] [CrossRef]
- Weeks, A.R.; Turelli, M.; Harcombe, W.R.; Reynolds, K.T.; Hoffmann, A.A. From parasite to mutualist: Rapid evolution of Wolbachia in natural populations of Drosophila. PLoS Biol. 2007, 5, 997–1005. [Google Scholar] [CrossRef]
- Ross, P.A.; Hoffmann, A.A. Fitness costs of Wolbachia shift in locally-adapted Aedes aegypti mosquitoes. Environ. Microbiol. 2022, 24, 5749–5759. [Google Scholar] [CrossRef]
- Kambhampati, S.; Rai, K.S. Mitochondrial DNA variation within and among populations of the mosquito Aedes albopictus. Genome 1991, 34, 288–292. [Google Scholar] [CrossRef]
- Artigas, P.; Reguera-Gomez, M.; Valero, M.A.; Osca, D.; da Silva Pacheco, R.; Rosa-Freitas, M.G.; Fernandes Silva-do-Nascimento, T.; Paredes-Esquivel, C.; Lucientes, J.; Mas-Coma, S.; et al. Aedes albopictus diversity and relationships in south-western Europe and Brazil by rDNA/mtDNA and phenotypic analyses: ITS-2, a useful marker for spread studies. Parasites Vectors 2021, 14, 333. [Google Scholar] [CrossRef]
- Hoffmann, A.A.; Turelli, M. Facilitating Wolbachia introductions into mosquito populations through insecticide-resistance selection. Proc. Biol. Sci. 2013, 280, 20130371. [Google Scholar] [CrossRef]
- Popovici, J.; Moreira, L.A.; Poinsignon, A.; Iturbe-Ormaetxe, I.; McNaughton, D.; O’Neill, S.L. Assessing key safety concerns of a Wolbachia-based strategy to control dengue transmission by Aedes mosquitoes. Mem. Inst. Oswaldo Cruz 2010, 105, 957–964. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cholvi, M.; Trelis, M.; Bueno-Marí, R.; Khoubbane, M.; Gil, R.; Marcilla, A.; Moretti, R. Wolbachia Infection through Hybridization to Enhance an Incompatible Insect Technique-Based Suppression of Aedes albopictus in Eastern Spain. Insects 2024, 15, 206. https://doi.org/10.3390/insects15030206
Cholvi M, Trelis M, Bueno-Marí R, Khoubbane M, Gil R, Marcilla A, Moretti R. Wolbachia Infection through Hybridization to Enhance an Incompatible Insect Technique-Based Suppression of Aedes albopictus in Eastern Spain. Insects. 2024; 15(3):206. https://doi.org/10.3390/insects15030206
Chicago/Turabian StyleCholvi, Maria, María Trelis, Rubén Bueno-Marí, Messaoud Khoubbane, Rosario Gil, Antonio Marcilla, and Riccardo Moretti. 2024. "Wolbachia Infection through Hybridization to Enhance an Incompatible Insect Technique-Based Suppression of Aedes albopictus in Eastern Spain" Insects 15, no. 3: 206. https://doi.org/10.3390/insects15030206
APA StyleCholvi, M., Trelis, M., Bueno-Marí, R., Khoubbane, M., Gil, R., Marcilla, A., & Moretti, R. (2024). Wolbachia Infection through Hybridization to Enhance an Incompatible Insect Technique-Based Suppression of Aedes albopictus in Eastern Spain. Insects, 15(3), 206. https://doi.org/10.3390/insects15030206