Critical Facets of European Corn Borer Adult Movement Ecology Relevant to Mitigating Field Resistance to Bt-Corn
Abstract
Simple Summary
Abstract
1. Introduction
1.1. European Corn Borer Voltinism and Pheromone Races
1.2. Pest Management
1.3. Insect Resistance Management
2. Field-Evolved Resistance to Bt-Corn in Canada
3. Adult European Corn Borer Movement and Dispersal
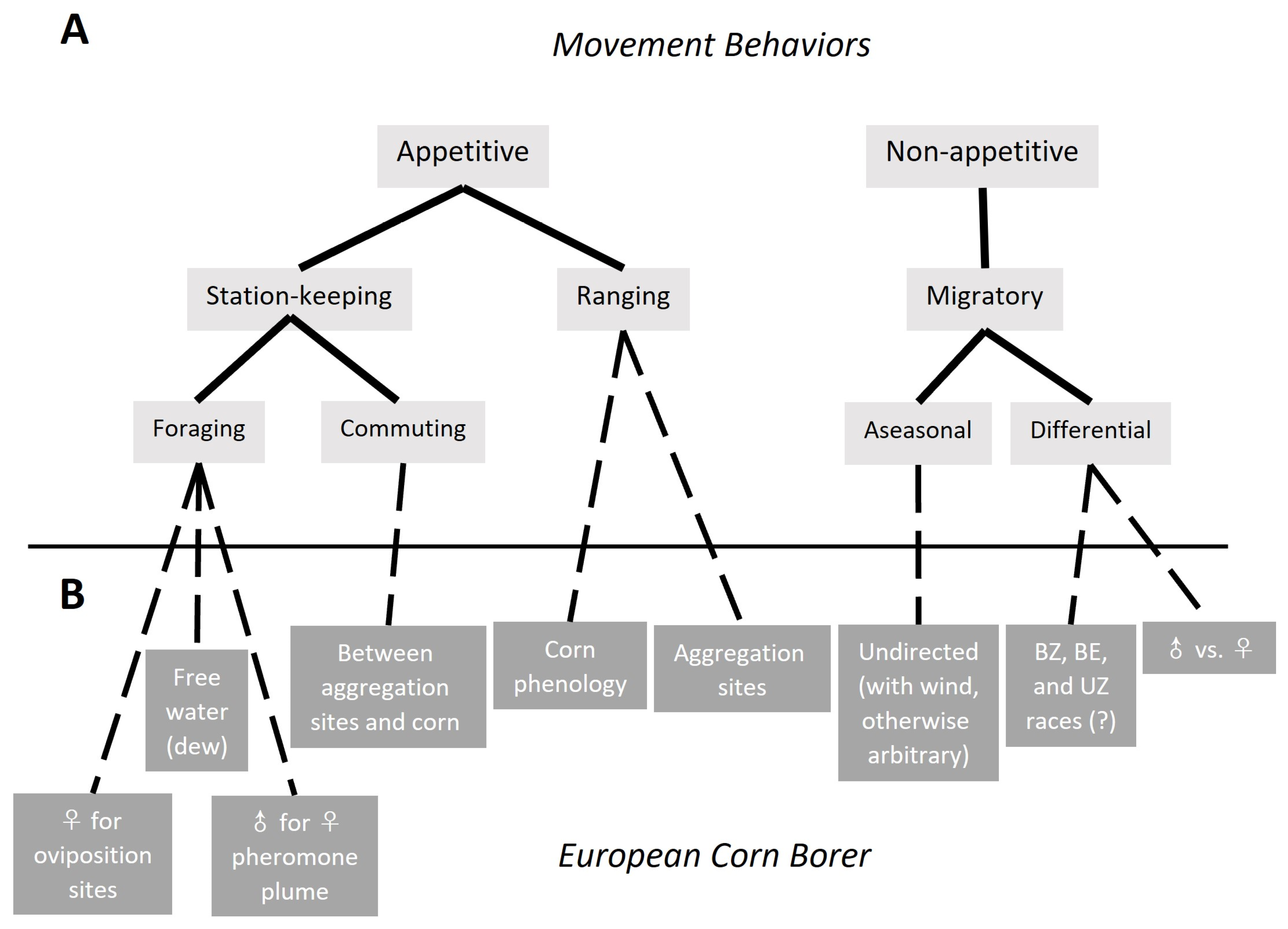
3.1. Types of Movement
3.1.1. Appetitive Flight: Station-Keeping and Ranging
3.1.2. Nonappetitive Flight: Migratory
3.2. Evidence for Migratory Flight by European Corn Borer
3.2.1. Range Expansion and Capture after Crossing Extensive Barriers
3.2.2. Mark-Release-Recapture
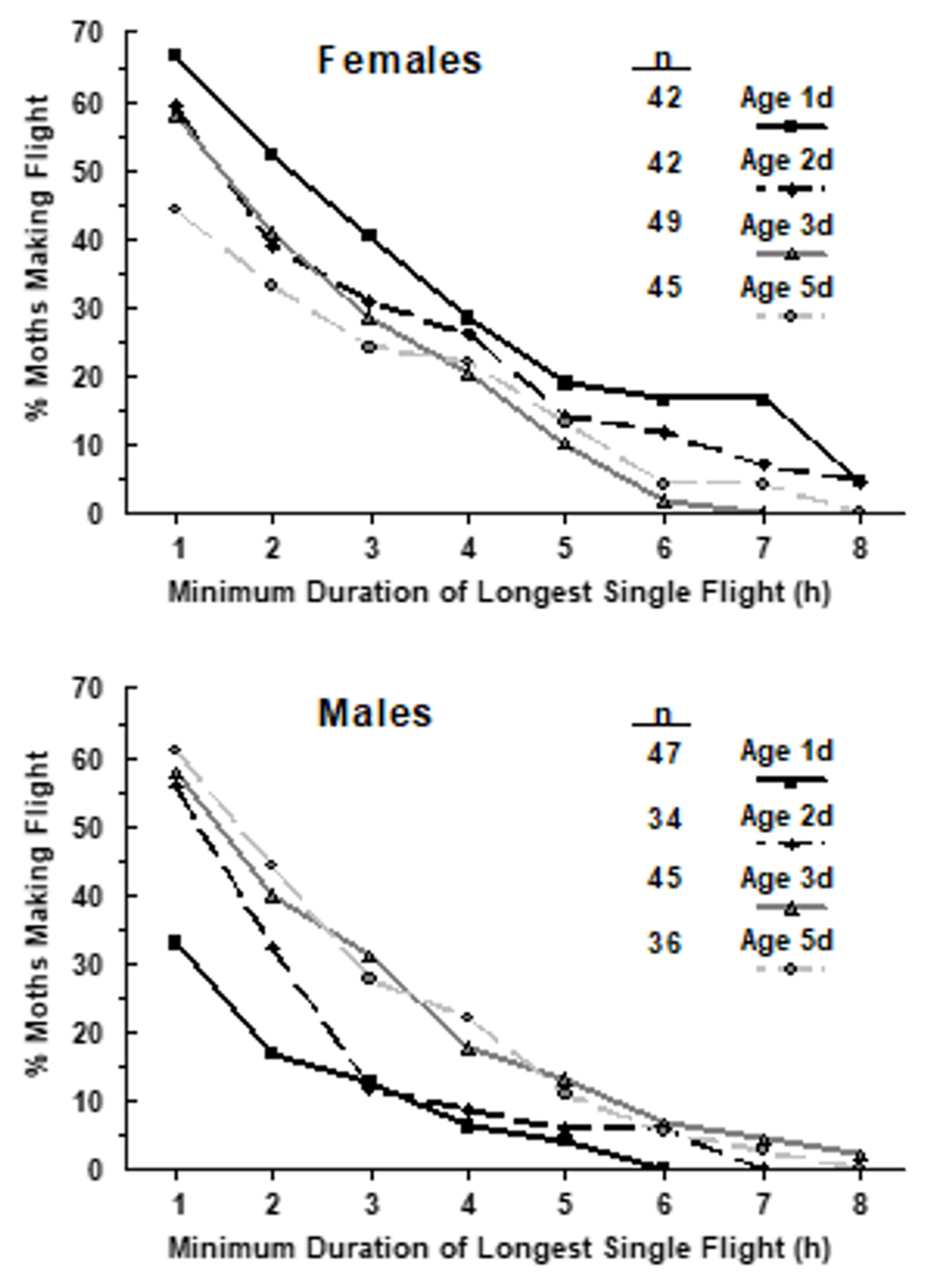
3.2.3. Flight Mill
3.2.4. Population Genetics and Gene Flow
| Pairwise Geographic Scale (km) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Region a | No. Sites | Min | Max | Genetic Marker System | Signif. IBD? | Overall FST or Pairwise FST Range b | % Signif. Pairwise FST’s | Notes | Citation |
| Eurasia: | Northern populations UZ, southern BZ. For temporal analyses: high stability of allele frequency distributions within each site over 4–6 generations. | Bourguet et al., 2000 [172] | |||||||
| France (Spatial) | (θ) | ||||||||
| Total | 29 | <1 | 863 | Allozyme | Yes | 0.011 * | 0 | ||
| Northeast | 11 | <1 | 286 | No | 0.015 * | ||||
| Northwest | 7 | 32 | 509 | Yes | 0.012 * | ||||
| South | 11 | <3 | 252 | No | 0.003 NS | ||||
| (Temporal) | |||||||||
| South | 6 | 0 | 64 | 0 (change in allele frequency) | |||||
| Eurasia: | (θ) | Bourguet et al., 2000 [177] | |||||||
| Northern France | 3 | 19 | 44 | Allozyme | No | 0.012 * | — | ||
| Eurasia: | (θ) | 6 and 3 samples for allozymes from Bourguet et al. [172] & [177], respectively. mtDNA samples from Bourguet et al. [172]. | Martel et al., 2003 [169] | ||||||
| Northern France | 9 | 19 | 503 | Allozyme | No | 0.009 * | — | ||
| 5 | mtDNA haplotype | — | 0.039 * | — | |||||
| North America: | 14 populations including BZ, UZ, & BE races. | Coates et al., 2004 [178] | |||||||
| USA: 8 states, ME to KS | 14 | 81 | 2639 | mtDNA haplotype | No | 0.024 * | 10.4 | ||
| BZ only | 10 | 81 | 2639 | — | — | 0 | |||
| Eurasia: | (θ) | Leniaud et al., 2006 [170] | |||||||
| France | 9 | 47 | 500 | Allozyme | No | 0 | |||
| Eurasia: | Malausa et al., 2007 [173] | ||||||||
| France | 14 | 3 | 768 | Microsat | No | 0–0.019 | 34.6 | ||
| Small scale, North | 7 | 3 | 16 | 0–0.014 | 10.0 | ||||
| North America: | Some sites may have included both UZ and BZ races. One site was far south of Corn Belt in LA. Significance of GST values was not reported. | Krumm et al., 2008 [171] | |||||||
| USA: 11 states, | (GST) | ||||||||
| western Corn Belt | 18 | 44 | 1900 | AFLP | No | 0.115 | — | ||
| North | 6 | 44 | 500 | No | 0.203 | ||||
| West | 6 | 108 | 303 | No | 0.171 | ||||
| South | 6 | 142 | 1028 | No | 0.153 | ||||
| North America: | Spatial samples along 2 transects (N-S, E-W). Temporal comparisons over 3 generations. Temporal maximum likelihood estimates of migration rate c (m) ranged from 0.04–0.54; geographic pattern of m reflected prevailing summer wind direction. | Kim et al., 2009 [94] | |||||||
| Central Corn Belt | |||||||||
| USA: 5 states, MN, IA, MO, NE, IL, (Spatial) | |||||||||
| Total | 13 | 16 | 720 | Microsat | No | 0–0.013 | 1.3 | ||
| Small scale, along | 6 | 16 | 80 | No | 0–0.007 | 0 | |||
| E-W transect, IA | |||||||||
| IA (Temporal) | |||||||||
| Between sites | 4 | 170 | 240 | — | 0–0.014 | 8.3 | |||
| Within sites | 4 | 0 | 0 | — | 0.001–0.002 | 0 | |||
| North America: | Estimated Wright’s genetic neighborhood radius as ~12 km. | Kim et al., 2011 [95] | |||||||
| USA: 8 states, New York to Colorado | 12 | 64 | 2180 | Microsat | Yes | 0–0.012 | 28.8 | ||
| Eurasia: | Frolov et al., 2012 [174] | ||||||||
| Russia | 6 | 71 | 679 | Microsat | — | 0–0.013 | 20.0 | ||
| Plus 7 France sites from [145] | 13 | 71 | 2741 | Yes | — | — | |||
| North America: | Samples from N-S transect of [90], 80-km intervals. | Levy et al., 2015 [33] | |||||||
| Central Corn Belt | |||||||||
| USA: 3 states, MN, IA, MO | 10 | 80 | 720 | SNP | NO | 0.002 NS | — | ||
| North America: | Coates et al., 2019 [44] | ||||||||
| Northeastern USA: 2 states, NY, PA: | |||||||||
| BZ race | 12 | 7.5 | 259 | SNP | — | 0–0.013 | 3.0 | ||
| Small scale (≤16 km) | 3 | 7.5 | 13.4 | — | 0–0.001 | 0 | |||
| BE race | 12 | 7.5 | 259 | — | 0–0.009 | 7.6 | |||
| Small scale (≤16 km) | 3 | 7.5 | 13.4 | — | 0–0.001 | 0 | |||
| BZ + BE (combined per location) | 12 | 7.5 | 259 | No | |||||
| Eurasia: | Li and Yang 2022 [179] | ||||||||
| Western China: Yili valley, Xinjiang | 3 | 53 | 204 | mtDNA haplotype | No | 0–0.100 | — | ||
| 3 | 53 | 204 | Nuclear haplotype | No | 0–0.057 | — | |||
3.2.5. Population Densities in a Cornfield Are Uncorrelated between Generations
