Morphological Characterization of the Antenna and Scent Patch of Three Danaus Species (Papilionoidea: Nymphalidae, Danainae)
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Microscopy
2.3. Terminology
2.4. Morphometric Measurements
2.5. Data Analysis
3. Results
3.1. Descriptions of the Wing Coloration and Pattern of the Three Danaus Species
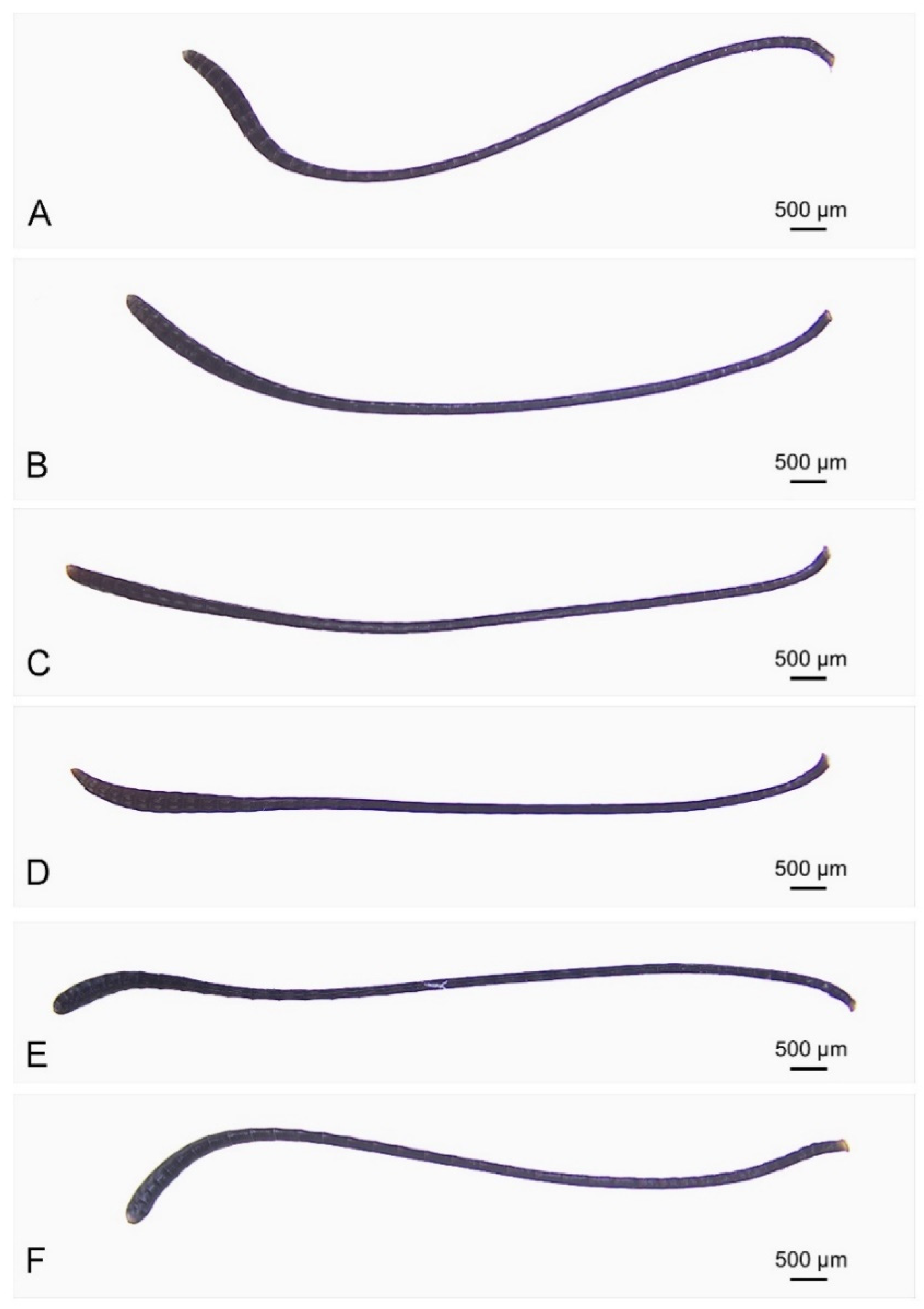
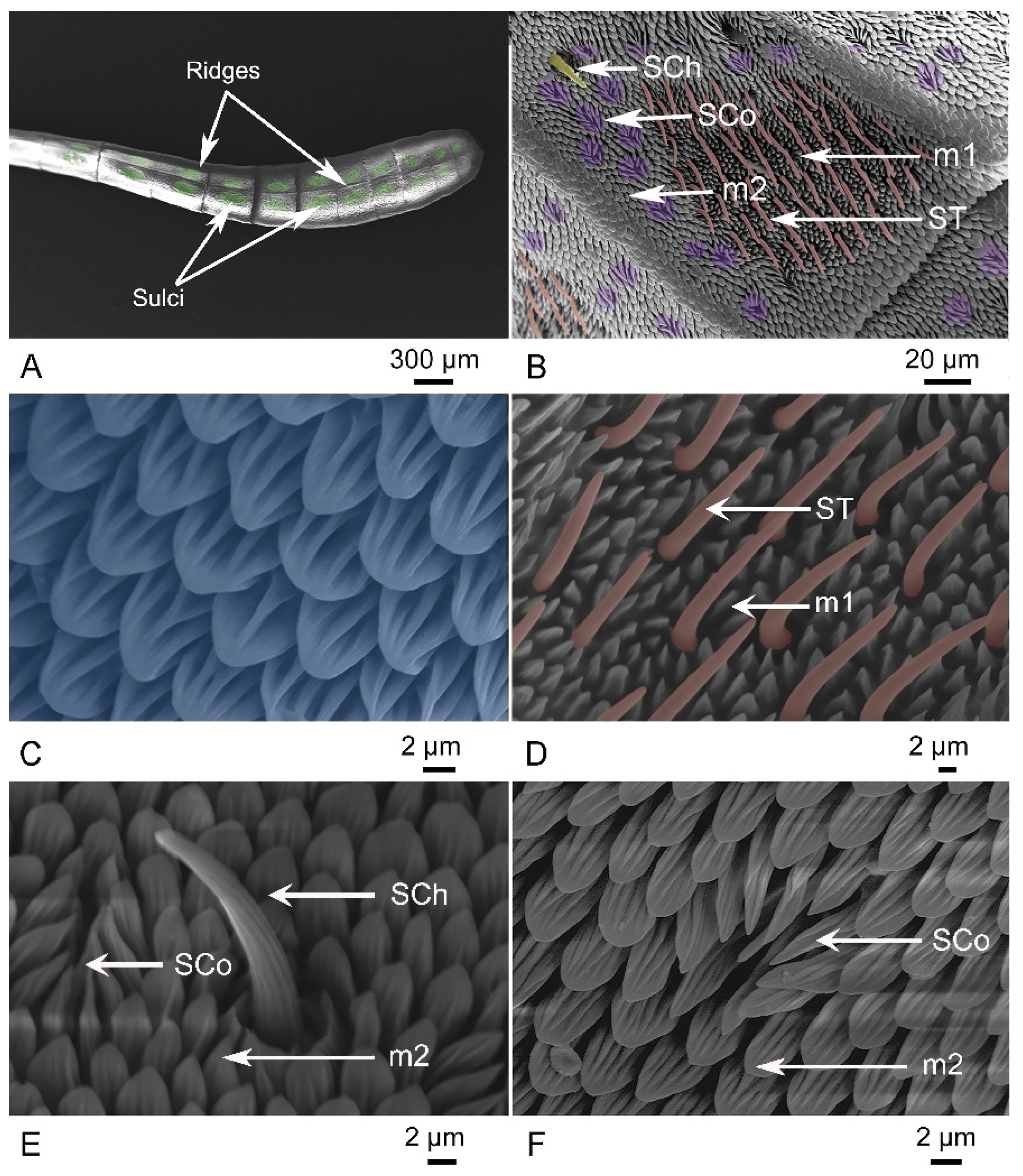
3.2. Comparative Morphology of Antennae
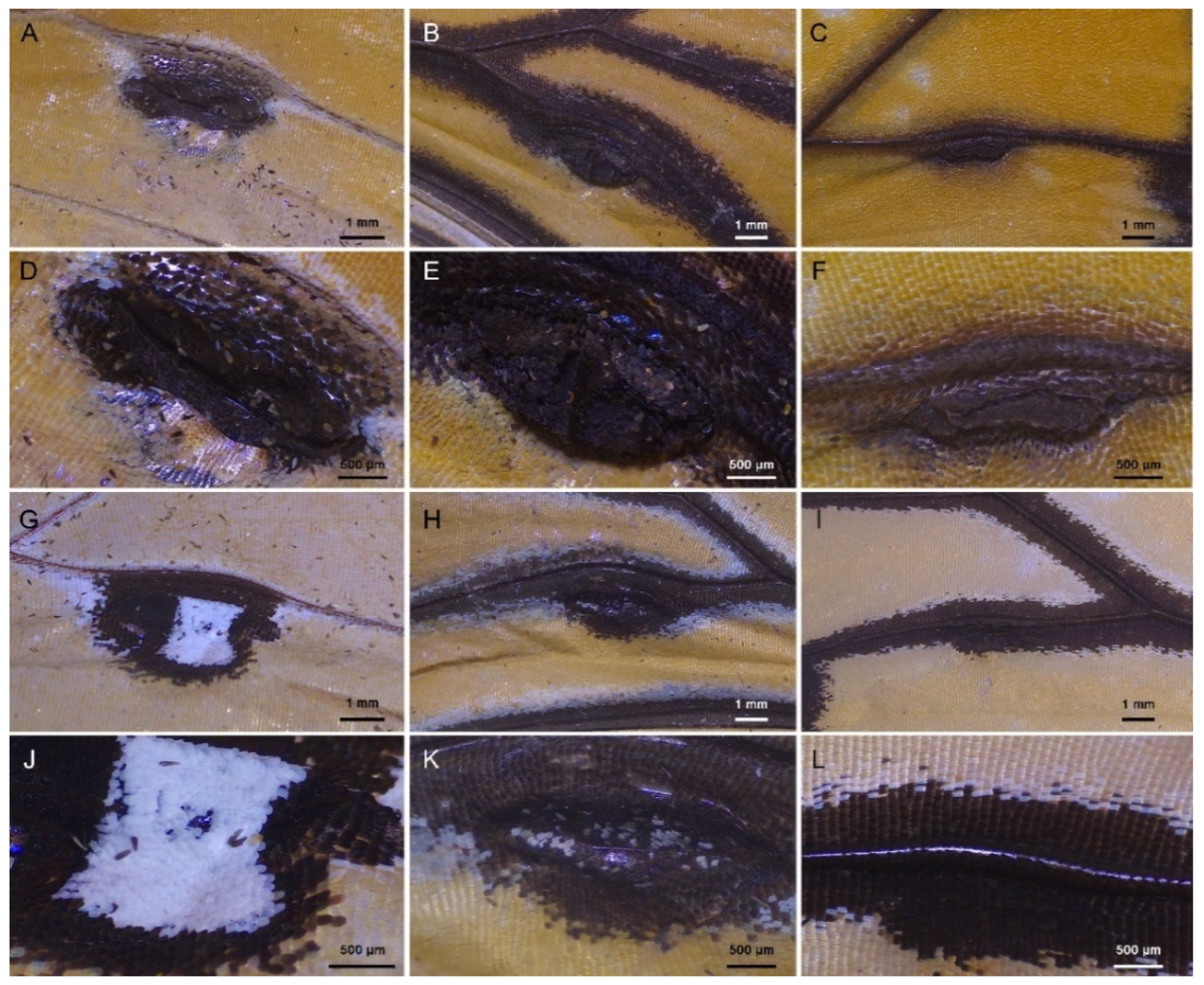
3.3. Comparative Morphology of Male Scent Patches
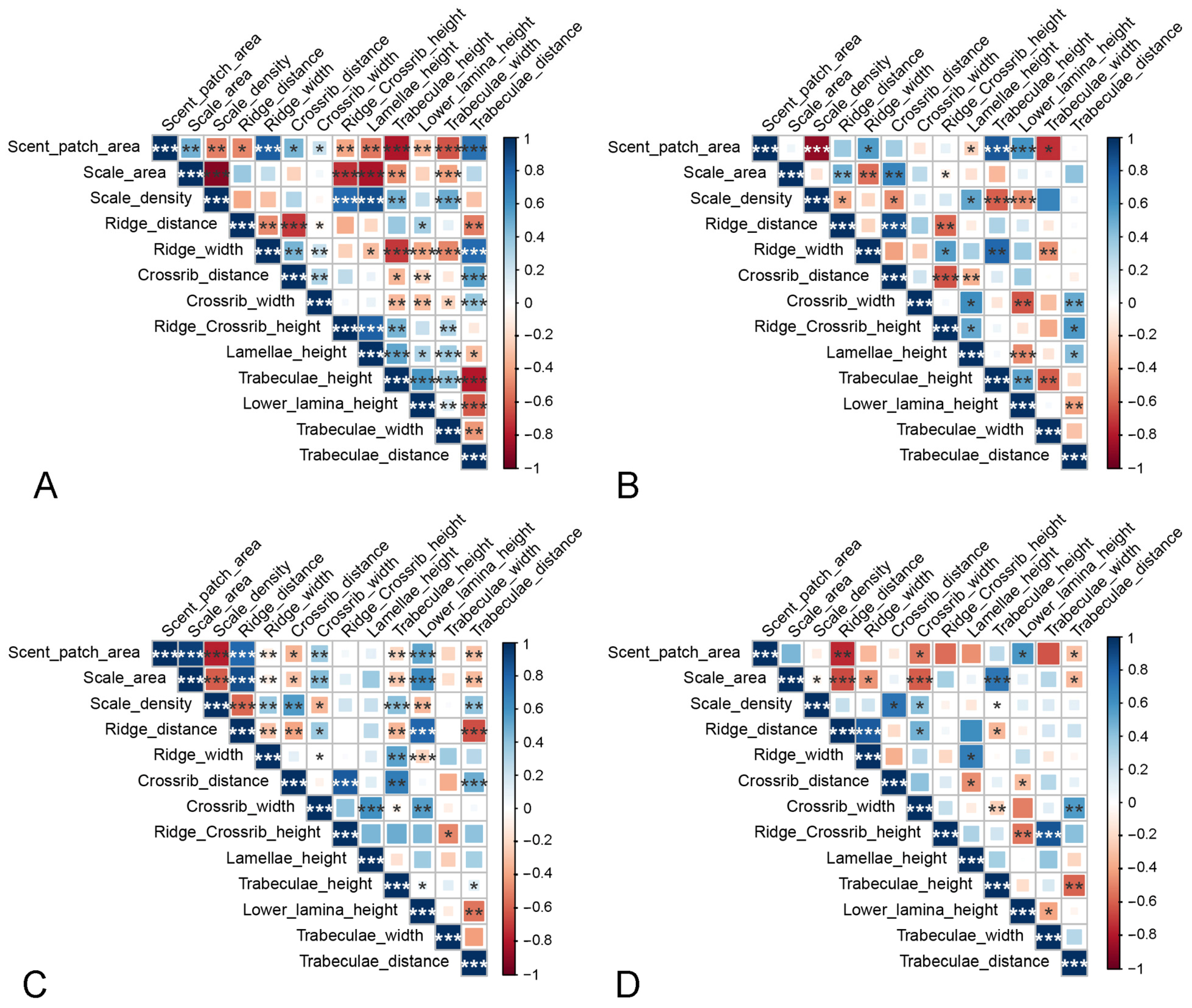
3.4. Correlations between the Morphological Characteristics of Antenna and Androconial Scales
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Smith, D.A.S.; Lushai, G.; Allen, J.A. A classification of Danaus butterflies (Lepidoptera: Nymphalidae) based upon data from morphology and DNA. Zool. J. Linn. Soc. 2005, 144, 191–212. [Google Scholar] [CrossRef][Green Version]
- Reichstein, T.; Von Euw, J.; Parsons, J.A.; Rothschild, M. Heart Poisons in the Monarch Butterfly: Some aposematic butterflies obtain protection from cardenolides present in their food plants. Science 1968, 161, 861–866. [Google Scholar] [CrossRef] [PubMed]
- Pocius, V.M.; Majewska, A.A.; Freedman, M.G. The role of experiments in monarch butterfly conservation: A review of recent studies and approaches. Ann. Entomol. Soc. Am. 2022, 115, 10–24. [Google Scholar] [CrossRef] [PubMed]
- Thomas, E. Courtship Behavior of the Monarch Butterfly, Danaus plexippus L. Ann. Entomol. Soc. Am. 1975, 68, 143–151. [Google Scholar] [CrossRef]
- Lundgren, L.; Bergström, G. Wing scents and scent-released phases in the courtship behavior of Lycaeides argyrognomon (Lepidoptera: Lycaenidae). J. Chem. Ecol. 1975, 1, 399–412. [Google Scholar] [CrossRef]
