DNA Barcoding Supports “Color-Pattern’’-Based Species of Stictochironomus from China (Diptera: Chironomidae)
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
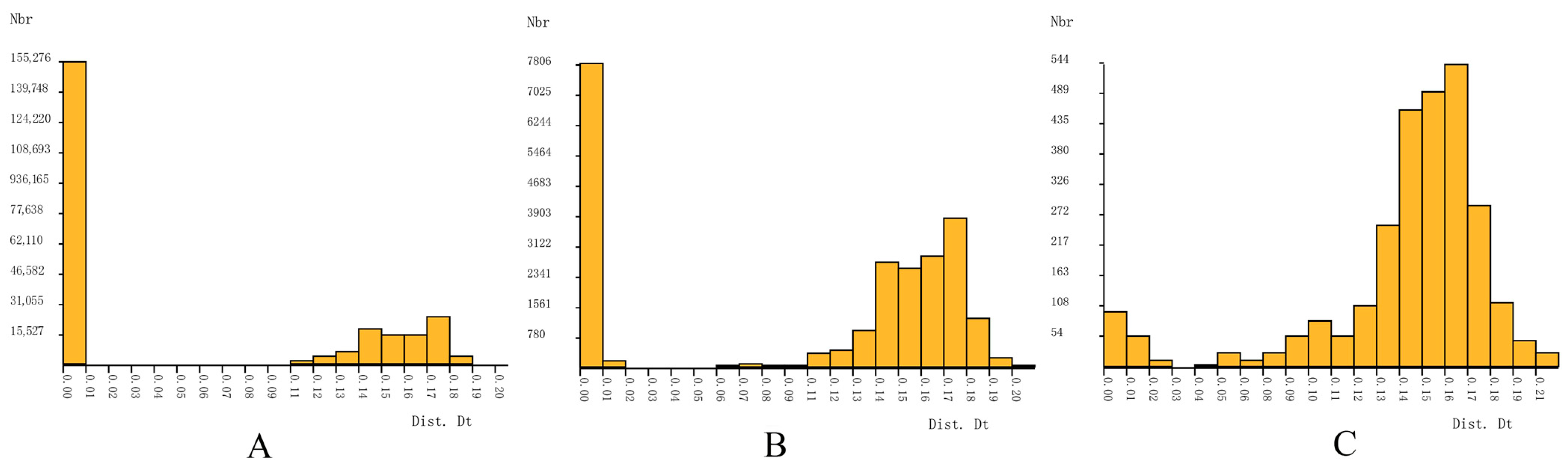
3.1. Barcode Analysis
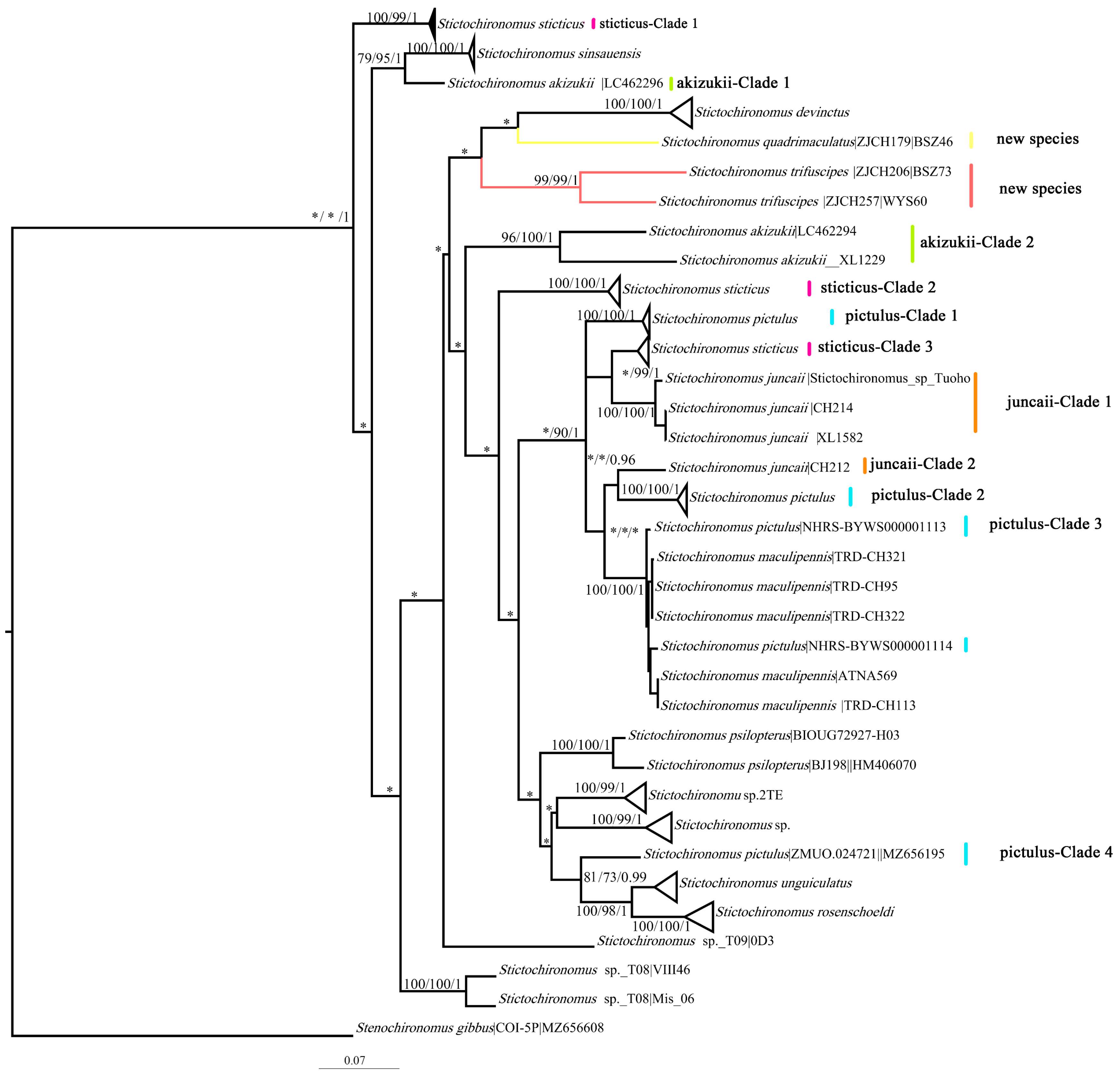
3.2. Phylogenetics and Species Delimitation
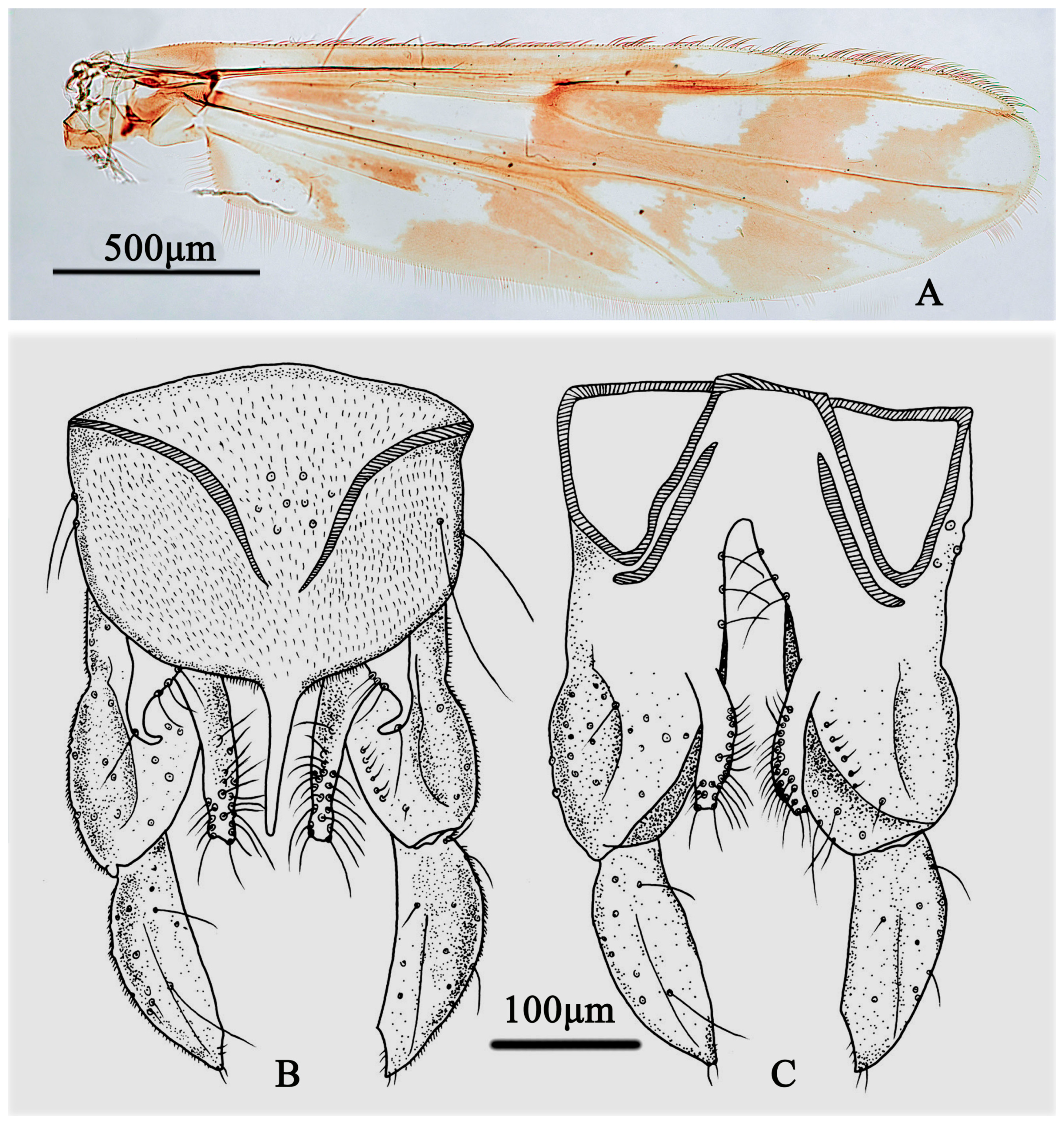
3.3. Taxonomy
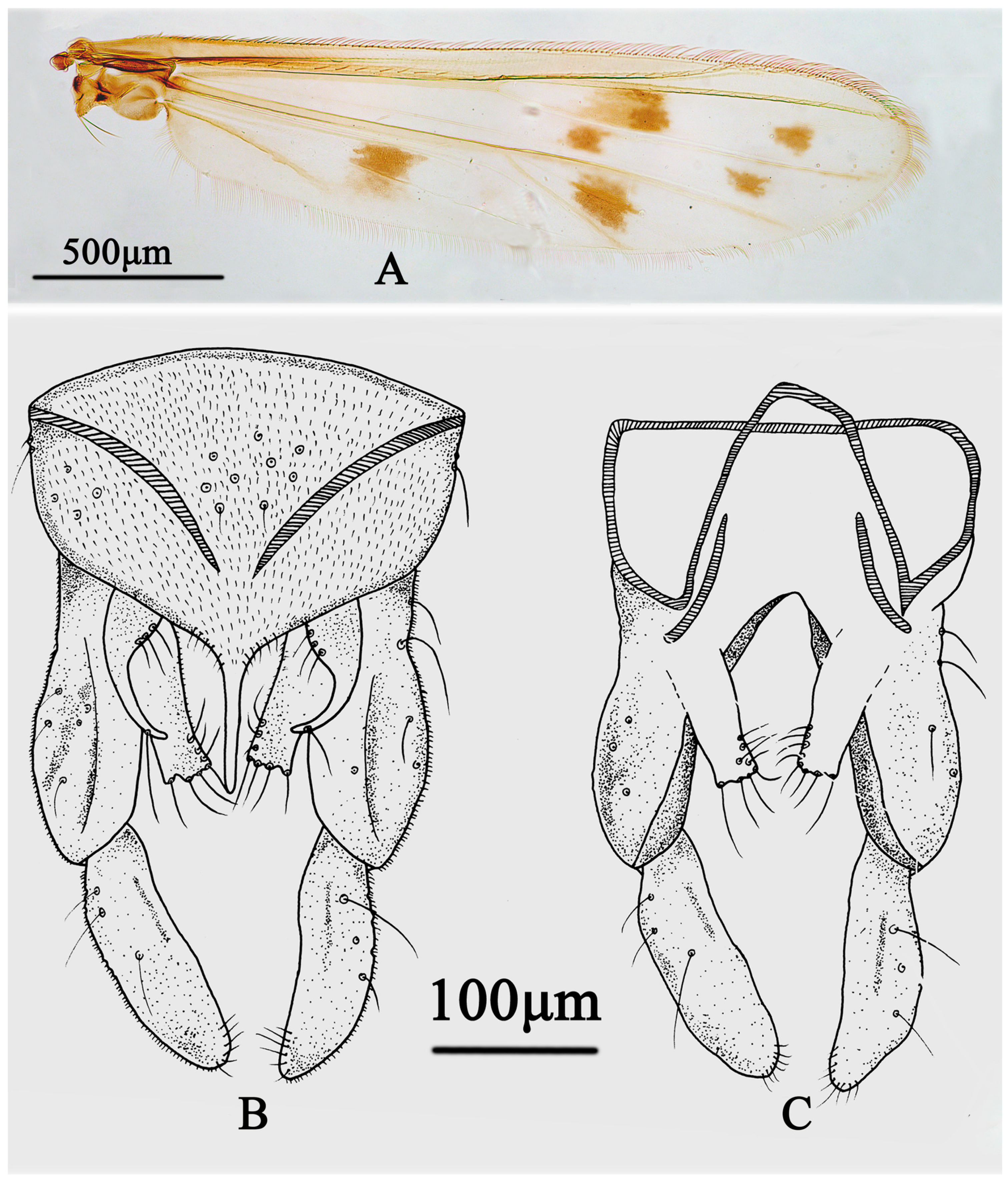
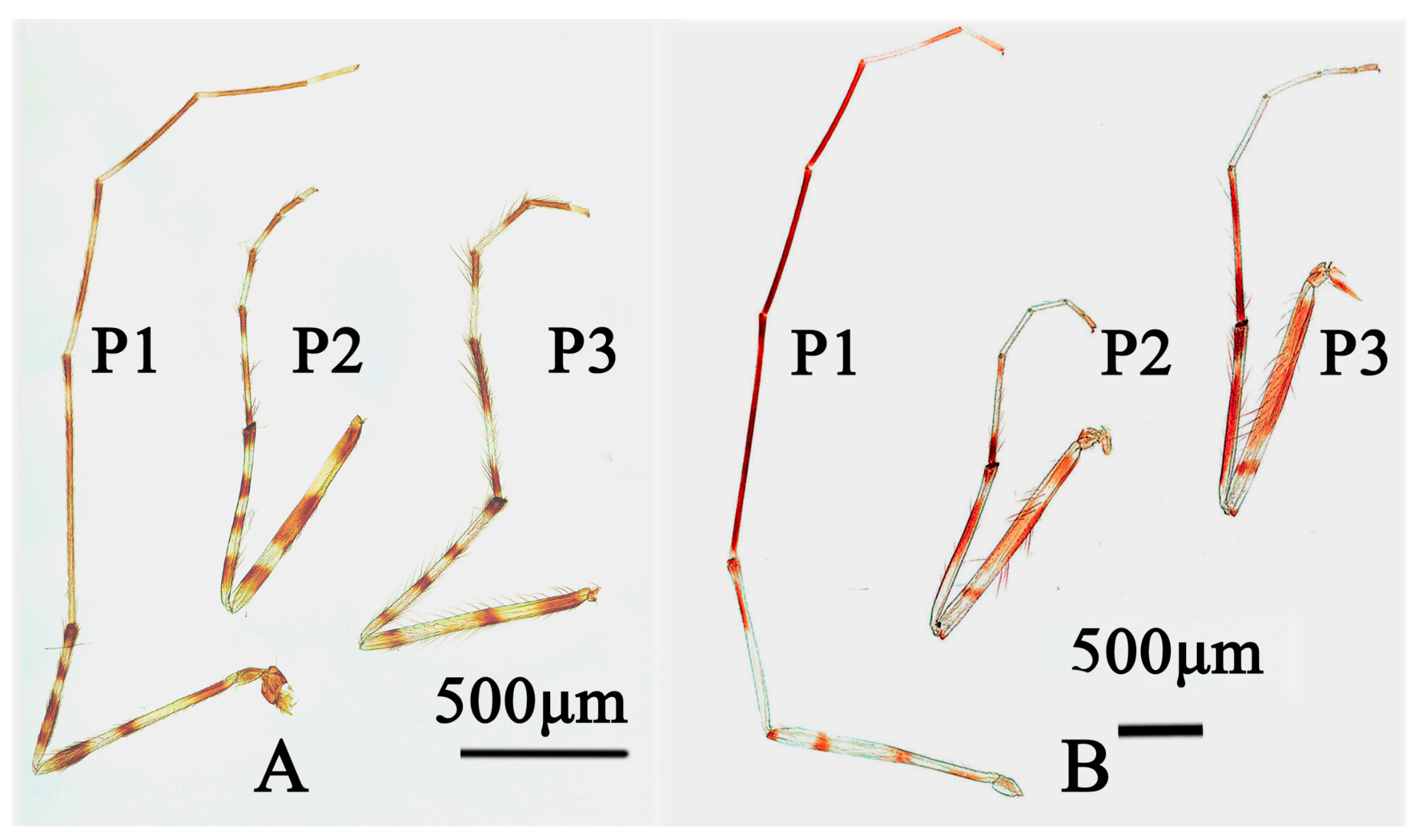
3.3.1. Stictochironomus quadrimaculatus Song and Qi, sp. nov.
3.3.2. Stictochironomus trifuscipes Song and Qi, sp. nov.
3.3.3. Stictochironomus akizukii (Tokunaga, 1940)
3.3.4. Stictochironomus juncaii Qi, Shi, and Wang, 2008
3.3.5. Stictochironomus multannulatus (Tokunaga, 1938)
3.3.6. Stictochironomus pictulus (Meigen, 1830)
3.3.7. Stictochironomus sticticus (Fabricius, 1781)
- 1.
- Wing with only one dark area around cross vein r-m··········································································································································································································································2
- -