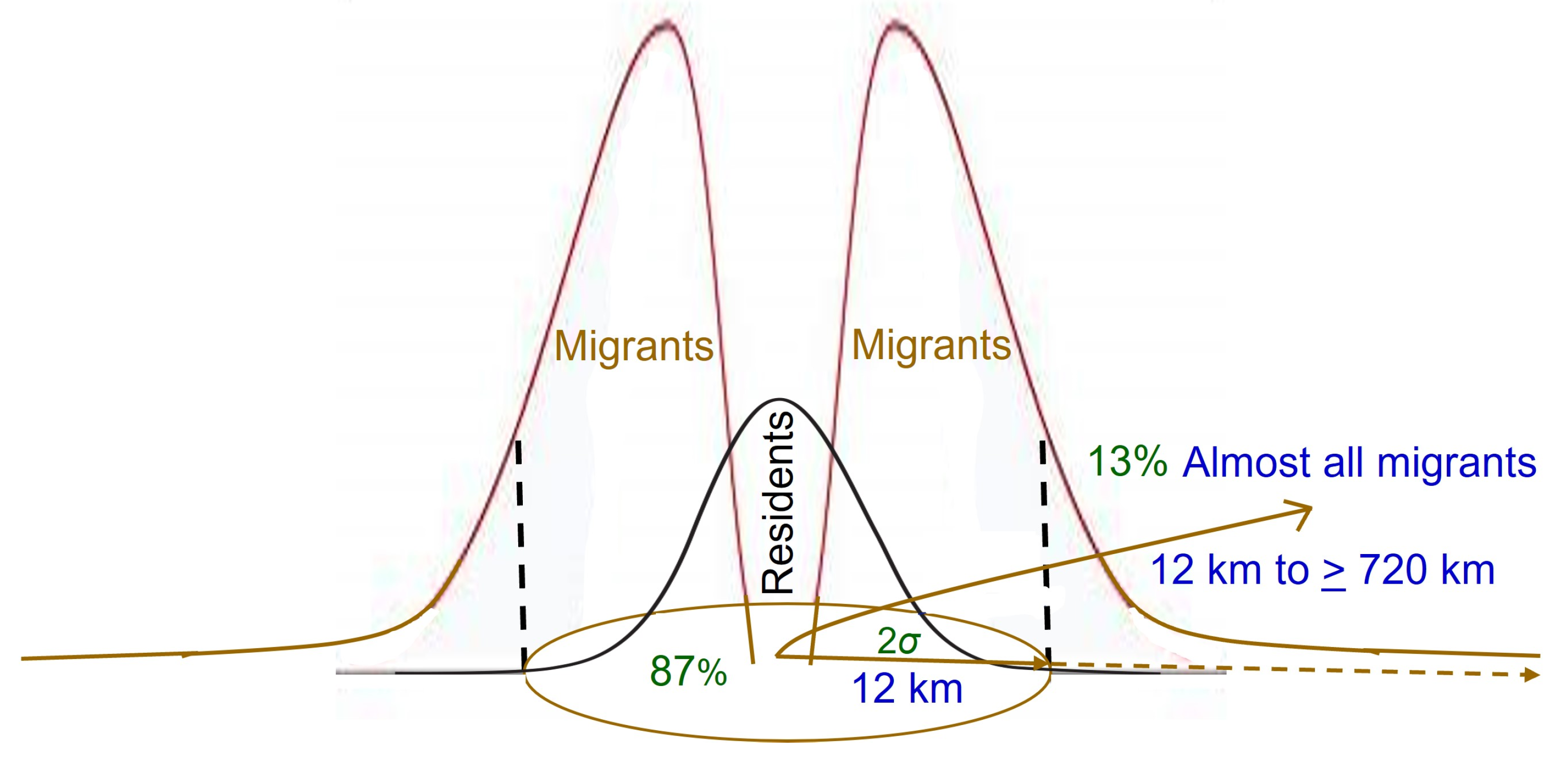
3.3. Proportion That Migrates
3.4. Timing of Mating Relative to Timing of Dispersal
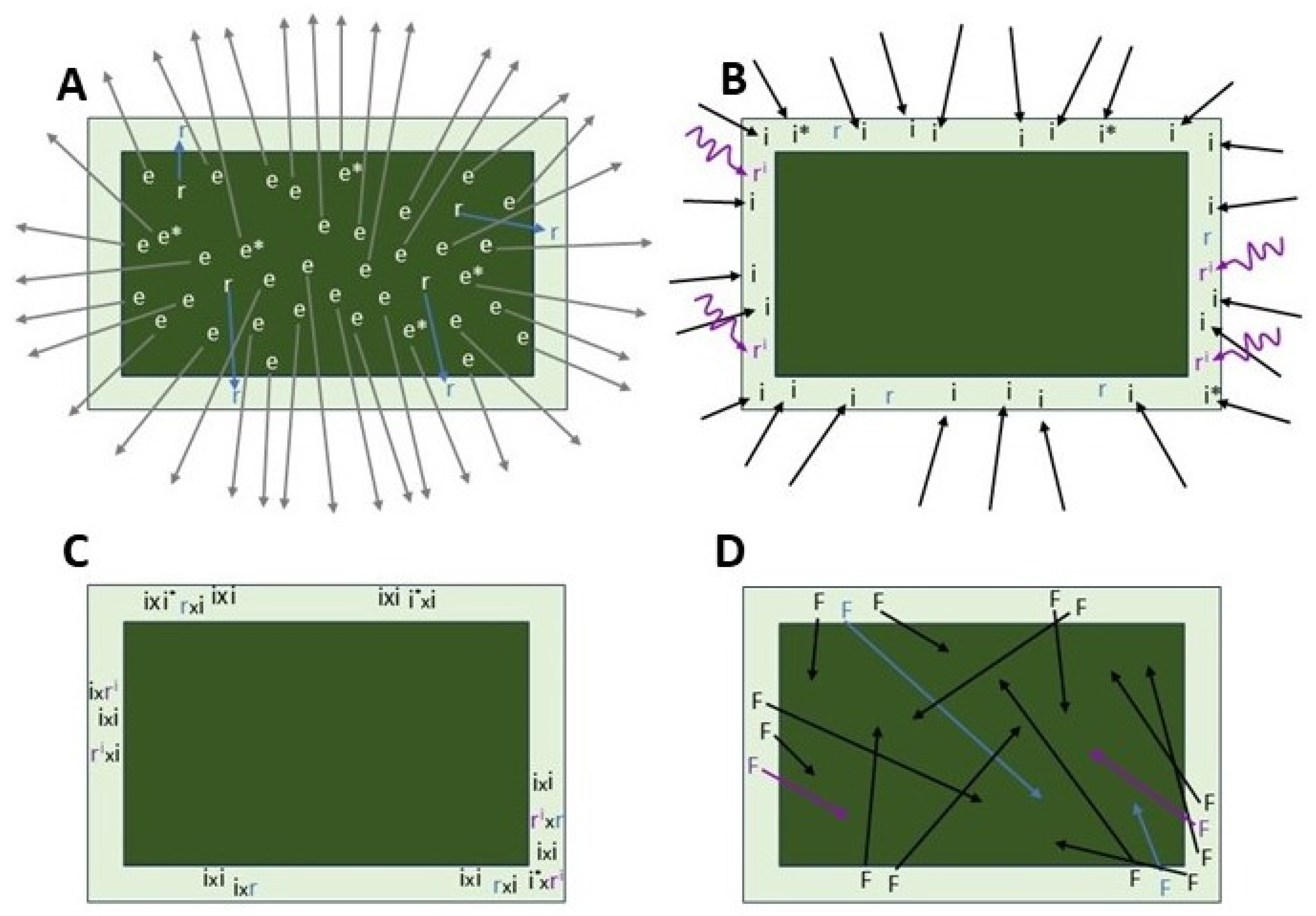
4. Conceptual Framework of European Corn Borer Adult Movement Ecology
5. Implications for Bt-Resistance Remediation and Mitigation
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Caffrey, D.; Worthley, L.A. Progress Report on the Investigations of the European Corn Borer; U.S. Department of Agriculture Bulletin, No. 1476; United States Government Printing Office: Washington, DC, USA, 1927. [CrossRef]
- Showers, W.B. Effect of diapause on the migration of the European corn borer into the southeastern United States. In Movement of Highly Mobile Insects: Concepts and Methodology in Research; Rabb, R.L., Kennedy, G.G., Eds.; University Graphics, North Carolina State University: Raleigh, NC, USA, 1979; pp. 420–430. [Google Scholar] [CrossRef]
- Mason, C.E.; Rice, M.E.; DiFonzo, C.D.; Porter, R.P.; Sappington, T.W.; Hunt, T.E.; Hellmich, R.L.; Bauté, T.S.; Andow, D.A.; Buntin, G.D.; et al. European Corn Borer Ecology, Management, and Its Interaction with Other Corn Pests; North Central Region Extension Publication No. NCR 327; Iowa State University: Ames, IA, USA, 2018. [Google Scholar]
- Hodgson, B.E. The Host Plants of the European Corn Borer in New England; U.S. Department of Agriculture Technical Bulletin, No. 77; United States Government Printing Office: Washington, DC, USA, 1928; Volume 77. [CrossRef]
- Anderson, T.E.; Kennedy, G.G.; Stinner, R.E. Distribution of the European corn borer, Ostrinia nubilalis (Hiibner), as related to oviposition preference of the spring colonizing generation in eastern North Carolina. Environ. Entomol. 1984, 13, 248–251. [Google Scholar] [CrossRef]
- Umeozor, O.C.; Bradley, J.R., Jr.; van Duyn, J.W.; Kennedy, G.G. Intercrop effects on the distribution of populations of the European corn borer, Ostrinia nubilalis, in maize. Entomol. Exp. Appl. 1986, 40, 293–296. [Google Scholar] [CrossRef]
- Eckenrode, C.J.; Webb, D.R. Establishment of various European corn borer (Lepidoptera: Pyralidae) races on selected cultivars of snap beans. J. Econ. Entomol. 1989, 82, 1169–1173. [Google Scholar] [CrossRef]
- Hitchner, E.M.; Ghidiu, G.M. Fruit size and infestation by European corn borer, Ostrinia nubilalis Hubner, in bell pepper. J. Vegetable Sci. 2006, 12, 101–107. [Google Scholar] [CrossRef]
- Chapman, A.V.; Kuhar, T.P.; Schultz, P.B.; Leslie, T.W.; Fleischer, S.J.; Dively, G.P.; Whalen, J. Integrating chemical and biological control of European corn borer in bell pepper. J. Econ. Entomol. 2009, 102, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Dively, G.P.; Venugopal, P.D.; Bean, D.; Whalen, J.; Holmstrom, K.; Kuhar, T.P.; Doughty, H.B.; Patton, T.; Cissel, W.; Hutchison, W.D. Regional pest suppression associated with widespread Bt maize adoption benefits vegetable growers. Proc. Natl. Acad. Sci. USA 2018, 115, 3320–3325. [Google Scholar] [CrossRef] [PubMed]
- Losey, J.E.; Calvin, D.D.; Carter, M.E.; Mason, C.E. Evaluation of noncorn host plants as a refuge in a resistance management program for European corn borer (Lepidoptera: Crambidae) on Bt-corn. Environ. Entomol. 2001, 30, 728–735. [Google Scholar] [CrossRef]
- Losey, J.E.; Carter, M.E.; Silverman, S.A. The effect of stem diameter on European corn borer behavior and survival: Potential consequences for IRM in Bt-corn. Entomol. Exp. Appl. 2002, 105, 89–96. [Google Scholar] [CrossRef]
- O’Rourke, M.E.; Sappington, T.W.; Fleischer, S.J. Managing resistance to Bt crops in a genetically variable insect herbivore, Ostrinia nubilalis. Ecol. Appl. 2010, 20, 1228–1236. [Google Scholar] [CrossRef]
- Lynch, R.E. European corn borer: Yield losses in relation to hybrid and stage of corn development. J. Econ. Entomol. 1980, 73, 159–164. [Google Scholar] [CrossRef]
- Bode, W.M.; Calvin, D.D. Yield-loss relationships and economic injury levels for European corn borer (Lepidoptera: Pyralidae) populations infesting Pennsylvania USA field corn. J. Econ. Entomol. 1990, 83, 1595–1603. [Google Scholar] [CrossRef]
- Mason, C.E.; Stromdahl, E.Y.; Pesek, J.D., Jr. Placement of pheromone traps within the vegetation canopy to enhance capture of male European corn borer (Lepidoptera: Pyralidae). J. Econ. Entomol. 1997, 90, 795–800. [Google Scholar] [CrossRef]
- Showers, W.B.; Reed, G.L.; Oloumi-Sadeghi, H. European com borer: Attraction of males to synthetic lure and to females of different strains. Environ. Entomol. 1974, 3, 51–58. [Google Scholar] [CrossRef]
- Showers, W.B.; Reed, G.L.; Robinson, J.F.; Derozari, M.B. Flight and sexual activity of the European corn borer. Environ. Entomol. 1976, 5, 1099–1104. [Google Scholar] [CrossRef]
- Showers, W.B.; Berry, E.C.; Kaster, L.V. Management of 2nd-generation European corn borer by controlling moths outside the cornfield. J. Econ. Entomol. 1980, 73, 88–91. [Google Scholar] [CrossRef]