- Simmons, R.B.; Weller, S.J.; Johnson, S.J. The evolution of androconia in mimetic tiger moths (Noctuoidea: Erebidae: Arctiinae: Ctenuchina and Euchromiina). Ann. Entomol. Soc. Am. 2012, 105, 801–816. [Google Scholar] [CrossRef]
- Mann, F.; Szczerbowski, D.; de Silva, L.; McClure, M.; Elias, M.; Schulz, S. 3-Acetoxy-fatty acid isoprenyl esters from androconia of the ithomiine butterfly Ithomia salapia. Beilstein J. Org. Chem. 2020, 16, 2776–2787. [Google Scholar] [CrossRef] [PubMed]
- Boppre, M.; Vanewright, R.I. Androconial systems in Danainae (Lepidoptera): Functional morphology of Amauris, Danaus, Tirumala and Euploea. Zool. J. Linn. Soc. 1989, 97, 101–133. [Google Scholar] [CrossRef]
- Myers, J. The structure of the antennae of the Florida Queen butterfly, Danaus gilippus berenice (Cramer). J. Morphol. 1968, 125, 315–328. [Google Scholar] [CrossRef]
- Boppre, M.; Petty, R.L.; Schneider, D.; Meinwald, J. Behaviorally mediated contacts between scent organs: Another prerequisite for pheromone production in Danaus chrysippus males (Lepidoptera). J. Comp. Physiol. 1978, 126, 97–103. [Google Scholar] [CrossRef]
- Honda, K.; Honda, Y.; Matsumoto, J.; Tsuruta, Y.; Yagi, W.; Omura, H.; Honda, H. Production and sex-pheromonal activity of alkaloid-derived androconial compounds in the danaine butterfly, Parantica sita (Lepidoptera: Nymphalidae: Danainae). Biol. J. Linn. Soc. 2016, 119, 1036–1059. [Google Scholar] [CrossRef]
- Tsai, C.C.; Childers, R.A.; Shi, N.N.; Ren, C.; Pelaez, J.N.; Bernard, G.D.; Pierce, N.E.; Yu, N.F. Physical and behavioral adaptations to prevent overheating of the living wings of butterflies. Nat. Commun. 2020, 11, 551. [Google Scholar] [CrossRef] [PubMed]