- Wing membrane with several dark spots································································································································································································································································3
- 2.
- Superior volsella with six or more basal setae; gonostylus slender and cylindraceous, with nine short setae concentrating in the apex; thorax and abdomen brown (Figures 8 and 9 in [48])··································································································································································································S. sticticus (Fabricius)
- -
- Superior volsella with four basal setae; gonostylus with twelve long setae along inner margin; thorax yellow with scutum, postnotum and scutellum brown; the abdominal tergites I–IV pale yellow, with apical brown band, tergites V–IX brown (Figure 35 in [46])························································································································································································S. akizukii (Tokunaga)
- 3.
- Wing with smoky spots ··························································································································································································································································································4
- -
- Wing with more than four clearly separated spots················································································································································································································································5
- 4.
- Inferior volsella with 14 setae; dorsocentrals 10–15, acrostichals 14–20, scutellars 28; fore tabia mostly black, with 1 subapical pale ring (Figure 39 in [46]) ···············································································S. multannulatus (Tokunaga)
- -
- Inferior volsella with 18–20 setae; dorsocentrals 17–20, acrostichals 8–12, scutellars 10; fore tibia mostly pale, with anterior 2/5 brownish (Figure 5B)······································S. trifuscipes Song and Qi sp. nov.
- 5.
- Posterior area of wing without smoky spots························································································································································································································································6
- -
- Posterior area of wing somewhat smoky, with five spots; dorsocentrals 18, acrostichals 12, scutellars 25; inferior volsella with 20 setae; tibiae with three dark rings and two pale rings (Figure 1 in [18]) ··································································································································································································S. juncaii Qi, Shi, and Wang
- 6.
- Wing with six spots; dorsocentrals 7, scutellars 5; inferior volsella with 12 setae; fore femur largely pale with three dark ring, tibia with four dark rings (Figure 5A) ····················································································································································································································S. quadrimaculatus Song and Qi sp. nov.
