In Vitro Evaluation of Essential Oils and Saturated Fatty Acids for Repellency against the Old-World Sand Fly, Phlebotomus papatasi (Scopoli) (Diptera: Psychodidae)
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sand Flies
2.2. Essential Oils and Chemical Compounds
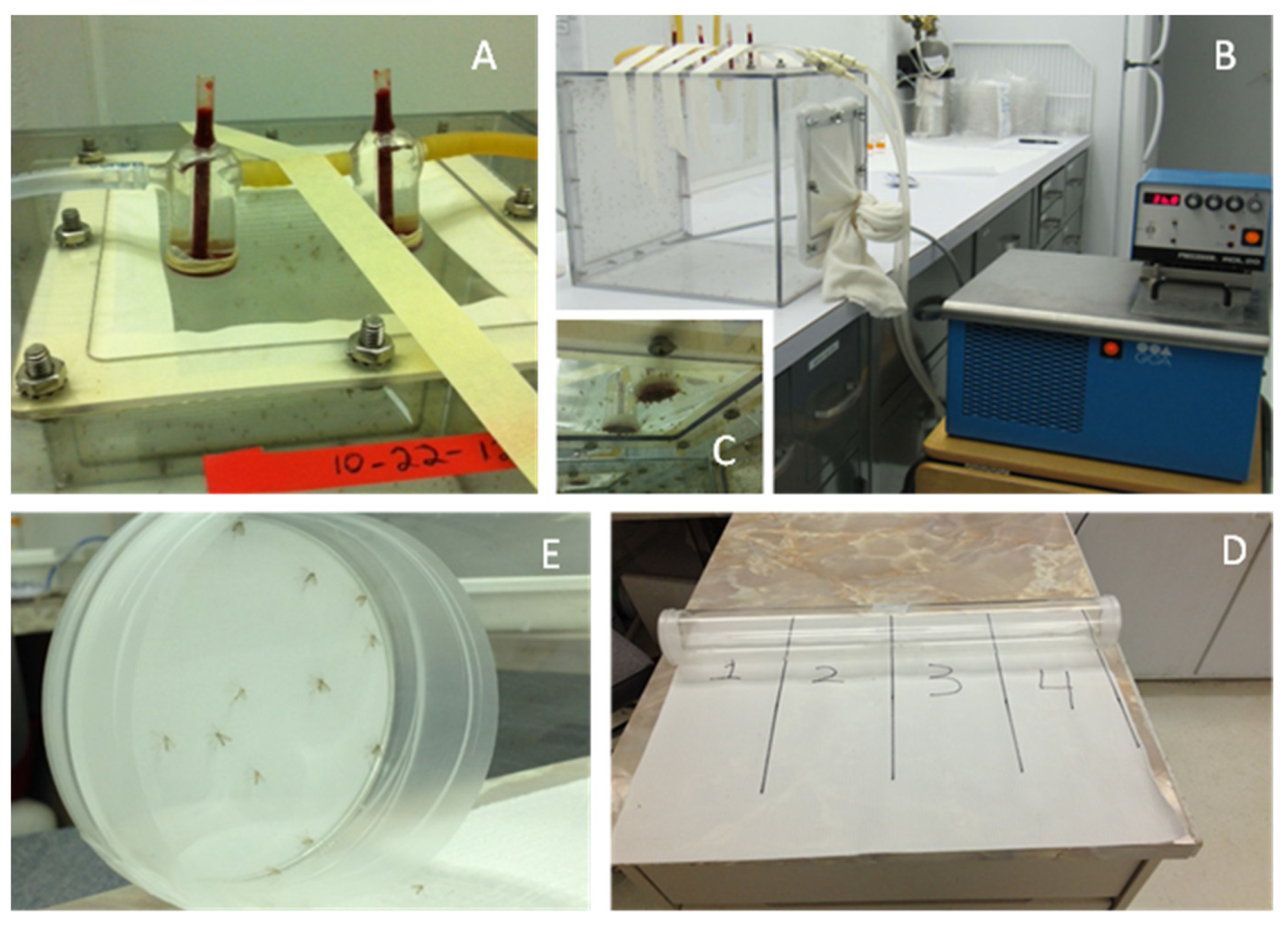
2.3. Static Air Repellency Bioassays
2.4. Inhibition of P. papatasi Acetylcholinesterase
2.5. Data Analysis
half)/(total # flies)] × 100%
flies on untreated paper + # flies on treated paper)] × 100%
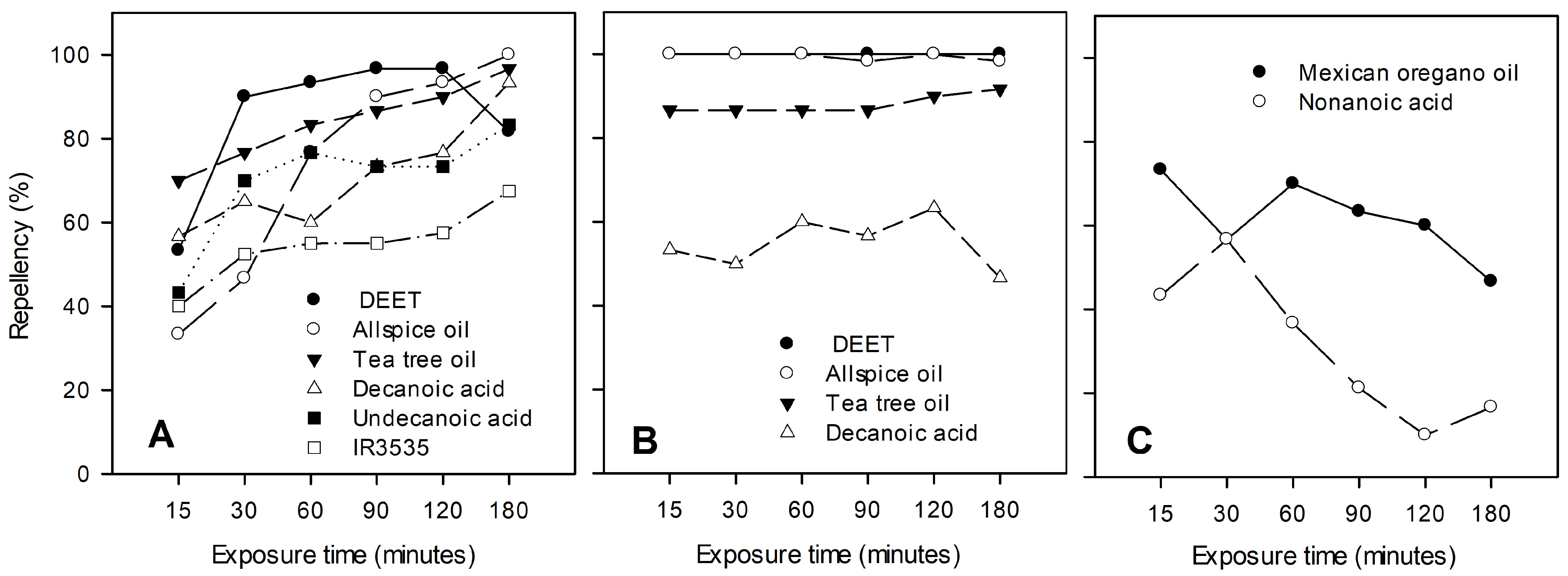