- Lou, C.H.; An, S.; Yang, R.H.; Zhu, H.R.; Shen, Q.C.; Jiang, M.D.; Fu, B.W.; Tao, P.; Song, C.Y.; Deng, T.; et al. Enhancement of infrared emissivity by the hierarchical microstructures from the wing scales of butterfly Rapala dioetas. APL Photonics 2021, 6, 036101. [Google Scholar] [CrossRef]
- Zhu, D.K.; Lu, J.F.; Zheng, M.J.; Wang, D.K.; Wang, J.Y.; Liu, Y.X.; Wang, X.H.; Zhang, M. Self-powered bionic antenna based on triboelectric nanogenerator for micro-robotic tactile sensing. Nano Energy 2023, 114, 108644. [Google Scholar] [CrossRef]
- Shi, Y.; Zhang, X.D.; Qiu, Q.X.; Gao, Y.R.; Huang, Z.M. Design of Terahertz detection antenna with fractal butterfly structure. IEEE Access 2021, 9, 113823–113831. [Google Scholar] [CrossRef]
- Zhou, Y. Monographia Rhopalocerorum Sinensium (Monograph of Chinese Butterflies); Henan Science and Technology Press: Zhengzhou, China, 2000; ISBN 9787534915741. [Google Scholar]
- Moradinour, Z.; Wiklund, C.; Jie, V.W.; Restrepo, C.E.; Gotthard, K.; Miettinen, A.; Perl, C.D.; Baird, E. Sensory organ investment varies with body size and sex in the butterfly Pieris napi. Insects 2021, 12, 1064. [Google Scholar] [CrossRef]
- Boddum, T.; Skals, N.; Hill, S.R.; Hansson, B.S.; Hillbur, Y. Gall midge olfaction: Pheromone sensitive olfactory neurons in Contarinia nasturtii and Mayetiola destructor. J. Insect Physiol. 2010, 56, 1306–1314. [Google Scholar] [CrossRef]
- Crook, D.J.; Mordue, A.J. Olfactory responses and sensilla morphology of the blackcurrant leaf midge Dasineura tetensi. Entomol. Exp. Et Appl. 1999, 91, 37–50. [Google Scholar] [CrossRef]
- Schneider, D. Insect antennae. Annu. Rev. Entomol. 1964, 9, 103–122. [Google Scholar] [CrossRef]
- Limberger, G.M.; Brugnera, R.; da Fonseca, D.B. Antennal morphology and sensilla ultrastructure of Ascia monuste (Linnaeus) (Lepidoptera: Pieridae). Micron 2021, 142, 103000. [Google Scholar] [CrossRef]