- -
- Wing with four spots; dorsocentrals 18–22, acrostichals 25–18, scutellars 28–32; inferior volsella with 20–23 setae; fore femur largely brown with a short preapical ring, tibia with three dark rings (Figure A15 in [56]) ··································································································································································································S. pictulus (Meigen)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Ac | acrostichal |
| AR | antennal ratio: length of the 13th/length of flagellomeres 1–12 |
| BV | length of (femur + tibia + ta1)/length of (ta2 + ta3 + ta4 + ta5) |
| Cu | cubitus |
| Dc | dorsocentrals |
| Fe | femur |
| HR | hypopygium ratio: length of gonocoxite/length of gonostylus |
| HV | hypopygium value: total length/10× length of gonostylus |
| IV | inner verticals |
| Ivo | inferior volsella |
| LR | leg ratio; length of ta1/length of tibia |
| M | media |
| MCu | cross-vein between media and cubitus |
| P1, P2, P3 | fore leg, mid leg, hind leg |
| R | radius |
| RM | crossvein between radius and media |
| Scts | scutellars |
| SV | SV-ratio: (length of femur + tibia)/length of ta1 |
| Svo | superior volsella |
| Ta | tarsomere |
| Ti | tibia |
| VR | venarum ratio: length of Cu/length of M |
References
- Shevtsova, E.; Hansson, C.; Janzen, D.H.; Kjærandsen, J. Stable structural color patterns displayed on transparent insect wings. Proc. Natl. Acad. Sci. USA 2011, 108, 668–673. [Google Scholar] [CrossRef]
- Brunetti, C.R. The generation and diversification of butterfly eyespot color patterns. Curr. Biol. 2001, 11, 1578–1585. [Google Scholar] [CrossRef]
- True, J.R. Insect melanism: The molecules matter. Trends Ecol. Evol. 2003, 18, 640–647. [Google Scholar] [CrossRef]
- Witt, J.D.S.; Threloff, D.L.; Hebert, P.D.N. DNA barcoding reveals extraordinary cryptic diversity in an amphipod genus: Implications for desert spring conservation. Mol. Ecol. 2006, 15, 3073–3082. [Google Scholar] [CrossRef]
- Moura, C.J.; Harris, D.J.; Cunha, M.R.; Rogers, A.D. DNA barcoding reveals cryptic diversity in marine hydroids (Cnidaria, Hydrozoa) from coastal and deep-sea environments. Zool. Scr. 2008, 37, 93–108. [Google Scholar] [CrossRef]
- Farias, M.C.L.; Filho, P.C.; Pontes, A.I.; Jacobina, U.P. DNA barcode reveals high cryptic diversity in the commercially important Penaeini shrimps (Decapoda, Penaeidae). Org. Divers. Evol. 2023, 23, 857–869. [Google Scholar] [CrossRef]
- Hebert, P.D.; Cywinska, A.; Ball, S.L.; de Waard, J.R. Biological identifications through DNA barcodes. Mol. Ecol. Resour. 2003, 270, 313–321. [Google Scholar] [CrossRef]
- Janzen, D.H.; Burns, J.M.; Cong, Q.; Hallwachs, W.; Dapkey, T.; Manjunath, R.; Hajibabaei, M.; Hebert, P.D.N. Nuclear genomes distinguish cryptic species suggested by their DNA barcodes and ecology. Proc. Natl. Acad. Sci. USA 2017, 114, 8313–8318. [Google Scholar] [CrossRef]
- Qi, X.; Wang, X.H.; Andersen, T.; Lin, X.L. A new species of Manoa Fittkau (Diptera: Chironomidae), with DNA barcodes from Xianju National Park, Oriental China. Zootaxa 2017, 4231, 398–408. [Google Scholar] [CrossRef]
- Song, C.; Zhu, B.Q.; Liu, W.; Qi, X. On the genus Polypedilum, subgenus Collartomyia, with description of P. (Col.) baishanzuensis sp. nov. from Baishanzu Nature Reserve, China (Diptera, Chironomidae). Zookeys 2021, 1065, 1–12. [Google Scholar] [CrossRef]
- Song, C.; Zhu, B.Q.; Moubayed-Breil, J.; Lei, T.; Qi, X. Taxonomic study on the genus Stenochironomus Kieffer from the Baishanzu Nature Reserve, China (Diptera, Chironomidae). ZooKeys 2022, 1104, 93–113. [Google Scholar] [CrossRef]
- Song, C.; Wang, L.; Lei, T.; Qi, X. New color-patterned species of Microtendipes Kieffer, 1913 (Diptera: Chironomidae) and a deep intraspecific divergence of species by DNA barcodes. Insects 2023, 14, 227. [Google Scholar] [CrossRef]
- Cranston, P.S.; Martin, J. 26. Family Chironomidae. In Catalog of the Diptera of the Australasian and Oceanian Regions; Evenhuis, N.L., Ed.; Bishop Museum Press: Honolulu, HI, USA, 1989; pp. 252–274. [Google Scholar]
- Epler, J.H.; Ekrem, T.; Cranston, P.S. The larvae of Holarctic Chironominae (Diptera: Chironomidae)-Keys and diagnoses. In Chironomidae of the Holarctic Region-Keys and Diagnoses; Andersen, T., Cranston, P.S., Epler, J.H., Eds.; Insect Systematics and Evolution Supplement: Lund, Sweden, 2013; Volume 66, pp. 387–556. [Google Scholar]