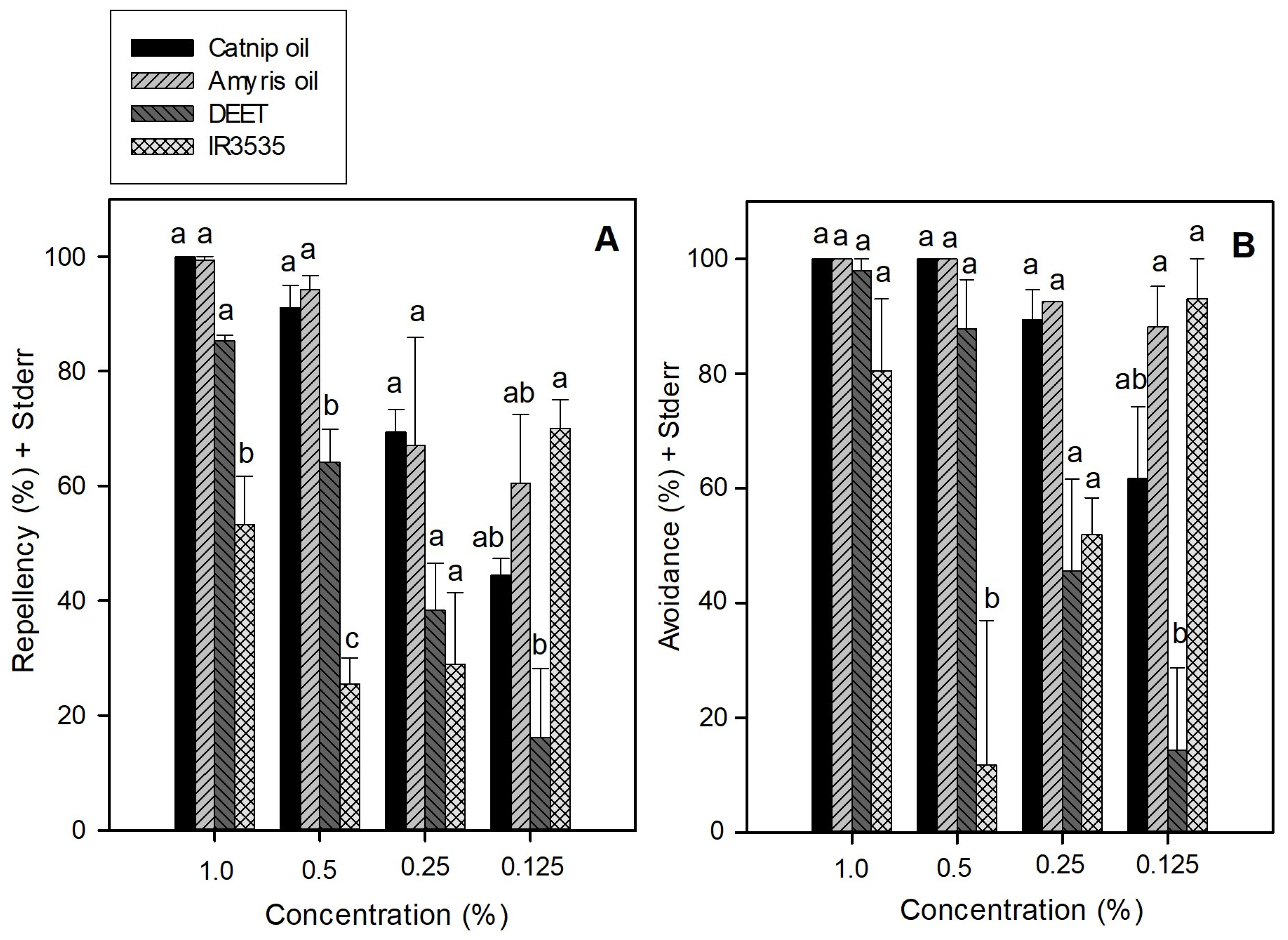
3. Results
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cecilio, P.; Cordeiro-da-Silva, A.; Oliveira, F. Basic information on the vectors of leishmaniasis and their interactions with Leishmania parasites. Commun. Biol. 2022, 5, 305. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control. Phlebotomine Sand Flies—Factsheet for Experts. 2022. Available online: https://www.ecdc.europa.eu/en/disease-vectors/facts/phlebotomine-sand-flies (accessed on 29 August 2022).