- DeRozari, M.B.; Showers, W.B.; Shaw, R.H. Environment and the sexual activity of the European corn borer. Environ. Entomol. 1977, 6, 657–665. [Google Scholar] [CrossRef]
- Sappington, T.W.; Showers, W.B. Comparison of three sampling methods for monitoring adult European corn borer (Lepidoptera: Pyralidae) population trends. J. Econ. Entomol. 1983, 76, 1291–1297. [Google Scholar] [CrossRef]
- Sappington, T.W.; Showers, W.B. Adult European corn borer (Lepidoptera: Pyralidae) flight activity in and away from aggregation sites. Environ. Entomol. 1983, 12, 1154–1158. [Google Scholar] [CrossRef]
- Derrick, M.E.; Showers, W.B. Relationship of adult European corn borer (Lepidoptera: Pyralidae) in action sites with egg masses in the cornfield. Environ. Entomol. 1990, 19, 1081–1085. [Google Scholar] [CrossRef]
- Hellmich, R.L.; Pingel, R.L.; Hansen, W.R. Influencing European corn borer (Lepidoptera: Crambidae) aggregation sites in small grain crops. Environ. Entomol. 1998, 27, 253–259. [Google Scholar] [CrossRef][Green Version]
- Pleasants, J.M.; Bitzer, R.J. Aggregation sites for adult European corn borers (Lepidoptera: Crambidae): A comparison of prairie and non-native vegetation. Environ. Entomol. 1999, 28, 608–617. [Google Scholar] [CrossRef]
- Sappington, T.W. First-flight adult European corn borer (Lepidoptera: Crambidae) distribution in roadside vegetation relative to cropping patterns and corn phenology. Environ. Entomol. 2005, 34, 1541–1548. [Google Scholar] [CrossRef]
- Dalecky, A.; Ponsard, S.; Bailey, R.I.; Pélissier, C.; Bourguet, D. Resistance evolution to Bt crops: Predispersal mating of European corn borers. PLoS Biol. 2006, 4, 1048–1057. [Google Scholar] [CrossRef]
- Bailey, R.I.; Bourguet, D.; le Pallec, A.H.; Ponsard, S. Dispersal propensity and settling preferences of European corn borers in maize field borders. J. Appl. Ecol. 2007, 44, 385–394. [Google Scholar] [CrossRef]
- Lee, D.A. Moth density and oviposition pattern of the European corn borer, Ostrinia nubilalis (Lepidoptera: Pyralidae), in Alberta. Environ. Entomol. 1988, 17, 220–224. [Google Scholar] [CrossRef]
- Hunt, T.E.; Higley, L.G.; Witkowski, J.F.; Young, L.J.; Hellmich, R.L. Dispersal of adult European corn borer (Lepidoptera: Crambidae) within and proximal to irrigated and non-irrigated corn. J. Econ. Entomol. 2001, 94, 1369–1377. [Google Scholar] [CrossRef]
- Qureshi, J.A.; Buschman, L.L.; Throne, J.E.; Ramaswamy, S.B. Adult dispersal of Ostrinia nubilalis Hübner (Lepidoptera: Crambidae) and its implications for resistance management in Bt-maize. J. Appl. Entomol. 2005, 129, 281–292. [Google Scholar] [CrossRef]
- Merrill, S.C.; Walter, S.M.; Peairs, F.B.; Schleip, E.M. The distribution of European corn borer (Lepidoptera: Crambidae) moths in pivot-irrigated corn. J. Econ. Entomol. 2013, 106, 2084–2092. [Google Scholar] [CrossRef] [PubMed]
- Levy, R.C.; Kozak, G.M.; Wadsworth, C.B.; Coates, B.S.; Dopman, E.B. Explaining the sawtooth: Latitudinal periodicity in a circadian gene correlates with shifts in generation number. J. Evol. Biol. 2015, 28, 40–53. [Google Scholar] [CrossRef] [PubMed]
- Eckenrode, C.J.; Robbins, P.S.; Andaloro, J.T. Variations in flight patterns of European corn borer (Lepidoptera: Pyralidae) in New York. Environ. Entomol. 1983, 12, 393–396. [Google Scholar] [CrossRef]
- Dopman, E.B.; Robbins, P.S.; Seaman, A. Components of reproductive isolation between North American pheromone strains of the European corn borer. Evolution 2010, 64, 881–902. [Google Scholar] [CrossRef]
- Levy, R.C.; Kozak, G.M.; Dopman, E.B. Non-pleiotropic coupling of daily and seasonal temporal isolation in the European corn borer. Genes 2018, 9, 180. [Google Scholar] [CrossRef]
- Wadsworth, C.B.; Okada, Y.; Dopman, E.B. Phenology-dependent cold exposure and thermal performance of Ostrinia nubilalis ecotypes. BMC Evol. Biol. 2020, 20, 34. [Google Scholar] [CrossRef]
- Glover, T.J.; Robbins, P.S.; Eckenrode, C.J.; Roelofs, W.L. Genetic control of voltinism characteristics in European corn borer races assessed with a marker gene. Arch. Insect Biochem. Physiol. 1992, 20, 107–117. [Google Scholar] [CrossRef]
- Catangui, M.A. Transgenic Bacillus thuringiensis corn hybrid performance against univoltine ecotype European corn borer (Lepidoptera: Crambidae) in South Dakota. J. Econ. Entomol. 2003, 96, 957–968. [Google Scholar] [CrossRef]
- Klun, J.A.; Chapman, O.L.; Mattes, K.C.; Wojthkowski, P.W.; Beroza, P.W.; Sonnet, P.E. Insect pheromones: Minor amount of opposite geometrical isomer critical to attraction. Science 1973, 181, 661–663. [Google Scholar] [CrossRef]
- Kochansky, J.; Cardé, R.T.; Liebherr, J.; Roelofs, W.L. Sex pheromone of the European com borer, Ostrinia nubilalis, in New York. J. Chem. Ecol. 1975, 1, 225–231. [Google Scholar] [CrossRef]