- Hazra, N.; Niitsuma, H.; Chaudhuri, P.K. Checklist of Chironomid Midges (Diptera: Chironomidae) of the Oriental Region; Occasional Paper No. 376; Records of the Zoological Survey of India: Kolkata, India, 2016; 204p. [Google Scholar]
- Konar, S. A new member of the Stictochironomus caffrarius group from West Bengal, India, with emendation of generic diagnosis (Diptera, Chironomidae). Zootaxa 2021, 5072, 173–181. [Google Scholar] [CrossRef]
- Mukherjee, T.; Mukherjee, B.; Hazra, N. Revision of the Oriental species of Polypedilum Kieffer (Diptera: Chironomidae) with their phylogenetic relationship. Zootaxa 2020, 4820, 31–69. [Google Scholar] [CrossRef]
- Qi, X.; Shi, S.D.; Wang, X.H. A new species and two new record species of the genus Stictochironomus Kieffer (Diptera: Chironomidae) from China. Entomotaxonomia 2008, 30, 280–286. [Google Scholar]
- Frohlich, D.; Torres-Jerez, I.; Bedford, I.; Markham, P.; Brown, J. A phylogeographical analysis of the Bemisia tabaci species complex based on mitochondrial DNA markers. Mol. Ecol. 1999, 8, 1683–1691. [Google Scholar] [CrossRef]
- Folmer, O.; Black, M.; Hoeh, W.; Lutz, R.; Vrijenhoek, R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotech. 1994, 3, 294–299. [Google Scholar]
- Song, C.; Lin, X.L.; Wang, Q.; Wang, X.H. DNA barcodes successfully delimit morphospecies in a superdiverse insect genus. Zool. Scr. 2018, 47, 311–324. [Google Scholar] [CrossRef]
- Sæther, O.A. Some Nearctic Podonominae, Diamesinae, and Orthocladiinae (Diptera: Chironomidae). Bull. Fish. Res. Board Can. 1969, 170, 1–154. [Google Scholar]
- Sæther, O.A. Glossary of chironomid morphology terminology (Diptera: Chironomidae). Entomol. Scand. Suppl. 1980, 14, 1–51. [Google Scholar]
- Ratnasingham, S.; Hebert, P.D.N. BOLD: The Barcode of Life Data System (www.barcodinglife.org). Mol. Ecol. Notes 2007, 7, 355–364. [Google Scholar] [CrossRef]
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Lanfear, R.; Frandsen, P.B.; Wright, A.M.; Senfeld, T.; Calcott, B. PartitionFinder2: New methods for selecting partitioned models of evolution for molecular and morphological phylogenetic analyses. Mol. Biol. Evol. 2017, 34, 772–773. [Google Scholar]
- Silvestro, D.; Michalak, I. raxmlGUI: A graphical front-end for RAxML. Org. Divers. Evol. 2012, 12, 335–337. [Google Scholar] [CrossRef]
- Ronquist, F.; Teslenko, M.; van der Mark, P.; Ayres, D.L.; Darling, A.; Hohna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian Phylogenetic Inference and Model Choice Across a Large Model Space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef]
- Rambaut, A.; Drummond, A.J.; Xie, D.; Baele, G.; Suchard, M.A. Posterior Summarization in Bayesian Phylogenetics Using Tracer 1.7. Syst. Biol. 2018, 67, 901–904. [Google Scholar] [CrossRef]
- Puillandre, N.; Brouillet, S.; Achaz, G. ASAP: Assemble species by automatic partitioning. Mol. Ecol. Resour. 2021, 21, 609–620. [Google Scholar] [CrossRef]
- Zhang, J.; Kapli, R.; Pavlidis, P.; Stamatakis, A. A general species delimitation method with applications to phylogenetic placements. Bioinformatics 2013, 29, 2869–2876. [Google Scholar] [CrossRef]
- Fujisawa, T.; Barraclough, T.G. Delimiting species using single-locus data and the Generalized Mixed Yule Coalescent (GMYC) approach: A revised method and evaluation on simulated datasets. Syst. Biol. 2013, 62, 707–724. [Google Scholar] [CrossRef]
- Drummond, A.J.; Suchard, M.A.; Xie, D.; Rambaut, A. Bayesian Phylogenetics with BEAUti and the BEAST 1.7. Mol. Biol. Evol. 2012, 29, 1969–1973. [Google Scholar] [CrossRef]
- Ekrem, T.; Willassen, E.; Stur, E. A comprehensive DNA sequence library is essential for identification with DNA barcodes. Mol. Phylogenet. Evol. 2007, 43, 530–542. [Google Scholar] [CrossRef]
- Lin, X.L.; Stur, E.; Ekrem, T. Exploring genetic divergence in a species-rich insect genus using 2790 DNA barcodes. PLoS ONE 2015, 10, e0138993. [Google Scholar] [CrossRef]
- Silva, F.L.; Wiedenbrug, S. Integrating DNA barcodes and morphology for species delimitation in the Corynoneura group (Diptera: Chironomidae: Orthocladiinae). Bull. Entomol. Res. 2014, 104, 65–78. [Google Scholar] [CrossRef]