- Ghiradella, H. Insect cuticular surface modifications: Scales and other structural formations. Adv. Insect Physiol. 2010, 38, 135–180. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, C.R.; Saalfeld, S.; Schmid, B.; Tinevez, J.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.F.; Li, F.F.; Cao, Y.; Liao, H.J. Universal cooling patterns of the butterfly wing scales hierarchy deduced from the heterogeneous thermal and structural properties of Tirumala limniace (Lepidoptera: Nymphalidae, Danainae). Insect Sci. 2022, 29, 1761–1772. [Google Scholar] [CrossRef] [PubMed]
- Gómez, V.R.C.; Carrasco, J.V. Morphological characteristics of antennal sensilla in Talponia batesi (Lepidoptera: Tortricidae). Ann. Entomol. Soc. Am. 2008, 101, 181–188. [Google Scholar] [CrossRef][Green Version]
- Nonglait, K.C.L.; Das, K.S.; Marwein, C.B.; Kharthangmaw, J.M.; Choudhury, S. Scanning electron microscopy study of the antennal sensilla of cob borer, Stenachroia elongella (Lepidoptera: Pyralidae). Microsc. Res. Tech. 2023, 86, 556–564. [Google Scholar] [CrossRef]
- Zhou, H.; Wu, W.J.; Zhnag, Z.F.; Zhnag, Y. Antennal sensilla of Apanteles cypris Nixon (Hymenoptera: Braconidae), a larval endoparasitoid of Cnaphalocrocis medinalis Guenée (Lepidoptera: Pyralidae). Microsc. Res. Tech. 2011, 74, 1199–1208. [Google Scholar] [CrossRef]
- Odendaal, F.J.; Ehrlich, P.R.; Thomas, F.C. Structure and function of the antennae of Euphydryas editha (Lepidoptera: Nymphalidae). J. Morphol. 1985, 184, 3–22. [Google Scholar] [CrossRef]
- Frank, D.L.; Leskey, T.C.; Bergh, J.C. Morphological characterization of antennal sensilla of the dogwood borer (Lepidoptera: Sesiidae). Ann. Entomol. Soc. Am. 2010, 103, 993–1002. [Google Scholar] [CrossRef]