- Coates, B.S.; Johnson, H.; Kim, K.S.; Hellmich, R.L.; Abel, C.A.; Mason, C.; Sappington, T.W. Frequency of hybridization between Ostrinia nubilalis E- and Z-pheromone races in regions of sympatry within the United States. Ecol. Evol. 2013, 3, 2459–2470. [Google Scholar] [CrossRef] [PubMed]
- Coates, B.S.; Dopman, E.B.; Wanner, K.W.; Sappington, T.W. Genomic mechanisms of sympatric ecological and sexual divergence in a model agricultural pest, the European corn borer. Curr. Opin. Insect Sci. 2018, 26, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Coates, B.S.; Kozak, G.M.; Kim, K.S.; Sun, J.; Wang, Y.; Fleischer, S.J.; Dopman, E.B.; Sappington, T.W. Influence of host plant, geography and pheromone strain on genomic differentiation in sympatric populations of Ostrinia nubilalis. Mol. Ecol. 2019, 28, 4439–4452. [Google Scholar] [CrossRef] [PubMed]
- Roelofs, W.L.; Du, J.W.; Tang, X.H.; Robbins, P.S.; Eckenrode, C.J. Three European corn borer populations in New York based on sex pheromones and voltinism. J. Chem. Ecol. 1985, 11, 829–839. [Google Scholar] [CrossRef] [PubMed]
- Wadsworth, C.B.; Li, X.; Dopman, E.B. A recombination suppressor contributes to ecological speciation in Ostrinia moths. Heredity 2015, 11, 593–600. [Google Scholar] [CrossRef] [PubMed]
- Kozak, G.M.; Wadsworth, C.B.; Kahne, S.C.; Bogdanowicz, S.M.; Harrison, R.G.; Coates, B.S.; Dopman, E.B. A combination of sexual and ecological divergence contributes to rearrangement spread during initial stages of speciation. Mol. Ecol. 2017, 26, 2331–2347. [Google Scholar] [CrossRef] [PubMed]
- Kozak, G.M.; Wadsworth, C.B.; Kahne, S.C.; Bogdanowicz, S.M.; Harrison, R.G.; Coates, B.S.; Dopman, E.B. Genomic basis of circannual rhythm in the European corn borer moth. Curr. Biol. 2019, 29, 3501–3509. [Google Scholar] [CrossRef] [PubMed]
- Kunerth, H.D.; Bogdanowicz, S.M.; Searle, J.B.; Harrison, R.G.; Coates, B.S.; Kozak, G.M.; Dopman, E.B. Consequences of coupled barriers to gene flow for the build-up of genomic differentiation. Evolution 2022, 76, 985–1002. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.L.; Farhan, Y.; Schaafsma, A.W. Practical resistance of Ostrinia nubilalis (Lepidoptera: Crambidae) to Cry1F Bacillus thuringiensis maize discovered in Nova Scotia, Canada. Sci. Rep. 2019, 9, 18247. [Google Scholar] [CrossRef] [PubMed]
- Rice, M.E.; Pilcher, C.D. Potential benefits and limitations of transgenic Bt corn for management of the European corn borer (Lepidoptera: Crambidae). Am. Entomol. 1998, 44, 75–78. [Google Scholar] [CrossRef]
- Hutchison, W.D.; Burkness, E.C.; Mitchell, P.D.; Moon, R.D.; Leslie, T.W.; Fleischer, S.J.; Abrahamsom, M.; Hamilton, K.L.; Steffey, K.L.; Gray, M.E.; et al. Areawide suppression of European corn borer with Bt maize reaps savings to non-Bt maize growers. Science 2010, 330, 222–225. [Google Scholar] [CrossRef]
- Bell, J.R.; Burkness, E.C.; Milne, A.E.; Onstad, D.W.; Abrahamson, M.; Hamilton, K.L.; Hutchison, W.D. Putting the brakes on a cycle: Bottom-up effects damp cycle amplitude. Ecol. Lett. 2012, 15, 310–318. [Google Scholar] [CrossRef]
- Milne, A.E.; Bell, J.R.; Hutchison, W.D.; van den Bosch, F.; Mitchell, P.D.; Crowder, D.; Parnell, S.; Whitmore, A.P. The effect of farmers’ decisions on pest control with Bt crops: A billion dollar game of strategy. PLoS Comput. Biol. 2015, 11, e1004483. [Google Scholar] [CrossRef]
- Dean, A.N.; Hodgson, E.W.; Rieck-Hinz, A.; Anderson, M. Needs assessment for corn insect pest management in Iowa. J. Integr. Pest Manag. 2021, 12, 27. [Google Scholar] [CrossRef]
- Siegfried, B.D.; Spencer, T.; Crespo, A.L.; Storer, N.P.; Head, G.P.; Owens, E.D.; Guyer, D. Ten years of Bt resistance monitoring in the European corn borer: What we know, what we don’t know, and what we can do better. Am. Entomol. 2007, 53, 208–214. [Google Scholar] [CrossRef]
- Kaçar, G.; Butrón, A.; Kontoglannatos, D.; Han, P.; Peñaflor, M.F.G.V.; Farinós, G.P.; Huang, F.; Hutchison, W.D.; de Souza, B.H.S.; Malvar, R.A.; et al. Recent trends in management strategies for two major maize borers: Ostrinia nubilalis and Sesamia nonagrioides. J. Pest Sci. 2023, 96, 879–901. [Google Scholar] [CrossRef]
- USEPA. Biopesticides Registration Action Document: Bacillus thuringiensis (Bt) Plant-Incorporated Protectants. 2001. Available online: https://www3.epa.gov/pesticides/chem_search/reg_actions/pip/bt_brad.htm (accessed on 2 February 2024).
- CFIA. DD2002-41: Determination of the Safety of Dow AgroSciences Canada Inc. and Pioneer Hi-Bred International’s Insect Resistant and Glufosinate–Ammonium Tolerant Corn (Zea mays L.) Line 1507. Canadian Food Inspection Agency. 2002. Available online: http://www.inspection.gc.ca/plants/plants-withnovel-traits/approved-under-review/decision-documents/dd2002-41/eng/1311872961735/1311873078751 (accessed on 2 February 2024).