- Jaffe, C.L.; Baneth, G.; Abdeen, Z.A.; Schlein, Y.; Warburg, A. Leishmaniasis in Israel and the Palestinian Authority. Trends Parasitol. 2004, 20, 328–332. [Google Scholar] [CrossRef]
- Janini, R.; Saliba, E.; Kamhawi, S. Species composition of sand flies and population dynamics of Phlebotomus papatasi (Diptera: Psychodidae) in the southern Jordan Valley, an endemic focus of cutaneous leishmaniasis. J. Med. Entomol. 1995, 32, 822–826. [Google Scholar] [CrossRef]
- Killick-Kendrick, R.; Leaney, A.J.; Peters, W.; Rioux, J.-A.; Bray, R.S. Zoonotic cutaneous leishmaniasis in Saudi Arabia: The incrimination of Phlebotomus papatasi as the vector in the Al-Hassa oasis. Trans. R. Soc. Trop. Med. Hyg. 1985, 79, 252–255. [Google Scholar] [CrossRef]
- Kravchenko, V.; Wasserberg, G.; Warburg, A. Bionomics of phlebotomine sand flies in the Galilee focus of cutaneous leishmaniasis in northern Israel. Med. Vet. Entomol. 2004, 18, 418–428. [Google Scholar] [CrossRef]
- Weeks, E.N.I.; Wasserberg, G.; Logan, J.L.; Agneessens, J.; Stewart, S.A.; Dewhirst, S. Efficacy of the insect repellent IR3535 on the sand fly Phlebotomus papatasi in human volunteers. J. Vector Ecol. 2019, 44, 290–292. [Google Scholar] [CrossRef]
- Weina, P.J.; Neafie, R.C.; Wortmann, G.; Polhemus, M.; Aronson, N.E. Old world leishmaniasis: An emerging infection among deployed US military and civilian workers. Clin. Infect. Dis. 2005, 39, 1674–1680. [Google Scholar] [CrossRef]
- Kitchen, L.W.; Lawrence, K.L.; Coleman, R.E. The role of the United States military in the development of vector control products, including insect repellents, insecticides, and bed nets. J. Vector Ecol. 2009, 34, 50–61. [Google Scholar] [CrossRef]
- Colacicco-Mayhugh, M.G.; Grieco, J.P.; Putnam, J.L.; Burkett, D.A.; Coleman, R.E. Impact of phlebotomine sand flies on United States Military Operations at Tallil Air Base, Iraq: 5. Impact of weather on sand fly activity. J. Med. Entomol. 2011, 48, 538–545. [Google Scholar] [CrossRef]
- Coleman, R.E.; Burkett, D.A.; Sherwood, V.; Caci, J.; Dennett, J.A.; Jennings, B.T.; Cushing, R.; Cushing, R.J.; Ploch, J.; Hopkins, G.; et al. Impact of phlebotomine sand flies on United State military operations at Tallil Air Base, Iraq: 6. Evaluation of insecticides for the control of sand flies. J. Med. Entomol. 2011, 48, 584–599. [Google Scholar] [CrossRef][Green Version]
- Jacobson, R.L. Leishmaniasis in an era of conflict in the Middle East. Vector-Borne Zoonotic Dis. 2011, 11, 247–258. [Google Scholar] [CrossRef]
- Bowles, D.E.; Britch, S.C.; Linthicum, K.J.; Johnson, R.N.; Linton, Y.-M.; White, G.B. Sand Flies—Significance, Surveillance, and Control in Contingency Operations (Diptera: Psychodidae: Phlebotominae); Armed Forces Pest Management Board Technical Guide No. 49; US Army Garrison-Forest Glen: Silver Spring, MD, USA, 2015; p. 65. Available online: https://www.acq.osd.mil/eie/afpmb/docs/techguides/tg49.pdf (accessed on 1 September 2023).
- Kalyanasundaram, M.; Srinivasaan, R.; Subramanian, S.; Panicker, K.N. Relative potency of DEPA as a repellent against the sand fly Phlebotomus papatasi. Med. Vet. Entomol. 1994, 8, 68–70. [Google Scholar] [CrossRef]
- Klun, J.A.; Khrimian, A.; Debboun, M. Repellent and deterrent effects of SS220, picardin, and DEET suppress human blood feeding by Aedes aegypti, Anopheles stephensi and Phlebotomus papatasi. J. Med. Entomol. 2006, 43, 34–39. [Google Scholar] [CrossRef]
- Paluch, G.; Grodnitzky, J.; Bartholomay, L.; Coats, J. Quantitative structure-activity relationship of botanical sesquiterpenes: Spatial and contact repellency to the yellow fever mosquito, Aedes aegypti. J. Agric. Food Chem. 2009, 57, 7618–7625. [Google Scholar] [CrossRef]
- Park, S.J.; Yu, M.H.; Kim, J.E.; Park, M.J.; Lee, I.S.; Lee, J.; Kim, B.H.; Lee, D.K.; Lee, S.P. Repellent efficacy and safety evaluation of IR3535 derivative against Aedes albopictus, Culex pipiens pallens and Aedes togoi. Entomol. Res. 2012, 42, 299–307. [Google Scholar] [CrossRef]
- Ali, A.; Cantrell, C.L.; Khan, I. A new in vitro bioassay system for the discovery and quantitative evaluation of mosquito repellents. J. Med. Entomol. 2017, 54, 1328–1336. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.Y. Essential oils as repellents against arthropods. Biomed. Res. Int. 2018, 2018, 6860271. [Google Scholar] [CrossRef]