- Sinclair, C.S.; Gresens, S.E. Discrimination of Cricotopus species (Diptera: Chironomidae) by DNA barcoding. Bull. Entomol. Res. 2008, 98, 555–563. [Google Scholar] [CrossRef]
- Song, C.; Wang, Q.; Zhang, R.L.; Sun, B.J.; Wang, X.H. Exploring the utility of DNA barcoding in species delimitation of Polypedilum (Tripodura) non-biting midges (Diptera: Chironomidae). Zootaxa 2016, 4079, 534–550. [Google Scholar] [CrossRef]
- Reistad, I. A Review of Fennoscandian Stictochironomus (Diptera: Chironomidae), with the Description of a Species New to Science. Master’s Thesis, Norwegian University of Science and Technology, Trondheim, Norway, 2023. [Google Scholar]
- Ekrem, T.; Stur, E.; Hebert, P.D.N. Females do count: Documenting Chironomidae (Diptera) species diversity using DNA barcoding. Org. Divers. Evol. 2010, 10, 397–408. [Google Scholar] [CrossRef]
- Schmidt, S.; Schmid-Egger, C.; Morinière, J.; Haszprunar, G.; Hebert, P.D. DNA barcoding largely supports 250 years of classical taxonomy: Identifications for Central European bees (Hymenoptera, Apoidea partim). Mol. Ecol. Resour. 2018, 15, 985–1000. [Google Scholar] [CrossRef]
- Zahiri, R.; Lafontaine, J.D.; Schmidt, B.C.; Dewaard, J.R.; Zakharov, E.V.; Hebert, P.D. A transcontinental challenge–a test of DNA barcode performance for 1,541 species of Canadian Noctuoidea (Lepidoptera). PLoS ONE 2014, 9, e92797. [Google Scholar] [CrossRef]
- Knebelsberger, T.; Landi, M.; Neumann, H.; Kloppmann, M.; Sell, A.F.; Campbell, P.D.; Laakmann, S.; Raupach, M.J.; Carvalho, G.R.; Costa, F.O. A reliable DNA barcode reference library for the identification of the North European shelf fish fauna. Mol. Ecol. Resour. 2014, 14, 1060–1071. [Google Scholar] [CrossRef]
- Na, K.B.; Bae, Y.J. New Species of Stictochironomus, Tanytarsus and Conchapelopia (Diptera: Chironomidae) from Korea. Entomol. Res. Bull. 2010, 26, 33–39. [Google Scholar]
- Sasa, M. Studies on chironomid midges in lakes of the Nikko National Park. Pt. II. Taxonomical and morphological studies on the chironomid species collected from lakes in the Nikko National Park. Res. Rep. Natl. Inst. Environ. Stud. 1984, 70, 16–215. [Google Scholar]
- Tokunaga, M. Chironomidae from Japan (Diptera), XII. New or little-known Ceratopogonidae and Chironomidae. Philipp. J. Sci. 1940, 72, 255–311. [Google Scholar]
- Sasa, M. Studies on the chironomids collected from lakes in the Mount Fuji Area (Diptera, Chironomidae). Res. Rep. NIES 1985, 83, 101–160. [Google Scholar]
- Wang, X.H. A revised checklist of Chironomidae from China (Diptera). In Late 20th Century Research on Chironomidae. An Anthology from the 13th International Symposium on Chironomidae; Hoffrichter, O., Ed.; Shaker Verlag, Achen: Freiburg, Germany, 2000; pp. 629–652. [Google Scholar]
- Tokunaga, M. Chironomidae from Japan (Diptera), XII. New or little–known midges, with descriptions of the metamorphoses of several species. Philipp. J. Sci. 1938, 65, 313–379. [Google Scholar]
- Sasa, M. Studies on the Chironomid Midges (Diptera, Chironomidae) of Shou River; Environmental Pollution Research Center: Niigata-shi, Japan, 1989; pp. 26–110. [Google Scholar]
- Yamamoto, M. Systematic study on the genus Stictochironomus from Japan (Diptera, Chironomidae). In Proceedings of the XVI International Congress Entomology Abstract, Kyoto, Japan, 3–9 August 1980; p. 24. [Google Scholar]
- Orel, O.V. Fauna of non-biting midges of subfamily Chironominae (Diptera, Chironomidae) of the Russian Far East. Freshw. Life 2016, 2, 185–196. [Google Scholar]
- Meigen, J.W. Systematische Beschreibung der bekannten Europäischen Zweiflügeligen Insekten; Schulz Press: Hamm, Germany, 1830; pp. 1–401. [Google Scholar] [CrossRef]
- Pinder, L.C.V. A key to the adult males of the British Chironomidae (Diptera) the non-biting midges. Freshw. Biol. Assoc. Sci. Publ. 1978, 37, 140. [Google Scholar]
- Sasa, M.; Suzuk, H. Studies on Chironomid midges collected in Hokkaido and Northen Honshu. Trop. Med. 1998, 40, 9–43. [Google Scholar]
- Fabricius, J.C. Species Insectorum: Exhibentes Differentias Specificas, Synonyma Avctorvm, Loca Natalia, Metamorphosin Adiectis Observationibvs, Descriptionibvs; Hamburgi et Kilonii Impensis C. E. Bohnii, 1781; Volume 2, p. 407. Available online: https://www.biodiversitylibrary.org/bibliography/36509 (accessed on 3 March 2024).