- Siegfried, B.D.; Hellmich, R.L. Understanding successful resistance management. GM Crops Food 2012, 3, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Alstad, D.N.; Andow, D.A. Managing the evolution of insect resistance to transgenic plants. Science 1995, 268, 1894–1896. [Google Scholar] [CrossRef]
- Glaser, J.A.; Matten, S.R. Sustainability of insect resistance management strategies for transgenic Bt corn. Biotechnol. Adv. 2003, 22, 45–69. [Google Scholar] [CrossRef]
- Bourguet, D. Resistance to Bacillus thuringiensis toxins in the European corn borer: What chance for Bt maize? Physiol. Entomol. 2004, 29, 251–256. [Google Scholar] [CrossRef]
- Bates, S.L.; Zhao, J.-Z.; Roush, R.T.; Shelton, A.M. Insect resistance management in GM crops: Past, present and future. Nat. Biotechnol. 2005, 23, 57–62. [Google Scholar] [CrossRef]
- Bourguet, D.; Desquilbet, M.; Lemarié, S. Regulating insect resistance management: The case of non-Bt corn refuges in the US. J. Environ. Manag. 2005, 76, 210–220. [Google Scholar] [CrossRef]
- Huang, F.; Andow, D.A.; Buschman, L.L. Success of the high-dose/refuge resistance management strategy after 15 years of Bt crop use in North America. Entom. Exp. App. 2011, 140, 1–16. [Google Scholar] [CrossRef]
- Tabashnik, B.E.; Carrière, Y. Surge in insect resistance to transgenic crops and prospects for sustainability. Nat. Biotechnol. 2017, 35, 926–935. [Google Scholar] [CrossRef]
- Onstad, D.W.; Mitchell, P.D.; Hurley, T.M.; Lundgren, J.G.; Porter, R.P.; Krupke, C.H.; Spencer, J.L.; DiFonzo, C.D.; Baute, T.S.; Hellmich, R.L.; et al. Seeds of change: Corn seed mixtures for resistance management and IPM. J. Econ. Entomol. 2011, 104, 343–352. [Google Scholar] [CrossRef]
- Head, G.P.; Greenplate, J. The design and implementation of insect resistance management programs for Bt crops. GM Crops Food 2012, 33, 144–153. [Google Scholar] [CrossRef]
- USEPA. Subpanel on Bacillus thuringiensis (Bt) Plant-Pesticides and Resistance Management. 1998. Available online: https://archive.epa.gov/scipoly/sap/meetings/web/pdf/finalfeb.pdf (accessed on 2 February 2024).
- Tabashnik, B.E.; Fabrick, J.A.; Carrière, Y. Global patterns of insect resistance to transgenic Bt crops: The first 25 years. J. Econ. Entomol. 2023, 116, 297–309. [Google Scholar] [CrossRef] [PubMed]
- Gould, F. Sustainability of transgenic insecticidal cultivars: Integrating pest genetics and ecology. Annu. Rev. Entomol. 1998, 43, 701–726. [Google Scholar] [CrossRef] [PubMed]
- Andow, D.A.; Pueppke, S.G.; Schaafsma, A.W.; Gassmann, A.J.; Sappington, T.W.; Meinke, L.J.; Mitchell, P.D.; Hurley, T.M.; Hellmich, R.L.; Porter, R.P. Early detection and mitigation of resistance to Bt maize by western corn rootworm (Coleoptera: Chrysomelidae). J. Econ. Entomol. 2016, 109, 1–12. [Google Scholar] [CrossRef] [PubMed]
- USEPA. White Paper on Resistance in Lepidopteran Pests of Bacillus thuringiensis (Bt) Plant-Incorporated Protectants in the United States. 2018. Available online: https://www.epa.gov/sites/production/files/2018-07/documents/position_paper_07132018.pdf (accessed on 2 February 2024).
- Tabashnik, B.E.; Mota-Sanchez, D.; Whalon, M.E.; Hollingworth, R.M.; Carrière, Y. Defining terms for proactive management of resistance to Bt crops and pesticides. J. Econ. Entomol. 2014, 107, 496–507. [Google Scholar] [CrossRef] [PubMed]
- Tabashnik, B.E.; Carrière, Y.; Wu, Y.; Fabrick, J.A. Global perspectives on field-evolved resistance to transgenic Bt crops: A special collection. J. Econ. Entomol. 2023, 116, 269–274. [Google Scholar] [CrossRef]
- Tabashnik, B.E.; Carrière, Y. Global patterns of resistance to Bt crops highlighting pink bollworm in the United States, China, and India. J. Econ. Entomol. 2019, 112, 2513–2523. [Google Scholar] [CrossRef] [PubMed]
- Gassmann, A.J.; Carrière, Y.; Tabashnik, B.E. Fitness costs of insect resistance to Bacillus thuringiensis. Annu. Rev. Entomol. 2009, 54, 147–163. [Google Scholar] [CrossRef]
- Carrière, Y.; Tabashnik, B.E. Fitness costs and incomplete resistance associated with delayed evolution of practical resistance to Bt crops. Insects 2023, 14, 214. [Google Scholar] [CrossRef] [PubMed]
- Huang, F. Dominance and fitness costs of insect resistance to genetically modified Bacillus thuringiensis crops. GM Crops Food 2021, 12, 192–211. [Google Scholar] [CrossRef] [PubMed]
- Crespo, A.L.B.; Spenser, T.A.; Tan, S.Y.; Siegfried, B.D. Fitness costs of Cry1Ab resistance in a field-derived strain of Ostrinia nubilalis (Lepidoptera: Crambidae). J. Econ. Entomol. 2010, 103, 1386–1393. [Google Scholar] [CrossRef] [PubMed]
- Siegfried, B.D.; Rangasamy, M.; Wang, H.; Spencer, T.; Haridas, C.V.; Tenhumberg, B.; Sumerford, D.V.; Storer, N.P. Estimating the frequency of Cry1F resistance in field populations of the European corn borer (Lepidoptera: Crambidae). Pest Manag. Sci. 2014, 70, 725–733. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.-Z.; Cao, J.; Collins, H.L.; Bates, S.L.; Roush, R.T.; Earle, E.D.; Shelton, A.M. Concurrent use of transgenic plants expressing a single and two Bacillus thuringiensis genes speeds insect adaptation to pyramided plants. Proc. Natl. Acad. Sci. USA 2005, 102, 8426–8430. [Google Scholar] [CrossRef] [PubMed]
- Carrière, Y.; Crickmore, N.; Tabashnik, B.E. Optimizing pyramided transgenic Bt crops for sustainable pest management. Nat. Biotechnol. 2015, 33, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Carrière, Y.; Fabrick, J.A.; Tabashnik, B.E. Can pyramids and seed mixtures delay resistance to Bt crops? Trends Biotechnol. 2016, 34, 291–302. [Google Scholar] [CrossRef]
- Miller, N.J.; Sappington, T.W. Role of dispersal in resistance evolution and spread. Curr. Opin. Insect Sci. 2017, 21, 68–74. [Google Scholar] [CrossRef]
- Smith, J.L.; Farhan, Y. Monitoring resistance of Ostrinia nubilalis (Lepidoptera: Crambidae) in Canada to Cry toxins produced by Bt corn. J. Econ. Entomol. 2023, 116, 916–926. [Google Scholar] [CrossRef]
- Alves, A.P.; Spencer, T.A.; Tabashnik, B.E.; Siegfried, B.D. Inheritance of resistance to the Cry1Ab Bacillus thuringiensis toxin in Ostrinia nubilalis (Lepidoptera: Crambidae). J. Econ. Entomol. 2006, 99, 494–501. [Google Scholar] [CrossRef]
- Sappington, T.W. Migratory flight of insect pests within a year-round distribution: European corn borer as a case study. J. Integrative Agric. 2018, 17, 1485–1505. [Google Scholar] [CrossRef]
- Farhan, Y.; Smith, J.L.; Sovic, M.G.; Michel, A.P. Genetic mutations linked to field-evolved Cry1Fa-resistance in the European corn borer, Ostrinia nubilalis. Sci. Rep. 2023, 13, 8081. [Google Scholar] [CrossRef] [PubMed]
- Taylor, C.E.; Quaglia, F.; Georghiou, G.P. Evolution of resistance to insecticides: A case study on the influence of migration and insecticide decay rates. J. Econ. Entomol. 1983, 76, 704–707. [Google Scholar] [CrossRef]
- Caprio, M.A.; Tabashnik, B.E. Gene flow accelerates local adaptation among finite populations: Simulating the evolution of insecticide resistance. J. Econ. Entomol. 1992, 85, 611–620. [Google Scholar] [CrossRef]
- Lenormand, T.; Raymond, M. Resistance management: The stable zone strategy. Proc. R. Soc. Lond. B 1998, 265, 1985–1990. [Google Scholar] [CrossRef]
- Kim, K.S.; Bagley, M.J.; Coates, B.S.; Hellmich, R.L.; Sappington, T.W. Spatial and temporal genetic analyses show high gene flow among European corn borer (Lepidoptera: Crambidae) populations across the central U.S. Corn Belt. Environ. Entomol. 2009, 38, 1312–1323. [Google Scholar] [CrossRef]
- Kim, K.S.; Coates, B.S.; Bagley, M.J.; Hellmich, R.L.; Sappington, T.W. Genetic structure and gene flow among European corn borer (Lepidoptera: Crambidae) populations from the Great Plains to the Appalachians of North America. Agric. For. Entomol. 2011, 13, 383–393. [Google Scholar] [CrossRef]
- O’Rourke, M.E.; Rienzo-Stack, K.; Power, A.G. A multi-scale, landscape approach to predicting insect populations in agroecosystems. Ecol. Appl. 2011, 21, 1782–1791. [Google Scholar] [CrossRef] [PubMed]
- Dorhout, D.L.; Sappington, T.W.; Rice, M.E. Evidence for obligate migratory flight behavior in young European corn borer (Lepidoptera: Crambidae) females. Environ. Entomol. 2008, 37, 1280–1290. [Google Scholar] [CrossRef]
- Bradburd, G.S.; Ralph, P.L. Spatial population genetics: It’s about time. Annu. Rev. Ecol. Evol. Syst. 2019, 50, 427–449. [Google Scholar] [CrossRef]