- Zhu, J.J.; Cermak, S.C.; Kenar, J.A.; Brewer, G.; Haynes, K.F.; Boxler, D.; Baker, P.D.; Wang, D.; Wang, C.; Li, A.Y.; et al. Better than DEET repellent compounds derived from coconut oil. Sci. Rep. 2018, 8, 14053. [Google Scholar] [CrossRef]
- Asadollahi, A.; Khoobdel, M.; Zahraei-Ramazani, A.; Azarmi, S.; Mosawi, S.H. Effectiveness of plant-based repellents against different Anopheles species: A systematic review. Malar. J. 2019, 18, 436. [Google Scholar] [CrossRef] [PubMed]
- Benelli, G.; Pavela, R. Beyond mosquitoes—Essential oil toxicity and repellency against bloodsucking insects. Industr. Crops Prod. 2018, 117, 382–392. [Google Scholar] [CrossRef]
- Demeter, S.; Lebbe, O.; Hecq, F.; Nicolis, S.C.; Kemene, T.K.; Martin, H.; Fauconnier, M.-L.; Hance, T. Insecticidal activity of 25 essential oils on the stored product pest, Sitophilus granaries. Foods 2021, 10, 200. [Google Scholar] [CrossRef]
- Liu, J.; Hua, J.; Qu, B.; Guo, X.; Wang, Y.; Shao, M.; Luo, S. Insecticidal terpenes from the essential oils of Artemisia nakaii and their inhibitory effects on acetylcholinesterase. Front. Plant Sci. 2021, 12, 720816. [Google Scholar] [CrossRef]
- Selles, S.M.A.; Kouidri, M.; González, M.G.; González, J.; Sánchez, M.; González-Coloma, A.; Sanchis, J.; Elhachimi, L.; Olmeda, A.S.; Tercero, J.M.; et al. Acaricidal and repellent effects of essential oils against ticks: A review. Pathogens 2021, 10, 1379. [Google Scholar] [CrossRef]
- Benelli, G.; Pavela, R. Repellence of essential oils and selected compounds against ticks—A systematic review. Acta Trop. 2018, 179, 47–54. [Google Scholar] [CrossRef]
- Sengül Demirak, M.S.; Canpolat, E. Plant-based bioinsecticides for mosquito control: Impact on insecticide resistance and disease transmission. Insects 2022, 13, 162. [Google Scholar] [CrossRef] [PubMed]
- Dinesh, D.S.; Kumari, S.; Kumar, V.; Das, P. The potentiality of botanicals and their products as an alternative to chemical insecticides to sand flies (Diptera: Psychodidae): A review. J. Vector Borne Dis. 2014, 51, 1–7. [Google Scholar]
- Pugliese, M.; Gaglio, G.; Passantino, A.; Brianti, E.; Napoli, E. Natural products against sand fly vectors of leishmaniosis: A systematic review. Vet. Sci. 2021, 8, 150. [Google Scholar] [CrossRef] [PubMed]
- Yaghoobi-Ershadi, M.R.; Akhavan, A.A.; Jahanifard, E.; Vatandoost, H.; Amin, G.H.; Moosavi, L.; Zahraei Ramazani, A.R.; Abdoli, H.; Arandian, M.H. Repellency effect of myrtle essential oil and DEET against Phlebotomus papatasi, under laboratory conditions. Iran. J. Public Health 2006, 35, 7–13. [Google Scholar]
- Kimutai, A.; Ngeiywa, M.; Mulaa, M.; Njagi, P.G.N.; Ingonga, J.; Nyamwamu, L.B.; Ombati, C.; Ngumbi, P. Repellent effects of the essential oils of Cymbopogon citratus and Tagetes minuta on the sand fly, Phlebotomus duboscqi. BMC Res. Notes 2017, 10, 98. [Google Scholar] [CrossRef] [PubMed]
- Ireri, L.N.; Kongoro, J.; Ngure, O.; Mutai, C.; Langat, B.; Tonui, W.; Kimutai, A.; Mucheru, O. The potential of the extracts of Tagetes minuta Linnaeus (Asteraceae), Acalypha fruticosa Forssk (Euphorbiaceae) and Tarchonanthus camphoratus L. (Compositae) against Phlebotomus duboscqi Neveu Lemaire (Diptera: Psychodidae), the vector for Leishmania major Yankinoff and Schokhor. J. Vector-Borne Dis. 2010, 47, 168–174. [Google Scholar]
- Samuel, M.; Zipporah, N.; Rosebella, M.; Zipporag, O.; Peter, N.; Philip, N.; Willy, T. Effect of leaf crude extracts of Tarchonanthus camphoratus (Asteraceae), Acalypha fruticose (Fabacea) and Tagetes minuta (Asteraceae) on fecundity of Phlebotomus duboscqi. Am. Int. J. Contemp. Res. 2012, 2, 194–200. [Google Scholar]
- Cantrell, C.L.; Ali, A.; Duke, S.O.; Khan, I. Identification of the mosquito biting deterrent constituents from the Indian folk remedy plant, Jatropha curcas. J. Med. Entomol. 2011, 48, 836–845. [Google Scholar] [CrossRef]
- Jones, A.M.P.; Klun, J.A.; Cantrell, C.L.; Ragone, D.; Chauhan, K.; Murch, S.J. Isolation and identification of mosquito (Aedes aegypti) biting deterrent fatty acids from flowers of the male breadfruit (Artocarpus altilis (Parkinson) Fosberg), a Hawaiian folk remedy. J. Agric. Food Chem. 2012, 60, 3867–3873. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Cantrell, C.L.; Duke, S.O.; Uli, B.; Khan, I. Aedes aegypti (Diptera: Culicidae) biting deterrence: Structure-activity relationship of saturated and unsaturated fatty acids. J. Med. Entomol. 2012, 49, 1370–1378. [Google Scholar] [CrossRef] [PubMed]