- Kimura, M. A simple method for estimating evolutionary rate of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 1980, 16, 111–120. [Google Scholar] [CrossRef]


| Nucleotide Position | Conserved Sites (%) | Variable Sites (%) | Informative Sites (%) | Adenine (%) | Thymine (%) | Cytosine (%) | Guanine (%) |
|---|---|---|---|---|---|---|---|
| 1 | 42.83 | 18.20 | 15.91 | 28.65 | 26.23 | 18.07 | 27.05 |
| 2 | 52.35 | 3.00 | 0.49 | 13.14 | 43.57 | 26.76 | 16.52 |
| 3 | 4.82 | 78.80 | 83.60 | 39.82 | 42.36 | 13.79 | 4.57 |
| Total | 61.70 | 38.30 | 33.90 | 27.02 | 37.39 | 19.54 | 16.05 |
| No. | Species | Distance (%) | No. | Species | Distance (%) |
|---|---|---|---|---|---|
| 1 | Stictochironomus akizukii | 15.56 | 9 | Stictochironomus sinsauensis | 0.77 |
| 2 | Stictochironomus devinctus | 2.42 | 10 | Stictochironomus sp. 2TE | 2.03 |
| 3 | Stictochironomus juncai | 14.4 | 11 | Stictochironomus sp. INVSJ | 0.21 |
| 4 | Stictochironomus maculipennis | 1.24 | 12 | Stictochironomus sp. T08 | 4.78 |
| 5 | Stictochironomus pictulus | 14.16 | 13 | Stictochironomus sp. T09 | * |
| 6 | Stictochironomus psilopterus | 3.16 | 14 | Stictochironomus sticticus | 16.81 |
| 7 | Stictochironomus quadrimaculatus | * | 15 | Stictochironomus trifuscipes | 10.85 |
| 8 | Stictochironomus rosenschoeldi | 2.68 | 16 | Stictochironomus unguiculatus | 2.02 |
| Fe | Ti | Ta1 | Ta2 | Ta3 | |
|---|---|---|---|---|---|
| P1 | 1050 | 670 | 1260 | 825 | 600 |
| P2 | 1090 | 870 | 550 | 255 | 200 |
| P3 | 1100 | 950 | 755 | 370 | 290 |
| Ta4 | Ta5 | LR | BV | SV | |
| P1 | 515 | 255 | 1.88 | 1.36 | 1.37 |
| P2 | 125 | 75 | 0.63 | 3.83 | 3.56 |
| P3 | 200 | 90 | 0.79 | 2.95 | 2.72 |
| Species | Wing Length (mm) | Spots Numbers and Location in Cell | ||||
|---|---|---|---|---|---|---|
| r4+5 | m1+2 | m3+4 | cu | an | ||
| S. ha | 3.04 | 2 downside | 0 | 1 downside | 1 downside | 1 downside |
| S. quadrimaculatus sp. nov | 1.8 | 2 in the middle | 2 in the middle | 1 in the middle | 1 in the middle | Absent |
| Species | AR | Inferior volsella Setae Number | P1-LR | Leg Pigmentation | ||
| P1-Fe | P1-Ti | F-Ta1 | ||||
| S. ha | 2.48 | 25–26 | 1.12 | 1 subapical pale ring | With 2 dark rings | Mostly pale |
| S. quadrimaculatus sp. nov | 1.44 | 12 | 1.88 | 3 dark rings | With 4 dark rings | Mostly brown |
| Fe | Ti | ta1 | ta2 | ta3 | |
|---|---|---|---|---|---|
| P1 | 1240–1300 | 940–950 | 1330–1410 | 820–850 | 620–640 |
| P2 | 1290–1300 | 950–1000 | 600–650 | 290–325 | 205–235 |
| P3 | 1310–1410 | 1000–1090 | 850–950 | 430–460 | 325–345 |
| ta4 | ta5 | LR | BV | SV | |
| P1 | 520–590 | 255–260 | 1.41–1.48 | 1.56–1.59 | 1.55–1.68 |
| P2 | 130–140 | 125–130 | 0.63–0.65 | 3.76–3.97 | 3.53–3.73 |
| P3 | 205–210 | 200–210 | 0.85–0.87 | 2.92–2.95 | 2.62–2.72 |
| Species | Color of Fore-Leg | Ac | Dc | Scts | Svo Setae | Ivo Setae | ||
|---|---|---|---|---|---|---|---|---|
| Femur | Tibia | TaI-III | ||||||
| S. multannulatus | most black, 2 pale rings | most dark, 2 pale rings | mostly pale | 14–20 | 10–15 | 24–28 | 3 inner, 1 lateral | 14 |
| S. simantomaculatus | most pale, 3 dark rings | most pale, 2 dark rings | absent | 18–20 | 24–24 | 28–34 | 2 inner, 0 lateral | 16 |
| S. trifuscipes sp. nov | most pale, dark rings | most pale, 3 dark rings | dark brown | 8–12 | 17–20 | 10–10 | 3 inner, 1 lateral | 18–20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, C.; Chen, G.; Wang, L.; Lei, T.; Qi, X. DNA Barcoding Supports “Color-Pattern’’-Based Species of Stictochironomus from China (Diptera: Chironomidae). Insects 2024, 15, 179. https://doi.org/10.3390/insects15030179
Song C, Chen G, Wang L, Lei T, Qi X. DNA Barcoding Supports “Color-Pattern’’-Based Species of Stictochironomus from China (Diptera: Chironomidae). Insects. 2024; 15(3):179. https://doi.org/10.3390/insects15030179
Chicago/Turabian StyleSong, Chao, Guanyu Chen, Le Wang, Teng Lei, and Xin Qi. 2024. "DNA Barcoding Supports “Color-Pattern’’-Based Species of Stictochironomus from China (Diptera: Chironomidae)" Insects 15, no. 3: 179. https://doi.org/10.3390/insects15030179
APA StyleSong, C., Chen, G., Wang, L., Lei, T., & Qi, X. (2024). DNA Barcoding Supports “Color-Pattern’’-Based Species of Stictochironomus from China (Diptera: Chironomidae). Insects, 15(3), 179. https://doi.org/10.3390/insects15030179