- Nathan, R.; Getz, W.M.; Revilla, E.; Holyoak, M.; Kadmon, R.; Saltz, D.; Smouse, P.E. A movement ecology paradigm for unifying organismal movement research. Proc. Natl. Acad. Sci. USA 2008, 105, 19052–19059. [Google Scholar] [CrossRef]
- Dingle, H.; Drake, V.A. What is migration? BioScience 2007, 57, 113–121. [Google Scholar] [CrossRef]
- Dingle, H. Migration: The Biology of Life on the Move, 2nd ed.; Oxford University Press: New York, NY, USA, 2014; ISBN 978-0-19-964039-3. [Google Scholar]
- Bridge, E.S.; Ross, J.D.; Contina, A.J.; Kelly, J.F. Using agent-based models to scale from individuals to populations. In Aeroecology; Chilson, P.B., Frick, W.F., Kelly, J.F., Liechti, F., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 259–275. [Google Scholar] [CrossRef]
- Acharya, L.; McNeil, J.N. Predation risk and mating behavior: The responses of moths to bat-like ultrasound. Behav. Ecol. 1998, 9, 552–558. [Google Scholar] [CrossRef]
- Van Dyck, H.; Baguette, M. Dispersal behaviour in fragmented landscapes: Routine or special movements? Basic Appl. Ecol. 2005, 6, 535–545. [Google Scholar] [CrossRef]
- Kira, M.T.; Guthrie, W.D.; Huggans, J.L. Effect of drinking water on production of eggs by the European corn borer. J. Econ. Entomol. 1969, 62, 1366–1368. [Google Scholar] [CrossRef]
- Foster, S.P.; Frèrot, B. Sex pheromone-mediated flight and landing behaviors of the European corn borer, Ostrinia nubilalis (Hübner). J. Chem. Ecol. 1994, 20, 2323–2343. [Google Scholar] [CrossRef] [PubMed]
- Kárpáti, Z.; Tasin, M.; Cardé, R.T.; Dekker, T. Early quality assessment lessens pheromone specificity in a moth. Proc. Natl. Acad. Sci. USA 2013, 110, 7377–7382. [Google Scholar] [CrossRef] [PubMed]
- Reardon, B.J.; Sappington, T.W. Effect of age and mating status on adult European corn borer (Lepidoptera: Crambidae) dispersal from small-grain aggregation plots. J. Econ. Entomol. 2007, 100, 1116–1123. [Google Scholar] [CrossRef] [PubMed]
- Tscharntke, T.; Brandl, R. Plant-insect interactions in fragmented landscapes. Annu. Rev. Entomol. 2004, 49, 405–430. [Google Scholar] [CrossRef]
- Reynolds, D.R.; Chapman, J.W.; Harrington, R. The migration of insect vectors of plant and animal viruses. Adv. Virus Res. 2006, 67, 453–517. [Google Scholar] [CrossRef]
- Spangler, S.M.; Calvin, D.D. Influence of sweet corn growth stages on European corn borer (Lepidoptera: Crambidae) oviposition. Environ. Entomol. 2000, 29, 1226–1235. [Google Scholar] [CrossRef]
- Pilcher, C.; Rice, M.E. Effect of planting dates and Bacillus thuringiensis corn on the population dynamics of European corn borer (Lepidoptera: Crambidae). J. Econ. Entomol. 2001, 94, 730–742. [Google Scholar] [CrossRef] [PubMed]
- Obopile, M.; Hammond, R.B. The influence of planting date, transgenic Bt maize and hybrid relative maturity on European corn borer Ostrinia nubilalis (Lepidoptera: Crambidae) ovipositional patterns. Entomol. Res. 2013, 43, 299–305. [Google Scholar] [CrossRef]
- Kennedy, G.G.; Margolies, D.C. Mobile arthropod pests: Management in diversified agroecosystems. Bull. Entomol. Soc. Am. 1985, 31, 21–27. [Google Scholar] [CrossRef]
- Savinelli, C.E.; Bacheler, J.S.; Bradley, J.R., Jr. Ovipositional preferences of the European corn borer (Lepidoptera: Pyralidae) for field corn and cotton under field cage conditions in North Carolina. Environ. Entomol. 1988, 17, 688–690. [Google Scholar] [CrossRef]
- Kennedy, J.S. A turning point in the study of insect migration. Nature 1961, 189, 785–791. [Google Scholar] [CrossRef]
- Kennedy, J.S. Migration, behavioural and ecological. In Migration: Mechanisms and Adaptive Significance; Rankin, M.A., Ed.; Contributions in Marine Science; Port Aransas Marine Laboratory, University of Texas Marine Science Institute: Port Aransas, TX, USA, 1985; Volume 27, pp. 5–26. Available online: http://hdl.handle.net/2152/18035 (accessed on 2 February 2024).
- Drake, V.A.; Gatehouse, A.G.; Farrow, R.A. Insect migration: A holistic conceptual model. In Insect Migration: Tracking Resources Through Space and Time; Drake, V.A., Gatehouse, A.G., Eds.; Cambridge University Press: Cambridge, UK, 1995; pp. 427–457. ISBN 0-521-44000-9. [Google Scholar]
- Chapman, J.W.; Reynolds, D.R.; Wilson, K. Long-range seasonal migration in insects: Mechanisms, evolutionary drivers and ecological consequences. Ecol. Lett. 2015, 18, 287–302. [Google Scholar] [CrossRef] [PubMed]
- Showers, W.B. Migratory ecology of the black cutworm. Annu. Rev. Entomol. 1997, 42, 393–425. [Google Scholar] [CrossRef] [PubMed]
- Nagoshi, R.N.; Rosas-García, N.M.; Meagher, R.L.; Fleischer, S.J.; Westbrook, J.K.; Sappington, T.W.; Hay-Roe, M.; Thomas, J.M.G.; Murúa, G.M. Haplotype profile comparisons between Spodoptera frugiperda (Lepidoptera: Noctuidae) populations from Mexico with those from Puerto Rico, South America, and the United States and their implications to migratory behavior. J. Econ. Entomol. 2015, 108, 135–144. [Google Scholar] [CrossRef]
- Wu, M.-F.; Qi, G.-J.; Chen, H.; Ma, J.; Liu, J.; Jiang, Y.-Y.; Lee, G.-S.; Otuka, A.; Hu, G. Overseas immigration of fall armyworm, Spodoptera frugiperda (Lepidoptera: Noctuidae), invading Korea and Japan in 2019. Insect Sci. 2022, 29, 505–520. [Google Scholar] [CrossRef]
- Reppert, S.M.; de Roode, J.C. Demystifying monarch butterfly migration. Curr. Biol. 2018, 28, R1009–R1022. [Google Scholar] [CrossRef]
- Taylor, O.R., Jr.; Lovett, J.P.; Gibo, D.L.; Weiser, E.L.; Thogmartin, W.E.; Semmens, D.J.; Diffendorfer, J.E.; Pleasants, J.M.; Pecoraro, S.D.; Grundel, R. Is the timing, pace, and success of the monarch migration associated with sun angle? Front. Ecol. Evol. 2019, 7, 442. [Google Scholar] [CrossRef]
- Satterfield, D.A.; Sillett, T.S.; Chapman, J.W.; Altizer, S.; Marra, P.P. Seasonal insect migrations: Massive, influential, and overlooked. Front. Ecol. Environ. 2020, 18, 335–344. [Google Scholar] [CrossRef]
- Chiang, H.C. Dispersion of the European corn borer (Lepidoptera: Pyralidae) in Minnesota and South Dakota, 1945 to 1970. Environ. Entomol. 1972, 1, 157–161. [Google Scholar] [CrossRef]
- Sappington, T.W.; Spencer, J.L. Movement ecology of adult western corn rootworm: Implications for management. Insects 2023, 14, 922. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, A.M.; Reynolds, D.R.; Sane, S.P.; Hu, G.; Chapman, J.W. Orientation in high-flying migrant insects in relation to flows: Mechanisms and strategies. Philos. Trans. R. Soc. B 2016, 371, 20150392. [Google Scholar] [CrossRef] [PubMed]
- Byrne, D.N. Migration and dispersal by the sweet potato whitefly, Bemisia tabaci. Agric. For. Meteorol. 1999, 97, 309–316. [Google Scholar] [CrossRef]
- Menz, M.H.M.; Reynolds, D.R.; Gao, B.; Chapman, J.W.; Wotton, K.R. Mechanisms and consequences of partial migration in insects. Front. Ecol. Evol. 2019, 7, 403. [Google Scholar] [CrossRef]
- Tigreros, N.; Davidowitz, G. Flight-fecundity tradeoffs in wing-monomorphic insects. Adv. Insect Physiol. 2019, 56, 1–41. [Google Scholar] [CrossRef]
- Wright, S. Isolation by distance under diverse systems of mating. Genetics 1946, 31, 39–59. [Google Scholar] [CrossRef]
- Rousset, F. Genetic differentiation and estimation of gene flow from F-statistics under isolation by distance. Genetics 1997, 145, 1219–1228. [Google Scholar] [CrossRef] [PubMed]
- Chiang, H.C. Fringe populations of the European corn borer, Pyrausta nubilalis: Their characteristics and problems. Ann. Entomol. Soc. Am. 1961, 54, 378–387. [Google Scholar] [CrossRef]
- Palmer, D.F.; Schenk, T.C.; Chiang, H.C. Dispersal and Voltinism Adaptation of the European corn borer in North America, 1917–1977; Minnesota Agricultural Experiment Station: St. Paul, MN, USA, 1985; AD-SB-2716; Available online: https://hdl.handle.net/11299/139529 (accessed on 2 February 2024).
- Gathmann, A.; Rothmeier, I. Dispersal of the European corn borer (Ostrinia nubilalis Hbn.) in southern Rhineland–Results of the infestation assessment 2002 and 2003. J. Plant Diseases Prot. 2005, 112, 200–203. Available online: https://www.jstor.org/stable/45154900 (accessed on 2 February 2024). (In German).
- Heidel, W. The European corn borer in Mecklenburg-Western Pomerania–spreading of the pest and strategies for control. Nachrichtenblatt des Deutschen Pflanzenschutzdienstes. 2007, 59, 270–273. Available online: https://www.openagrar.de/receive/openagrar_mods_00062322 (accessed on 2 February 2024). (In German).