- Chang, X.Q.; Zhang, S.; Lv, L.; Wang, M.Q. Insight into the ultrastructure of antennal sensilla of Mythimna separata (Lepidoptera: Noctuidae). J. Insect Sci. 2015, 15, 124. [Google Scholar] [CrossRef]
- Xu, J.; Deng, C.P.; Lu, W.F.; Wu, S.N. Ultrastructure of antennal sensilla in adults of Dioryctria rubella Hampson (Lepidoptera: Pyralidae). Insects 2021, 12, 821. [Google Scholar] [CrossRef]
- Yuan, X.Q.; Gao, K.; Yuan, F.; Zhang, Y.L. Ultrastructure of antennal sensilla of four skipper butterflies in Parnara sp. and Pelopidas sp. (Lepidoptera, Hesperiidae). Zookeys 2014, 399, 17–27. [Google Scholar] [CrossRef]
- Jian, M.L.; Zhang, L.L.; Mao, R.Q. Studies on the antennal sensilla of Chilades pandava by scanning electron microscopy. J. South China Agric. Univ. 2011, 32, 52–56. [Google Scholar]
- Lan, X.N.; Xiang, S.S.; Zhu, H. Research progress of the types and functions of insect antennal sensilla. J. Environ. Entomol. 2023, 45, 1197–1216. [Google Scholar]
- Okumura, Y.; Ozeki, Y.; Itoh, T.; Ohta, S.; Omura, H. Volatile terpenoids from male wings lacking scent scales in Anthocharis scolymus (Lepidoptera: Pieridae). Appl. Entomol. Zool. 2016, 51, 385–392. [Google Scholar] [CrossRef]
- Pan, Y.; Yu, Z.S.; Yuan, X.Q. Ultrastructure of androconia and surrounding scales of nine species of Hesperiidae (Lepidoptera). Zookeys 2022, 1084, 65–81. [Google Scholar] [CrossRef]
- Caelsson, M.A.; Schäpers, A.; Nässel, D.R.; Janz, N. Organization of the olfactory system of Nymphalidae butterflies. Chem. Senses 2013, 38, 355–367. [Google Scholar] [CrossRef]
- Osotsi, M.I.; Zhang, W.; Zada, I.; Gu, J.J.; Liu, Q.L.; Zhang, D. Butterfly wing architectures inspire sensor and energy applications. Natl. Sci. Rev. 2021, 8, nwaa107. [Google Scholar] [CrossRef] [PubMed]
- Ehlers, S.; Schulz, S. The scent chemistry of butterflies. Nat. Prod. Rep. 2023, 40, 794–818. [Google Scholar] [CrossRef] [PubMed]