- Cantrell, C.L.; Zaki, M.A.; Reichley, A.; Sink, M.; Kim, S.J.; Ali, A. Biting deterrency of undecanoic acid and dodecanoic acid ester analogs against Aedes aegypti. Pest Manag. Sci. 2021, 77, 3737–3743. [Google Scholar] [CrossRef]
- Maia, M.F.; Moore, S.J. Plant-based insect repellents: A review of their efficacy, development and testing. Malar. J. 2011, 10, S11. [Google Scholar] [CrossRef]
- Swale, D.R.; Sun, B.; Tong, F.; Bloomquist, J.R. Neurotoxicity and mode of action of N,N-diethyl-meta-toluamide (DEET). PLoS ONE 2014, 9, ee103713. [Google Scholar] [CrossRef]
- Cilek, J.E.; Petersen, J.L.; Hallmon, C.F. Comparative efficacy of IR3535 and DEET as repellents against adult Aedes aegypti and Culex quinquefasciatus. J. Am. Mosq. Control Assoc. 2004, 20, 299–304. [Google Scholar]
- Moreau, E.; Mikulska-Ruminska, K.; Goulu, M.; Perrier, S.; Deshayes, C.; Stankiewicz, M.; Apaire-Marchais, V.; Nowak, W.; Lapied, B. Orthosteric muscarinic receptor activation by the insect repellent IR3535 opens new prospects in insecticide-based vector control. Sci. Rep. 2020, 10, 6842. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, B.; Pradhan, R.N.; Nath, D.K. Cellular and molecular basis of IR3535 perception in Drosophila. Pest Manag. Sci. 2021, 78, 793–802. [Google Scholar] [CrossRef]
- Naucke, T.J.; Lorentz, S.; Grunewald, H.-W. Laboratory testing of the insect repellents IR3535 and DEET against Phlebotomus mascittii and P. duboscqi (Diptera: Psychodidae). Int. J. Med. Microbiol. 2006, 296, 230–232. [Google Scholar] [CrossRef]
- Cardoso, A.d.S.; Santos, E.G.G.; Lima, A.d.S.; Temeyer, K.B.; Perez de Leon, A.A.; Junior, L.M.C.; Soares, A.M.d.S. Terpenes on Rhipicephalus (Boophilus) microplus: Acaricidal activity and acetylcholinesterase inhibition. Vet. Parasitol. 2020, 280, 109090. [Google Scholar] [CrossRef]
- Duque, J.E.; Urbina, D.L.; Vesga, L.C.; Ortiz-Rodriquez, L.A.; Vanegas, T.S.; Stashenko, E.E.; Mendez-Sanchez, S.C. Insecticidal activity of essential oils from American native plants against Aedes aegypti (Diptera: Culicidae): An introduction to their possible mechanism of action. Sci. Rep. 2023, 13, 2989. [Google Scholar] [CrossRef]
- Farag, M.A.; Ezzat, S.M.; Salama, M.M.; Tadros, M.G.; Serya, R.A.T. Anti-cholinesterase activity of essential oils and their major constituents from four Ocimum species. Z. Naturforschung C 2016, 71, 393–402. [Google Scholar] [CrossRef]
- Hung, N.H.; Quan, P.M.; Satyal, P.; Dai, D.N.; Hoa, V.V.; Huy, N.G.; Giang, L.D.; Ha, N.T.; Huong, L.T.; Hien, V.T.; et al. Acetylcholinesterase inhibitory activities of essential oils from Vietanmese traditional medicinal plants. Molecules 2022, 27, 7092. [Google Scholar] [CrossRef]
- Miyazawa, M.; Watanabe, H.; Umemoto, K.; Kameoka, H. Inhibition of acetylcholinesterase activity by oils of Mentha species. J. Agric. Food Chem. 1998, 46, 3431–3434. [Google Scholar] [CrossRef]
- Orban, I.; Kartal, M.; Kan, Y.; Sener, B. Activity of essential oils and individual components against acetyl- and butyrylcholinesterase. Z. Naturforsch. C J. Biosci. 2008, 63, 547–553. [Google Scholar] [CrossRef]
- Ranjan, N.; Kumari, M. Acetylcholinesterase inhibition by medicinal plants: A review. Ann. Plant Sci. 2017, 6, 1640–1644. [Google Scholar] [CrossRef]
- Xiang, C.P.; Han, J.X.; Li, X.C.; Li, Y.H.; Zhang, Y.; Chen, L.; Qu, Y.; Hao, C.Y.; Li, H.Z.; Yang, C.R.; et al. Chemical composition and acetylcholinesterase inhibitory activity of essential oils form Piper species. J. Agric. Food Chem. 2017, 65, 3702–3710. [Google Scholar] [CrossRef]
- Koloski, C.W.; Cassone, B.J. Transcriptional profilinf of Dermacentor variabilis (Acari: Ixodidae) provides insights into the role of the Haller’s organ in spatial DEET recognition. Ticks Tick-Borne Dis. 2022, 13, 101827. [Google Scholar] [CrossRef]
- Koloski, C.W.; LeMoine, C.M.R.; Klonowski, A.R.; Smith, C.M.; Cassone, B.J. Molecular evidence for the inhibition of cytochrome p450s and cholinesterases in ticks by the repellent DEET. Ticks Tick-Borne Dis. 2019, 10, 515–522. [Google Scholar] [CrossRef]
- Temeyer, K.B.; Brake, D.K.; Tuckow, A.P.; Li, A.Y.; Pérez de León, A.A.; Bloomquist, J.R. Acetylcholinesterase of the sand fly, Phlebotomus papatasi (Scopoli): cDNA sequence, baculovirus expression, and biochemical properties. Parasit. Vectors 2013, 6, 31. [Google Scholar] [CrossRef][Green Version]