- Shigesada, N.; Kawasaki, K.; Takeda, Y. Modeling stratified diffusion in biological invasions. Am. Nat. 1995, 146, 229–251. [Google Scholar] [CrossRef]
- Liebhold, A.M.; Tobin, P.C. Population ecology of insect invasions and their management. Annu. Rev. Entomol. 2008, 53, 387–408. [Google Scholar] [CrossRef]
- Blackburn, T.M.; Lockwood, J.L.; Cassey, P. The influence of numbers on invasion success. Mol. Ecol. 2015, 24, 1942–1953. [Google Scholar] [CrossRef]
- Saccaggi, D.L.; Wilson, J.R.U.; Terblanche, J.S. Propagule pressure helps overcome adverse environmental conditions during population establishment. Curr. Res. Insect Sci. 2021, 1, 100011. [Google Scholar] [CrossRef]
- Zenni, R.D.; Nuñez, M.A. The elephant in the room: The role of failed invasions in understanding invasion biology. Oikos 2013, 122, 801–815. [Google Scholar] [CrossRef]
- Ciosi, M.; Miller, N.J.; Toepfer, S.; Estoup, A.; Guillemaud, T. Stratified dispersal and increasing genetic variation during the invasion of Central Europe by the western corn rootworm, Diabrotica virgifera virgifera. Evol. Appl. 2011, 4, 54–70. [Google Scholar] [CrossRef] [PubMed]
- Pedgley, D.E. Weather influences on pest movement. In Handbook of Pest Management; Ruberson, J.R., Ed.; Marcel Dekker, Inc.: New York, NY, USA, 1999; pp. 57–78. ISBN 0-8247-9433-8. [Google Scholar]
- Mikkola, K. Direction of insect migrations in relation to the wind. In Insect Flight: Dispersal and Migration; Danthanarayana, W., Ed.; Springer: Berlin/Heidelberg, Germany, 1986; pp. 152–171. ISBN 3-540-16502-9. [Google Scholar]
- Bretherton, R.F.; Chalmers-Hunt, J.M. Immigration of Lepidoptera to the British Isles in 1988. Entomol. Record J. Variation 1989, 101, 153–159, 225–230. [Google Scholar]
- Colenutt, S.R. Evergestis limbata (L.) (Lep: Pyralidae) new to mainland Britain. Entomol. Record J. Variation 1995, 107, 197. [Google Scholar]
- Langmaid, J.R.; Young, M.R. Microlepidoptera review of 2005. Entomol. Record J. Variation 2006, 118, 241–265. [Google Scholar]
- Pedgley, D.E.; Yathom, S. Windborne moth migration over the Middle East. Ecol. Entomol. 1993, 18, 67–72. [Google Scholar] [CrossRef]
- Showers, W.B.; Hellmich, R.L.; Derrick-Robinson, M.E.; Hendrix, W.H. Aggregation and dispersal behavior of marked and released European corn borer (Lepidoptera: Crambidae) adults. Environ. Entomol. 2001, 30, 700–710. [Google Scholar] [CrossRef]
- Reardon, B.J.; Sumerford, D.V.; Sappington, T.W. Dispersal of newly-eclosed European corn borer adults (Lepidoptera: Crambidae) from corn into small-grain aggregation plots. J. Econ. Entomol. 2006, 99, 1641–1650. [Google Scholar] [CrossRef]
- Schneider, C. The influence of spatial scale on quantifying insect dispersal: An analysis of butterfly data. Ecol. Entomol. 2003, 28, 252–256. [Google Scholar] [CrossRef]
- Carrière, Y.; Dutilleul, P.; Ellers-Kirk, C.; Pedersen, B.; Haller, S.; Antilla, L.; Dennehy, T.J.; Tabashnik, B.E. Sources, sinks, and the zone of influence of refuges for managing insect resistance to Bt crops. Ecol. Appl. 2004, 14, 1615–1623. [Google Scholar] [CrossRef]
- Oloumi-Sadeghi, H.; Showers, W.B.; Reed, G.L. European corn borer: Lack of synchrony of attraction to sex pheromone and capture in light traps. J. Econ. Entomol. 1975, 68, 663–667. [Google Scholar] [CrossRef]
- Laurent, P.; Frérot, B. Monitoring of European corn borer with pheromone-baited traps: Review of trapping system basics and remaining problems. J. Econ. Entomol. 2007, 100, 1797–1807. [Google Scholar] [CrossRef] [PubMed]
- Derrick, M.E.; Van Duyn, J.W.; Sorenson, C.E.; Kennedy, G.G. Effect of pheromone trap placement on capture of male European corn borer (Lepidoptera: Pyralidae) in three North Carolina crops. Environ. Entomol. 1992, 21, 240–246. [Google Scholar] [CrossRef]
- Reardon, B.J.; Sumerford, D.V.; Sappington, T.W. Impact of trap design, windbreaks, and weather on captures of European corn borer (Lepidoptera: Crambidae) in pheromone-baited traps. J. Econ. Entomol. 2006, 99, 2002–2009. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dorhout, D.L.; Sappington, T.W.; Lewis, L.C.; Rice, M.E. Flight behaviour of European corn borer infected with Nosema pyrausta. J. Appl. Entomol. 2011, 135, 25–37. [Google Scholar] [CrossRef]
- Kuang, X.Q.; Calvin, D.D.; Knapp, M.C.; Poston, F.L. Female European corn borer (Lepidoptera: Crambidae) ovarian developmental stages: Their association with oviposition and use in a classification system. J. Econ. Entomol. 2004, 97, 828–835. [Google Scholar] [CrossRef]
- Drake, V.A.; Reynolds, D.R. Radar Entomology: Observing Insect Flight and Migration; CABI: Wallingford, UK, 2012; ISBN 978-1-84593-556-6. [Google Scholar]
- Wright, S. Evolution in Mendelian populations. Genetics 1931, 16, 97–159. [Google Scholar] [CrossRef] [PubMed]
- Holsinger, K.E.; Weir, B.S. Genetics in geographically structured populations: Defining, estimating and interpreting FST. Nat. Rev. Genet. 2009, 10, 639–650. [Google Scholar] [CrossRef] [PubMed]
- Wright, S. Evolution and Genetics of Populations; University of Chicago Press: Chicago, IL, USA, 1969; Volume 2. [Google Scholar]
- Broquet, T.; Petit, E.J. Molecular estimation of dispersal for ecology and population genetics. Annu. Rev. Ecol. Evol. Syst. 2009, 40, 193–216. [Google Scholar] [CrossRef]
- Lowe, W.H.; Allendorf, F.W. What can genetics tell us about population connectivity? Mol. Ecol. 2010, 19, 3038–3051. [Google Scholar] [CrossRef]
- Kim, K.S.; Sappington, T.W. Population genetics strategies to characterize long-distance dispersal of insects. J. Asia-Pac. Entomol. 2013, 16, 87–97. [Google Scholar] [CrossRef]
- Slatkin, M. Gene flow and the geographic structure of natural populations. Science 1987, 236, 787–792. [Google Scholar] [CrossRef]
- Hutchison, D.W.; Templeton, A.R. Correlation of pairwise genetic and geographic distance measures: Inferring the relative influences of gene flow and drift on the distribution of genetic variability. Evolution 1999, 53, 1898–1914. [Google Scholar] [CrossRef]
- Martel, C.; Réjasse, A.; Rousset, F.; Bethenod, M.T.; Bourget, D. Host-plant-associated genetic differentiation in northern French populations of the European corn borer. Heredity 2003, 90, 141–149. [Google Scholar] [CrossRef]
- Leniaud, L.; Audiot, P.; Bourguet, D.; Frérot, B.; Genestier, G.; Lee, S.F.; Malausa, T.; Le Pallec, A.H.; Souqual, M.C.; Ponsard, S. Genetic structure of European and Mediterranean maize borer populations on several wild and cultivated host plants. Entomol. Exp. Appl. 2006, 120, 51–62. [Google Scholar] [CrossRef]
- Krumm, J.T.; Hunt, T.E.; Skoda, S.R.; Hein, G.L.; Lee, D.J.; Clark, P.L.; Foster, J.E. Genetic variability of the European corn borer, Ostrinia nubilalis, suggests gene flow between populations in the Midwestern United States. J. Insect Sci. 2008, 8, 72. [Google Scholar] [CrossRef]
- Bourguet, D.; Bethenod, M.T.; Pasteur, N.; Viard, F. Gene flow in the European corn borer Ostrinia nubilalis: Implications for the sustainability of transgenic insecticidal maize. Proc. R. Soc. Lond. B 2000, 267, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Malausa, T.; Dalecky, A.; Ponsard, S.; Audiot, P.; Streiff, R.; Chaval, Y.; Bourguet, D. Genetic structure and gene flow in French populations of two Ostrinia taxa: Host races or sibling species? Mol. Ecol. 2007, 16, 4210–4222. [Google Scholar] [CrossRef] [PubMed]
- Frolov, A.N.; Audiot, P.; Bourguet, D.; Kononchuk, A.G.; Malysh, J.M.; Ponsard, S.; Streiff, R.; Tokarev, Y.S. From Russia with lobe: Genetic differentiation in trilobed uncus Ostrinia spp. follows food plant, not hairy legs. Heredity 2012, 108, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Whitlock, M.C. Estimating effective population size and migration rates from genetic samples over space and time. Genetics 2003, 163, 429–446. [Google Scholar] [CrossRef] [PubMed]
- Shirk, A.J.; Cushman, S.A. Spatially-explicit estimation of Wright’s neighborhood size in continuous populations. Front. Ecol. Evol. 2014, 2, 62. [Google Scholar] [CrossRef]
- Bourguet, D.; Bethenod, M.T.; Trouvé, C.; Viard, F. Host-plant diversity of the European corn borer Ostrinia nubilalis: What value for sustainable transgenic insecticidal Bt maize? Proc. R. Soc. Lond. B 2000, 267, 1177–1184. [Google Scholar] [CrossRef] [PubMed]
- Coates, B.S.; Sumerford, D.V.; Hellmich, R.L. Geographic and voltinism differentiation among North American Ostrinia nubilalis (European corn borer) mitochondrial cytochrome c oxidase haplotypes. J. Insect Sci. 2004, 4, 35. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Yang, Z. Multilocus evidence provides insight into the demographic history and asymmetrical gene flow between Ostrinia furnacalis and Ostrinia nubilalis (Lepidoptera: Crambidae) in the Yili area, Xinjiang, China. Ecol. Evol. 2022, 12, e9504. [Google Scholar] [CrossRef] [PubMed]