- Borges, E.D.; Bonfantti, D.; Ribeiro, C.A.D.; Zarbin, P.H.G. Structures related to pheromone storage in alar androconia and the female abdominal scent gland of Heliconius erato phyllis, Heliconius ethilla narcaea, and Heliconius besckei (Lepidoptera: Nymphalidae: Heliconiinae). J. Morphol. 2020, 281, 388–401. [Google Scholar] [CrossRef] [PubMed]


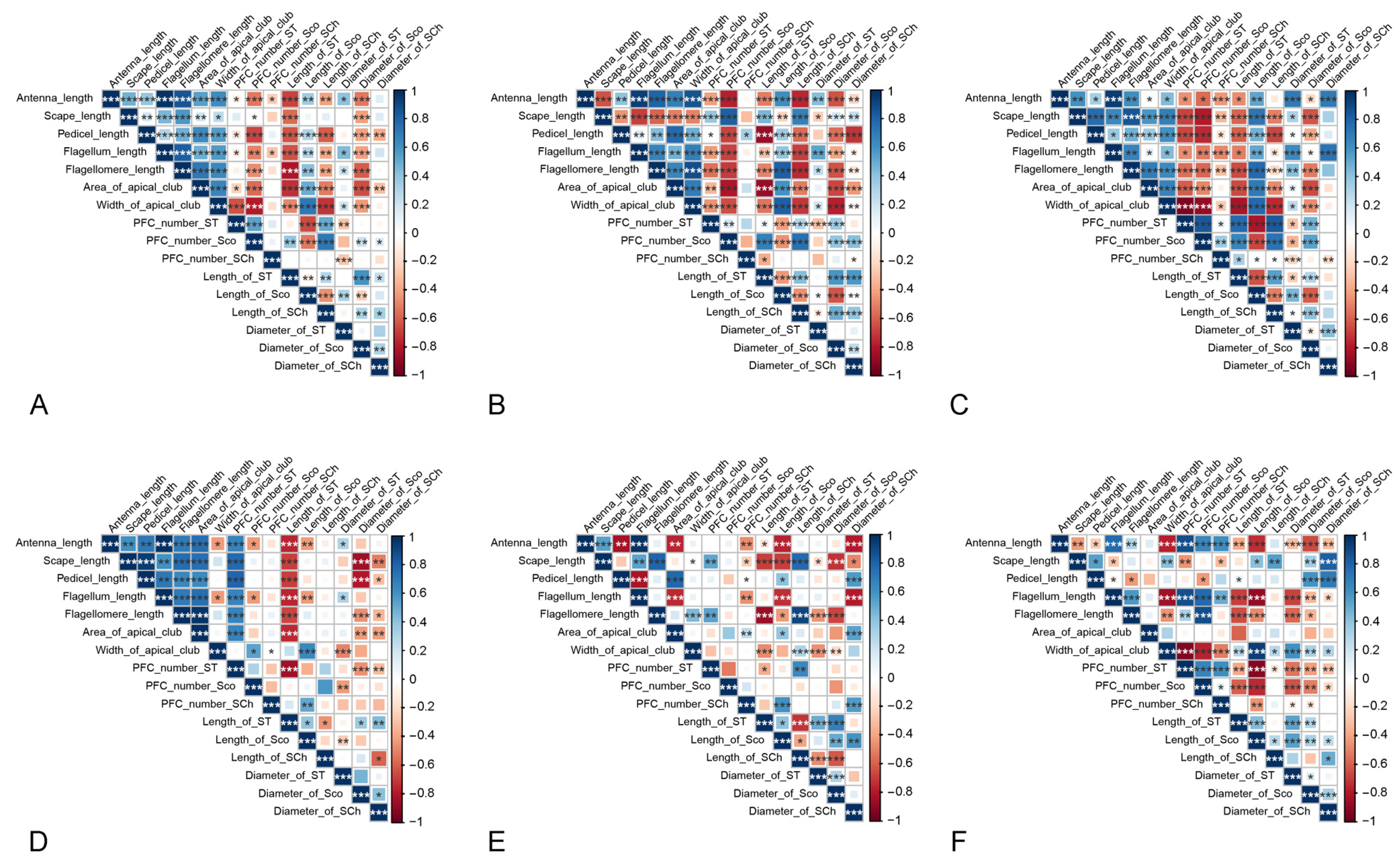
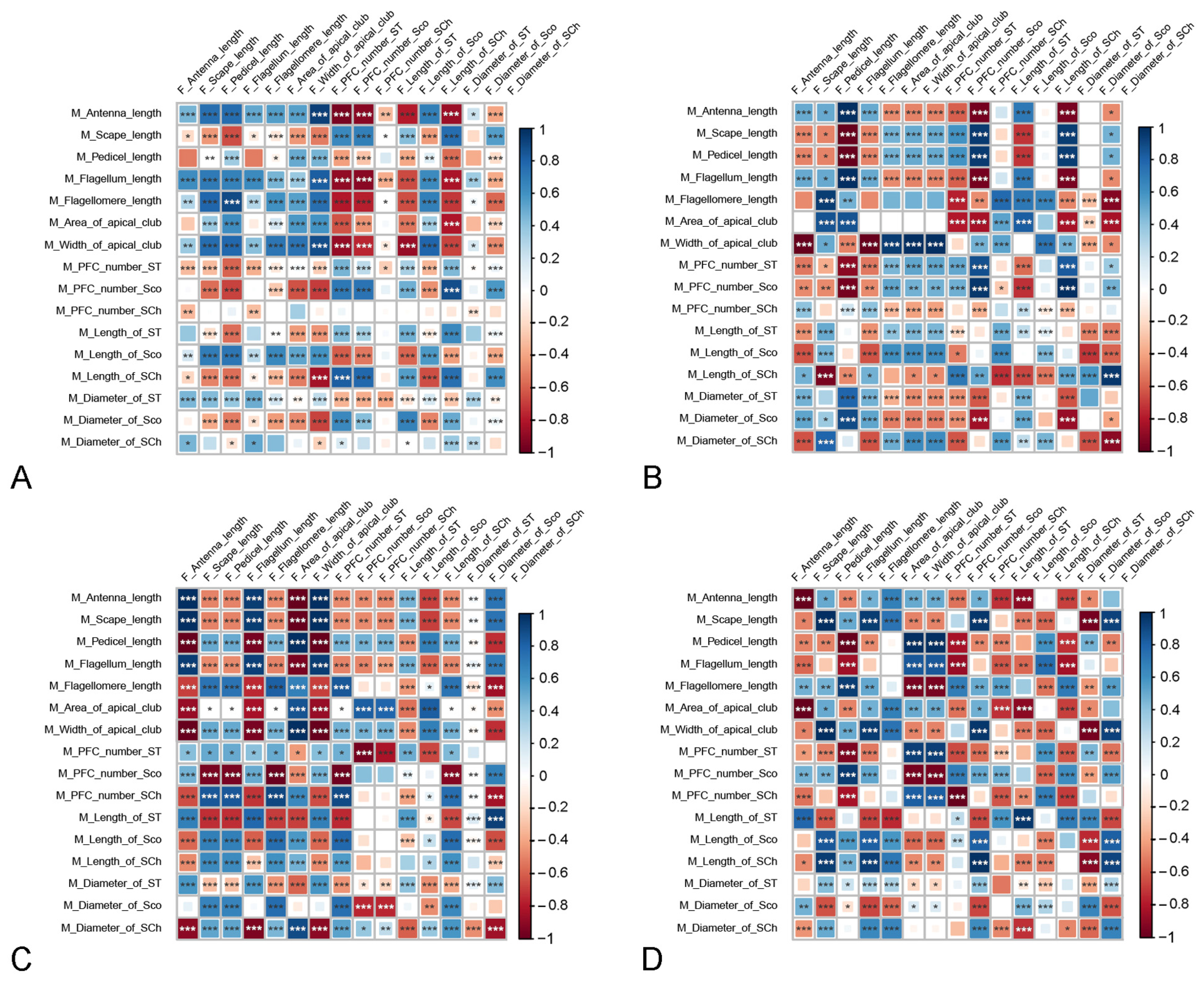

| Item | D. chrysippus | D. genutia | D. plexippus | |||
|---|---|---|---|---|---|---|
| Male | Female | Male | Female | Male | Female | |
| Antenna length (mm) | 28.82 ± 0.16 Ba | 25.66 ± 0.18 Bb | 28.33 ± 0.16 Ba | 28.13 ± 0.13 Bb | 31.11 ± 0.18 Aa | 30.41 ± 0.21 Ab |
| Scape length (mm) | 0.42 ± 0.02 Aa | 0.35 ± 0.01 Ab | 0.45 ± 0.01 Aa | 0.35 ± 0.01 Ab | 0.40 ± 0.01 Ab | 0.43 ± 0.02 Aa |
| Pedicel length (mm) | 0.41 ± 0.01 Aa | 0.32 ± 0.06 Ab | 0.29 ± 0.01 Ba | 0.30 ± 0.02 Ba | 0.33 ± 0.01 Ab | 0.37 ± 0.03 Aa |
| Flagellum length (mm) | 27.95 ± 0.24 Ba | 24.98 ± 0.18 Bb | 27.60 ± 0.16 Ba | 27.49 ± 0.15 Bb | 30.38 ± 0.18 Aa | 29.79 ± 0.04 Ab |
| Flagellomere length (mm) | 0.75 ± 0.01 Ba | 0.65 ± 0.02 Bb | 0.72 ± 0.01 Ba | 0.67 ± 0.03 Bb | 0.77 ± 0.02 Aa | 0.75 ± 0.01 Aa |
| Area of apical club (mm2) | 3.81 ± 0.10 Aa | 3.42 ± 0.15 Ab | 3.18 ± 0.13 Ba | 3.23 ± 0.21 Ba | 3.85 ± 0.28 Aa | 3.72 ± 0.34 Aa |
| Width of apical club (mm) | 0.85 ± 0.04 Ba | 0.86 ± 0.03 Ba | 0.75 ± 0.04 Ca | 0.73 ± 0.03 Cb | 0.98 ± 0.01 Ab | 1.02 ± 0.02 Aa |
| PFC number of ST | 121 ± 2 ABb | 130 ± 6 ABa | 131 ± 6 Aa | 129 ± 5 Aa | 127 ± 6 Ba | 114 ± 1 Bb |
| PFC number of Sco | 285 ± 16 Ba | 291 ± 9 Ba | 347 ± 19 Aa | 336 ± 27 Aa | 282 ± 17 Ba | 261 ± 4 Bb |
| PFC number of SCh | 7 ± 1 Aa | 7 ± 1 Aa | 7 ± 1 Aa | 7 ± 1 Aa | 7 ± 1 Aa | 6 ± 1 Aa |