- Temeyer, K.B.; Tong, F.; Totrov, M.M.; Tuckow, A.P.; Chen, Q.-H.; Carlier, P.R.; Perez de Leon, A.A.; and Bloomquist, J.R. Acetylcholinesterase of the sand fly, Phlebotomus papatasi (Scopoli): Construction, expression and biochemical properties of the G119S mutant. Parasit. Vectors 2014, 7, 577. [Google Scholar] [CrossRef]
- Wirtz, R.A.; Rowton, E.D.; Hallam, J.A.; Perkins, P.V.; Rutledge, L.C. Laboratory testing of repellents against the sand fly Phlebotomus papatasi (Diptera: Psychodidae). J. Med. Entomol. 1986, 23, 64–67. [Google Scholar] [CrossRef] [PubMed]
- Lawyer, P.; Killick-Kendrick, M.; Rowland, T.; Rowton, E.; Volf, P. Laboratory colonization and mass rearing of phlebotomine sand flies (Diptera, Psychodidae). Parasite 2017, 24, 42. [Google Scholar] [CrossRef]
- Young, D.G.J.; Perkins, P.V.; Endris, R.G. A larval diet for rearing Phlebotomine sand flies (Diptera: Psychodidae). J. Med. Entomol. 1981, 18, 466. [Google Scholar] [CrossRef]
- Denlinger, D.S.; Durham, S.; Li, A.Y.; Lawyer, P.G.; Andersen, J.L.; Bernhardt, S.A. Comparison of in vivo and in vitro methods for blood feeding of Phlebotomus papatasi (Diptera: Psychodidae) in the laboratory. J. Med. Entomol. 2016, 53, 1112–1116. [Google Scholar] [CrossRef]
- Temeyer, K.B.; Schlechte, K.G.; Dandeneau, L.B. Sand fly colony crash tentatively attributed to nematode infestation. J. Med. Entomol. 2020, 57, 1301–1304. [Google Scholar] [CrossRef]
- Martinez-Velazquez, M.; Castillo-Herrera, G.; Rosario-Cruz, R.; Flores-Fernandez, J.M.; Lopez-Ramirez, J.; Hernandez-Gutierrez, R.; Lugo-Cervantes, E. Acaricidal effect and chemical composition of essential oils extracted from Cuminum cyminum, Pimenta dioica and Ocimum basilicum against the cattle tick Rhipicephalus (Boophilus) microplus (Acari: Ixodidae). Parasitol. Res. 2011, 108, 481–487. [Google Scholar] [CrossRef]
- Martinez-Velazquez, M.; Rosario-Cruz, R.; Castillo-Herrera, G.; Flores-Fernandez, J.M.; Alvarez, A.H.; Lugo-Cervantes, E. Acaricidal effect of essential oils from Lippia graveolens (Lamiales: Verbenaceae), Rosmarinus officinalis (Lamiales: Lamiaceae), and Allium sativum (Liliales: Liliaceae) against Rhipicephalus (Boophilus) microplus (Acari: Ixodidae). J. Med. Entomol. 2011, 48, 822–827. [Google Scholar] [CrossRef]
- Paluch, G.E.; Zhu, J.; Bartholomay, L.; Coats, J.R. Amyris and Siam-wood essential oils: Insect activity of sesquiterpenes. In Pesticides in Household, Structural and Residential Pest Management; ACS Symposium Series; Peterson, C., Stout, D., Eds.; American Chemical Society: Washington, DC, USA, 2009; pp. 5–18. Volume 1015, Chapter 2. [Google Scholar] [CrossRef]
- Peterson, C.; Coats, J. Insect repellents—Past, present and future. Pestic. Outlook 2001, 12, 154–158. [Google Scholar] [CrossRef]
- Schultz, G.; Peterson, C.; Coats, J. Natural insect repellents: Activity against mosquitoes and cockroaches. In Natural Products for Pest Management; Rimando, A.M., Duke, S.O., Eds.; American Chemical Society: Washington, DC, USA, 2006; pp. 168–181, Chapter 13. [Google Scholar] [CrossRef]
- Farag, S.M.; Essa, E.E.; Alharbi, S.A.; Alfarraj, S.; Abu El-Hassan, G.M.M. Agro-waste derived compounds (flax and black seed peels): Toxicological effect against the West Nile virus vector, Culex pipiens L. with special reference to GC-MS analysis. Saudi J. Biol. Sci. 2021, 28, 5261–5267. [Google Scholar] [CrossRef] [PubMed]
- Démares, F.; Coquerel, Q.; Richoux, G.; Linthicum, K.; Bloomquist, J. Fatty acid and related potassium Kv2 channel blockers: Toxicity and physiological actions on mosquitoes. Insects 2018, 9, 155. [Google Scholar] [CrossRef] [PubMed]
- Santos, E.G.G.; Bezerra, W.A.S.; Temeyer, K.B.; Pérez de León, A.A.; Costa-Junior, L.M.; Soares, A.M.S. Effects of essential oils on native and recombinant acetylcholinesterases of Rhipicephalus microplus. Braz. J. Vet. Parasitol. 2021, 30, e002221. [Google Scholar] [CrossRef] [PubMed]
- Alves, J.A.; Mantovani, A.L.L.; Martins, M.H.G.; Abrao, F.; Lucarini, R.; Crotti, A.E.M.; Martins, C.H.G. Antimycobacterial activity of some commercially available plant-derived essential oils. Chem. Nat. Compd. 2015, 51, 353–355. [Google Scholar] [CrossRef]
- Kačániová, M.; Terentjeva, M.; Štefániková, J.; Žiarovská, J.; Savitskaya, T.; Ginshpan, D.; Kowalczewski, P.L.; Vukovic, N.; Tvrdá, E. Chemical composition and antimicrobial activity of selected essential oils against Staphylococcus spp. Isolated from human semen. Antibiotics 2020, 9, 765. [Google Scholar] [CrossRef] [PubMed]