- Chiang, H.C.; Hodson, A.C. Population fluctuations of the European corn borer, Pyrausta nubilalis, at Waseca, Minnesota, 1948–70. Environ. Entomol. 1972, 1, 7–16. [Google Scholar] [CrossRef]
- Showers, W.B.; DeRozari, M.B.; Reed, G.L.; Shaw, R.H. Temperature-related climatic effects on survivorship of the European corn borer. Environ. Entomol. 1978, 7, 717–723. [Google Scholar] [CrossRef]
- Pilcher, C.; Rice, M.E. Management of European corn borer (Lepidoptera: Crambidae) and corn rootworms (Coleoptera: Chrysomelidae) with transgenic corn: A survey of farmer perceptions. Am. Entomol. 1998, 44, 36–44. [Google Scholar] [CrossRef]
- Hyde, J.; Martin, M.A.; Preckel, P.V.; Edwards, C.R. The economics of Bt corn: Valuing protection from the European corn borer. Rev. Agric. Econ. 1999, 21, 442–454. [Google Scholar] [CrossRef]
- Brust, G.E.; King, L.R. Effects of crop rotation and reduced chemical inputs on pests and predators in maize agroecosystems. Agric. Ecosys Environ. 1994, 48, 77–89. [Google Scholar] [CrossRef]
- Phelan, P.L.; Mason, J.F.; Stinner, B.R. Soil-fertility management and host preference by European corn borer, Ostrinia nubilalis (Hiibner), on Zea mays L.: A comparison of organic and conventional chemical farming. Agric. Ecosys Environ. 1995, 56, 1–8. [Google Scholar] [CrossRef]
- Tollefson, J.J.; Calvin, D.D. Sampling arthropod pests in field corn. In Handbook of Sampling Methods for Arthropods in Agriculture; Pedigo, L.P., Buntin, G.D., Eds.; CRC Press: Boca Raton, FL, USA, 1994; pp. 433–473. ISBN 0-8493-2923-X. [Google Scholar]
- Crawford, H.G.; Spencer, G.J. The European corn borer control measures. J. Econ. Entomol. 1922, 15, 231–236. [Google Scholar] [CrossRef]
- Umeozor, O.C.; Van Duyn, J.W.; Bradley, J.R., Jr.; Kennedy, G.G. Comparison of the effect of minimum-tillage treatments on the overwintering emergence of European corn borer (Lepidoptera: Pyralidae) in cornfields. J. Econ. Entomol. 1985, 78, 937–939. [Google Scholar] [CrossRef]
- Sappington, T.W.; Showers, W.B. Effects of precipitation and wind on populations of adult European corn borer (Lepidoptera: Pyralidae). Environ. Entomol. 1983, 12, 1193–1196. [Google Scholar] [CrossRef]
- Hudon, M.; LeRoux, E.J. Variation between samples of immature stages, and of mortalities from some factors, of the European corn borer, Ostrinia nubilalis (Hübner) (Lepidoptera: Pyralidae), on sweet corn in Quebec. Can. Entomol. 1961, 93, 867–888. [Google Scholar] [CrossRef]
- LeRoux, E.J.; Paradis, R.O.; Hudon, M. Major Mortality factors in the population dynamics of the eye-spotted bud moth, the pistol casebearer, the fruit-tree leaf roller, and the European corn borer in Quebec. Mem. Entomol. Soc. Can. 1963, 95, 67–82. [Google Scholar] [CrossRef]
- Yu, H.; Nason, J.D.; Ge, X.; Zeng, J. Slatkin’s Paradox: When direct observation and realized gene flow disagree. A case study in Ficus. Mol. Ecol. 2010, 19, 4441–4453. [Google Scholar] [CrossRef] [PubMed]
- Jones, A. Reconciling field observations of dispersal with estimates of gene flow. Mol. Ecol. 2010, 19, 4379–4382. [Google Scholar] [CrossRef]
- Reid, C. The Origin of the British Flora; Dulau & Company: London, UK, 1899. [Google Scholar]
- Clark, J.S.; Fastie, C.; Hurtt, G.; Jackson, S.T.; Johnson, C.; King, G.A.; Lewis, M.; Lynch, J.; Pacala, S.; Prentice, C.; et al. Reid’s paradox of rapid plant migration: Dispersal theory and interpretation of paleoecological records. BioScience 1998, 48, 13–24. [Google Scholar] [CrossRef]
- Caswell, H.; Lensink, R.; Neubert, M.G. Demography and dispersal: Life table response experiments for invasion speed. Ecology 2003, 84, 1968–1978. [Google Scholar] [CrossRef]
- Marko, P.B. ‘What’s larvae got to do with it?’ Disparate patterns of post-glacial population structure in two benthic marine gastropods with identical dispersal potential. Mol. Ecol. 2004, 13, 597–611. [Google Scholar] [CrossRef]
- Chapman, B.B.; Brönmark, C.; Nilsson, J.-Å.; Hansson, L.-A. Partial migration: An introduction. Oikos 2011, 120, 1761–1763. [Google Scholar] [CrossRef]
- Chapman, B.B.; Brönmark, C.; Nilsson, J.-Å.; Hansson, L.-A. The ecology and evolution of partial migration. Oikos 2011, 120, 1764–1775. [Google Scholar] [CrossRef]
- Asplen, M.K. Proximate drivers of migration and dispersal in wing-monomorphic insects. Insects 2020, 11, 61. [Google Scholar] [CrossRef]
- Hidalgo, J.; de Casas, R.R.; Muñoz, M.Á. Environmental unpredictability and inbreeding depression select for mixed dispersal syndromes. BMC Evol. Biol. 2016, 16, 71. [Google Scholar] [CrossRef]
- Liu, B.R. Biphasic range expansions with short- and long-distance dispersal. Theor. Ecol. 2021, 14, 409–427. [Google Scholar] [CrossRef]
- Caton, B.P.; Fang, H.; Manoukis, N.C.; Pallipparambil, G.R. Quantifying insect dispersal distances from trapping detections data to predict delimiting survey radii. J. Appl. Entomol. 2021, 146, 203–216. [Google Scholar] [CrossRef]
- Royer, L.; McNeil, J.N. Changes in calling behaviour and mating success in the European corn borer (Ostrinia nubilalis), caused by relative humidity. Entomol. Exp. Appl. 1991, 61, 131–138. [Google Scholar] [CrossRef]
- Hu, Y.; Andow, D.A. Field observations of Ostrinia nubilalis eclosion and post-eclosion activity of females around their natal plants. Insect Sci. 2011, 18, 712–718. [Google Scholar] [CrossRef]
- Campagne, P.; Smouse, P.E.; Pasquet, R.; Silvain, J.-F.; Le Ru, B.; Van den Berg, J. Impact of violated high-dose refuge assumptions on evolution of Bt resistance. Evol. Appl. 2016, 9, 596–607. [Google Scholar] [CrossRef]
- Carrière, Y.; Onstad, D.W. The role of landscapes in insect resistance management. In Insect Resistance Management: Biology, Economics, and Prediction, 3rd ed.; Onstad, D.W., Knolhoff, L.M., Eds.; Academic Press, Elsevier Ltd.: London, UK, 2023; pp. 329–379. [Google Scholar] [CrossRef]
- Carrière, Y.; Crowder, D.W.; Tabashnik, B.E. Evolutionary ecology of insect adaptation to Bt crops. Evol. Appl. 2010, 3, 561–573. [Google Scholar] [CrossRef] [PubMed]
- Sorenson, C.E.; Kennedy, G.G.; Schal, C.; Walgenbach, J.F. Geographical variation in pheromone response of the European corn borer, Ostrinia nubilalis (Lepidoptera: Crambidae), in North Carolina: A 20-y perspective. Environ. Entomol. 2005, 34, 1057–1062. [Google Scholar] [CrossRef]
- Dopman, E.B. Genetic hitchhiking associated with life history divergence and colonization of North America in the European corn borer moth. Genetica 2011, 139, 565–573. [Google Scholar] [CrossRef] [PubMed]
- Lassance, J.M.; Bogdanowicz, S.M.; Wanner, K.W.; Löfstedt, C.; Harrison, R.G. Gene genealogies reveal differentiation at sex pheromone olfactory receptor loci in pheromone strains of the European corn borer, Ostrinia nubilalis. Evolution 2011, 65, 1583–1593. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, J.A.; Mason, C.E.; Pesek, J. Dispersal and movement behavior of neonate European corn borer (Lepidoptera: Crambidae) on non-Bt and transgenic Bt corn. J. Econ. Entomol. 2010, 103, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Prasifka, J.R.; Hellmich, R.L.; Crespo, A.L.B.; Siegfried, B.D.; Onstad, D.W. Video-tracking and on-plant tests show Cry1Ab of resistance influences behavior and survival of neonate Ostrinia nubilalis following exposure to Bt maize. J. Insect Behav. 2010, 23, 1–11. [Google Scholar] [CrossRef]
- Mallet, J.; Porter, P. Preventing insect adaptation to insect-resistant crops: Are seed mixtures or refugia the best strategy? Proc. R. Soc. Lond. B 1992, 250, 165–169. [Google Scholar] [CrossRef]
- Hughson, S.A.; Spencer, J.L. Emergence and abundance of western corn rootworm (Coleoptera: Chrysomelidae) in Bt cornfields with structured and seed blend refuges. J. Econ. Entomol. 2015, 108, 114–125. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sappington, T.W. Critical Facets of European Corn Borer Adult Movement Ecology Relevant to Mitigating Field Resistance to Bt-Corn. Insects 2024, 15, 160. https://doi.org/10.3390/insects15030160
Sappington TW. Critical Facets of European Corn Borer Adult Movement Ecology Relevant to Mitigating Field Resistance to Bt-Corn. Insects. 2024; 15(3):160. https://doi.org/10.3390/insects15030160
Chicago/Turabian StyleSappington, Thomas W. 2024. "Critical Facets of European Corn Borer Adult Movement Ecology Relevant to Mitigating Field Resistance to Bt-Corn" Insects 15, no. 3: 160. https://doi.org/10.3390/insects15030160
APA StyleSappington, T. W. (2024). Critical Facets of European Corn Borer Adult Movement Ecology Relevant to Mitigating Field Resistance to Bt-Corn. Insects, 15(3), 160. https://doi.org/10.3390/insects15030160