| Length of ST (μm) | 17.40 ± 0.36 Bb | 20.99 ± 0.63 Ba | 19.36 ± 0.51 Ab | 21.87 ± 0.49 Aa | 18.04 ± 0.46 Bb | 18.83 ± 0.71 Ba |
| Length of Sco (μm) | 16.10 ± 0.47 Ba | 16.33 ± 0.72 Ba | 15.49 ± 0.30 Bb | 16.15 ± 0.48 Ba | 16.68 ± 0.68 Ab | 18.34 ± 0.28 Aa |
| Length of SCh (μm) | 26.47 ± 1.43 Ba | 25.98 ± 1.47 Ba | 34.20 ± 1.21 Aa | 30.72 ± 1.56 Ab | 25.33 ± 1.92 B | 25.38 ± 0.87 B |
| Diameter of ST (μm) | 1.79 ± 0.13 Ba | 1.77 ± 0.08 Ba | 1.82 ± 0.08 ABa | 1.90 ± 0.10 ABa | 1.89 ± 0.06 Ab | 1.96 ± 0.06 Aa |
| Diameter of Sco (μm) | 8.40 ± 0.31 ABb | 8.96 ± 0.12 ABa | 9.27 ± 0.19 Ab | 9.26 ± 0.23 Aa | 8.27 ± 0.43 Bb | 8.82 ± 0.42 Ba |
| Diameter of SCh (μm) | 3.53 ± 0.17 Ba | 3.76 ± 0.22 Ba | 4.83 ± 0.18 Aa | 4.93 ± 0.08 Aa | 4.69 ± 0.10 Ab | 4.90 ± 0.20 Aa |
| Item | Androconial Scales on the Dorsal Wing Surface | Scent Patch Scales on the Ventral Wing Surface | ||||
|---|---|---|---|---|---|---|
| D. chrysippus | D. genutia | D. plexippus | D. chrysippus | D. genutia | D. plexippus | |
| Density (number/mm2) | 557.91 ± 11.99 Ba | 400.15 ± 9.19 Ca | 643.32 ± 4.78 Aa | 348.72 ± 14.04 Ab | 320.51 ± 8.88 Bb | 263.25 ± 7.50 Cb |
| Ridge distance (μm) | 1.53 ± 0.03 Cb | 1.92 ± 0.16 Aa | 1.71 ± 0.09 Bb | 1.81 ± 0.14 Ba | 1.94 ± 0.13 ABa | 2.08 ± 0.18 Aa |
| Ridge width (nm) | 445.61 ± 29.79 Aa | 363.92 ± 19.38 Ba | 327.26 ± 26.97 Ca | 281.34 ± 33.77 Bb | 332.76 ± 27.07 Ab | 256.75 ± 15.45 Bb |
| Crossrib distance (nm) | 590.39 ± 54.24 Aa | 477.50 ± 47.00 Bb | 519.55 ± 30.54 Bb | 616.65 ± 78.79 Ba | 872.06 ± 75.04 Aa | 709.15 ± 72.33 Ba |
| Crossrib width (nm) | 248.80 ± 20.61 Aa | 235.69 ± 14.97 Aa | 235.45 ± 12.49 Aa | 164.13 ± 12.56 Bb | 220.01 ± 14.77 Ab | 125.08 ± 15.35 Cb |
| Lamellae height (μm) | 0.84 ± 0.07 Bb | 0.73 ± 0.06 Ca | 1.74 ± 0.21 Aa | 0.99 ± 0.14 Aa | 0.62 ± 0.19 Ba | 0.64 ± 0.07 Bb |