- Van Beek, T.A.; Kleis, R.; Posthumus, M.A.; Veldhutzen, A.V. Essential oil of Amyris balsamifera. Phytochem. 1989, 28, 1909–1911. [Google Scholar] [CrossRef]
- Beigi, M.; Torki-Harchegani, M.; Pirbalouti, A.G. Quantity and chemical composition of essential oil of peppermint (Mentha piperita L.) leaves under different drying methods. Int. J. Food Prop. 2018, 21, 267–276. [Google Scholar] [CrossRef]
- Schmidt, E.; Bail, S.; Buchhauer, G.; Stoilova, I.; Atanasova, T.; Stoyanova, A.; Krastanov, A.; Jirovetz, L. Chemical composition, olfactory evaluation and antioxidant effects of essential oil from Mentha piperita. Nat. Prod. Commun. 2009, 4, 1107–1112. [Google Scholar] [CrossRef]
- Taherpour, A.A.; Khaef, S.; Yari, A.; Nikeafshar, S.; Fathi, M.; Ghamban, S. Chemical composition analysis of the essential oil of Mentha piperita L. from Kermanshah, Iran by hydrodistillation and HS/SPME methods. J. Anal. Sci. Technol. 2017, 8, 11. [Google Scholar] [CrossRef]
- Barnard, D.R. Repellency of essential oils to mosquitoes. J. Med. Entomol. 1999, 36, 625–629. [Google Scholar] [CrossRef]
- Kumar, S.; Wahub, N.; Warikoo, R. Bioefficacy of Mentha piperita essential oil against dengue fever mosquito Aedes aegypti L. Asian J. Trop. Biomed. 2011, 1, 85–88. [Google Scholar] [CrossRef]
- Patience, G.S.; Karirekinyana, G.; Galli, F.; Patience, M.A.; Kubwabo, C.; Collin, G.; Bizimana, J.C.; Boffito, D.C. Sustainable manufacture of insect repellents derived from Nepeta cataria. Sci. Rep. 2018, 8, 2235. [Google Scholar] [CrossRef]
- Ahl, H.S.-A.; Naguib, N.Y.; Hussein, M. Evaluation growth and essential oil content of catmint and lemon catnip plants as new cultivated medicinal plants in Egypt. Ann. Agri. Sci. 2018, 63, 201–205. [Google Scholar] [CrossRef]
- Bourrel, C.; Perineau, F.; Michel, G.; Bessiere, J.M. Catnip (Nepeta cataria L.) essential oil: Analysis of chemical constituents, bacteriostatic and fungistatic properties. J. Essent. Oil Res. 1963, 5, 159–167. [Google Scholar] [CrossRef]
- Kim, J.H.; Jung, D.H.; Park, H.K. The composition of essential oil from Nepeta cataria and its effect on microorganism. J. Ecol. Field Biol. 2006, 29, 81–387. [Google Scholar] [CrossRef]
- Mohammadi, S.; Saharkhiz, M.J. Changes in essential oil content and composition of catnip (Nepeta cataria L.) during different developmental stages. J. Essent. Oil Bear. Plants 2011, 14, 396–400. [Google Scholar] [CrossRef]
- Zomorodian, K.; Saharkhiz, M.J.; Shariati, S.; Pakshir, K.; Rahimi, M.J.; Khashei, R. Chemical composition and antimicrobial activities of essential oils from Nepeta cataria L. against common causes of food-borne infections. ISRN Pharm 2012, 591953. [Google Scholar] [CrossRef]
- Reichert, W.; Park, H.C.; Juliani, H.R.; Simon, J.E. ‘CR9’: A new highly aromatic catnip Nepeta cataria L. cultivar rich in Z.E-Nepetalactone. HortSci. 2016, 51, 588–591. [Google Scholar] [CrossRef]
- Reichert, W.; Ejercito, J.; Guda, T.; Dong, X.; Wu, Q.; Ray, A.; Simon, J.E. Repellency assessment of Nepeta cataria essential oils and isolated nepetalactones on Aedes aegypti. Sci. Rep. 2019, 9, 1524. [Google Scholar] [CrossRef]
- Simmons, D.; Gobble, C.; Chickos, J. Vapor pressure and enthalpy of vaporization of oil of catnip by correlation gas chromatography. J. Chem. Therm. 2016, 92, 126–131. [Google Scholar] [CrossRef]
- N’guessan, R.; Knols, B.G.J.; Pennetier, C.; Rowland, M. DEET microencapsulation: A slow-release formulation enhancing the residual efficacy of bed nets against malaria vectors. Trans. R. Soc. Trop. Med. Hyg. 2008, 102, 259–262. [Google Scholar] [CrossRef] [PubMed]
- Melo, N.; Capek, M.; Arenas, O.M.; Afify, A.; Yilmaz, A.; Potter, C.J.; Laminette, P.J.; Para, A.; Gallio, M.; Stensmyr, M.C. The irritant receptor TRPA1 mediates the mosquito repellent effect of catnip. Curr. Biol. 2021, 31, 1988–1994. [Google Scholar] [CrossRef] [PubMed]
- Badshah, S.L.; Jehan, R. Density-functional theory of the catnip molecule, nepetalactone. Mol. Cell. Biochem. 2022, 477, 1139–1153. [Google Scholar] [CrossRef]
- Birkett, M.A.; Hassanali, A.; Hoglund, S.; Pettersson, J.; Pickett, J.A. Repellent activity of catmint, Nepeta cataria, and iridoid nepetalactone isomers against Afro-tropical mosquitoes, ixodid ticks and red poultry mites. Phytochemistry 2011, 72, 109–114. [Google Scholar] [CrossRef]