| Ridge/crossrib height (nm) | 339.69 ± 51.53 Ba | 264.33 ± 41.77 Cb | 405.97 ± 42.57 Aa | 292.55 ± 27.00 Ab | 313.47 ± 30.05 Aa | 247.17 ± 35.46 Bb |
| Trabeculae height (μm) | 1.02 ± 0.17 Cb | 1.41 ± 0.22 Bb | 2.17 ± 0.28 Aa | 2.29 ± 0.18 Aa | 1.71 ± 0.21 Ba | 1.64 ± 0.27 Bb |
| Lower lamina height (nm) | 312.17 ± 38.96 Ba | 326.43 ± 29.62 Ba | 362.87 ± 35.13 Aa | 137.39 ± 9.76 Bb | 187.67 ± 21.25 Ab | 190.82 ± 31.83 Ab |
| Trabeculae width (nm) | 229.65 ± 33.81 Ba | 237.02 ± 23.24 Bb | 273.57 ± 24.47 Aa | 137.60 ± 18.83 Bb | 272.53 ± 44.51 Aa | 129.37 ± 21.73 Bb |
| Trabeculae distance (nm) | 969.31 ± 117.84 Ab | 784.08 ± 76.61 Bb | 652.74 ± 117.15 Ab | 1785.40 ± 280.41 Aa | 1209.47 ± 131.21 Ba | 798.00 ± 81.29 Ca |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Ding, L.; Wang, T.; Liao, H.; Tang, C. Morphological Characterization of the Antenna and Scent Patch of Three Danaus Species (Papilionoidea: Nymphalidae, Danainae). Insects 2024, 15, 121. https://doi.org/10.3390/insects15020121
Yang Y, Ding L, Wang T, Liao H, Tang C. Morphological Characterization of the Antenna and Scent Patch of Three Danaus Species (Papilionoidea: Nymphalidae, Danainae). Insects. 2024; 15(2):121. https://doi.org/10.3390/insects15020121
Chicago/Turabian StyleYang, Yaqi, Linyun Ding, Tong Wang, Huaijian Liao, and Chufei Tang. 2024. "Morphological Characterization of the Antenna and Scent Patch of Three Danaus Species (Papilionoidea: Nymphalidae, Danainae)" Insects 15, no. 2: 121. https://doi.org/10.3390/insects15020121
APA StyleYang, Y., Ding, L., Wang, T., Liao, H., & Tang, C. (2024). Morphological Characterization of the Antenna and Scent Patch of Three Danaus Species (Papilionoidea: Nymphalidae, Danainae). Insects, 15(2), 121. https://doi.org/10.3390/insects15020121