- Hassaballa, I.B.; Matoke-Muhia, D.; Masiga, D.K.; Sole, C.L.; Torto, B.; Tchouassi, D.P. Behavioural responses of Phlebotomus duboscqi to plant-derived volatile organic compounds. Med. Vet. Entomol. 2021, 35, 625–632. [Google Scholar] [CrossRef]
- Debboun, M.; Frances, S.P.; Strickman, D. Insect Repellents Handbook, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2020. [Google Scholar]
| Repellency (%) | Avoidance (%) | Mortality (%) | ||||
|---|---|---|---|---|---|---|
| Chemical (1%) | Mean (Stderr) | Mean (Stderr) | Mean (Stderr) | |||
| Catnip (Nepeta cataria) oil | 100.0 (0.0) | a | 100.0 (0.0) | a | 0 | b |
| Amyris (Amyris balsamifera) oil | 99.4 (0.6) | a | 100.0 (0.0) | a | 0 | b |
| Century tea tree (Melaleuca alternifolia) oil | 88.9 (4.4) | a | 99.3 (0.7) | a | 5.0 (2.9) | b |
| Peppermint (Mentha piperita) oil | 88.3 (4.4) | a | 98.1 (1.9) | a | 5.0 (2.9) | b |
| DEET | 85.3 (1.0) | ab | 98.0 (2.0) | a | 1.7 (1.7) | b |
| Allspice (Pimenta dioica) oil | 73.3 (1.7) | ab | 93.1 (3.8) | a | 3.3 (3.3) | b |
| Decanoic acid | 70.8 (8.5) | ab | 94.6 (4.0) | a | 0 | b |
| Undecanoic acid | 70.0 (3.5) | ab | 100 (0.0) | a | 16.7 (6.0) | ab |
| Mexican oregano (Lippia graveolens) oil | 61.7 (17.8) | ab | 90.2 (2.8) | a | 15.0 (10.4) | b |
| Dodecanoic acid | 55.0 (13.5) | ab | 90.9 (3.1) | a | 0 | b |
| IR3535 | 54.6 (8.9) | ab | 80.5 (12.5) | a | 7.5 (4.3) | b |
| Nonanoic acid | 30.8 (28.1) | bc | 98.6 (1.4) | a | 53.3 (24.2) | a |
| Solvent (acetone) only | −10.7 (9.1) | c | −15.8 (13.7) | b | 0 | b |
| Inhibitor Concentration (µM) | % Residual rPpAChE1 Activity (Compared to No Inhibitor) ± Std. Dev. * | |||
|---|---|---|---|---|
| Nonanoic Acid | Undecanoic Acid | IR3535 | DEET | |
| 0.0508 | 99.7 ± 1.8 | 101.6 ± 1.2 | 106.9 ± 1.2 | 97.6 ± 0.6 |
| 0.152 | 102.1 ± 0.7 | 100.8 ± 0.4 | 107.2 ± 2.2 | 96.6 ± 0.4 |
| 0.457 | 102.9 ± 1.1 | 102.7 ± 0.5 | 107.5 ± 0.9 | 97.5 ± 0.7 |
| 1.37 | 103.7 ± 0.5 | 101.9 ± 1.1 | 100.0 ± 7.0 | 94.5 ± 0.5 |
| 4.12 | 103.3 ± 0.8 | 102.6 ±0.3 | 105.0 ± 0.4 | 96.8 ± 0.7 |
| 12.3 | 103.7 ± 1.5 | 102.5 ± 0.5 | 104.5 ± 0.6 | 95.1 ± 1.8 |
| 37 | 102.2 ± 0.5 | 101.9 ± 0.9 | 106.4 ± 2.1 | 94.7 ± 1.3 |
| 111 | 101 ± 1.7 | 101.3 ± 0.7 | 105.7 ± 4.0 | 90.8 ± 3.0 |
| 333 | 98.5 ± 1.5 | 100.3 ± 1.4 | 103.0 ± 3.9 | 77.1 ± 13.1 |
| 1000 | 96.0 ± 2.3 | 98.2 ± 0.4 | 103.8 ± 5.0 | 93.2 ± 1.4 |
| Material | Concentration at Which Spatial Repellency Fell Below 50% |
|---|---|
| Catnip (Nepeta cataria) oil | 0.125% (11.65 µg/cm2) |
| Amyris (Amyris balsamifera) oil | 0.06125% (9.825 µg/cm2) |
| Century tea tree (Melaleuca alternifolia) oil | ND * |
| Peppermint (Mentha piperita) oil | 0.03125% (4.9125 µg/cm2) |
| DEET | 0.25% (39.31 µg/cm2) |
| Allspice (Pimenta dioica) oil | ND |
| Decanoic acid (C10:0) | ND |
| Undecanoic acid (C11:0) | ND |
| Mexican oregano (Lippia graveolens) oil | ND |
| Dodecanoic acid (C12:0) | ND |
| IR3535 | 0.5% (78.62 µg/cm2) |
| Nonanoic acid (C9:0) | 1% |
| Solvent (acetone) only control | ND |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Temeyer, K.B.; Schlechte, K.G.; Coats, J.R.; Cantrell, C.L.; Rosario-Cruz, R.; Lohmeyer, K.H.; Pérez de León, A.A.; Li, A.Y. In Vitro Evaluation of Essential Oils and Saturated Fatty Acids for Repellency against the Old-World Sand Fly, Phlebotomus papatasi (Scopoli) (Diptera: Psychodidae). Insects 2024, 15, 155. https://doi.org/10.3390/insects15030155
Temeyer KB, Schlechte KG, Coats JR, Cantrell CL, Rosario-Cruz R, Lohmeyer KH, Pérez de León AA, Li AY. In Vitro Evaluation of Essential Oils and Saturated Fatty Acids for Repellency against the Old-World Sand Fly, Phlebotomus papatasi (Scopoli) (Diptera: Psychodidae). Insects. 2024; 15(3):155. https://doi.org/10.3390/insects15030155
Chicago/Turabian StyleTemeyer, Kevin B., Kristie G. Schlechte, Joel R. Coats, Charles L. Cantrell, Rodrigo Rosario-Cruz, Kimberly H. Lohmeyer, Adalberto A. Pérez de León, and Andrew Y. Li. 2024. "In Vitro Evaluation of Essential Oils and Saturated Fatty Acids for Repellency against the Old-World Sand Fly, Phlebotomus papatasi (Scopoli) (Diptera: Psychodidae)" Insects 15, no. 3: 155. https://doi.org/10.3390/insects15030155
APA StyleTemeyer, K. B., Schlechte, K. G., Coats, J. R., Cantrell, C. L., Rosario-Cruz, R., Lohmeyer, K. H., Pérez de León, A. A., & Li, A. Y. (2024). In Vitro Evaluation of Essential Oils and Saturated Fatty Acids for Repellency against the Old-World Sand Fly, Phlebotomus papatasi (Scopoli) (Diptera: Psychodidae). Insects, 15(3), 155. https://doi.org/10.3390/insects15030155









