Genetic Diversity and DNA Barcoding of Thrips in Bangladesh
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
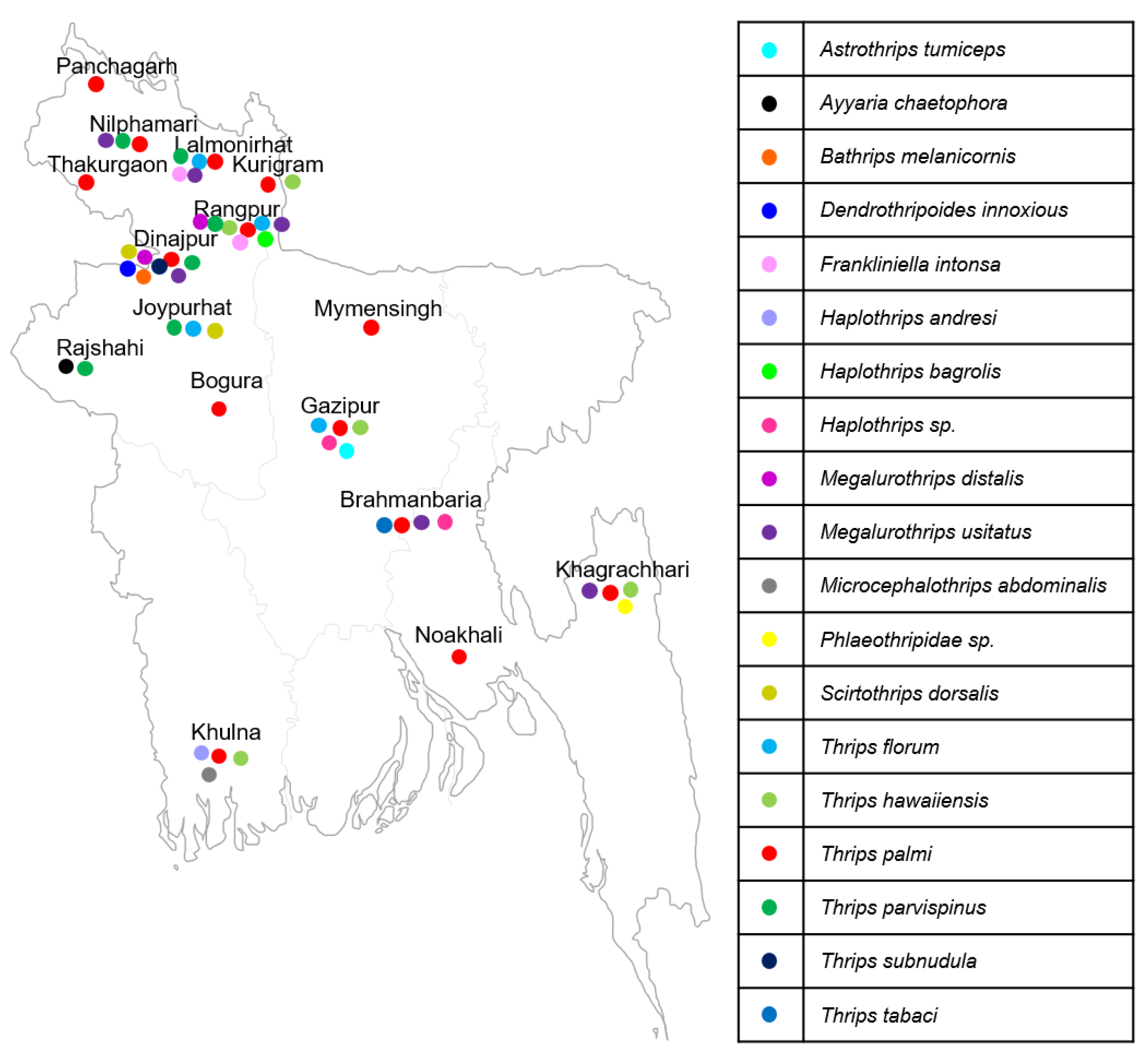
2.1. Thrips Collection
2.2. DNA Extraction
2.3. PCR Amplification
2.4. DNA Sequence and Barcoding Analysis
2.5. Phylogenetic Analysis
2.6. Population Structure and Genetic Analysis
3. Results
3.1. Identification of Thrips Species in Bangladesh
3.2. Barcode Divergence of Thrips Species
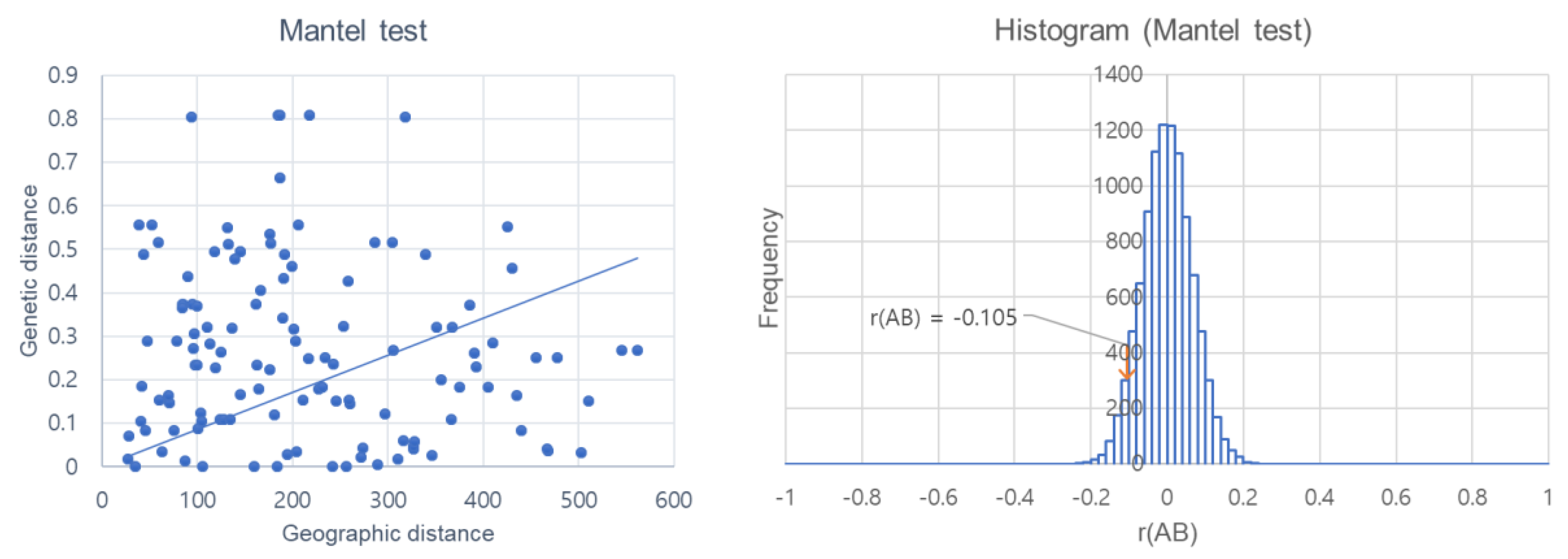
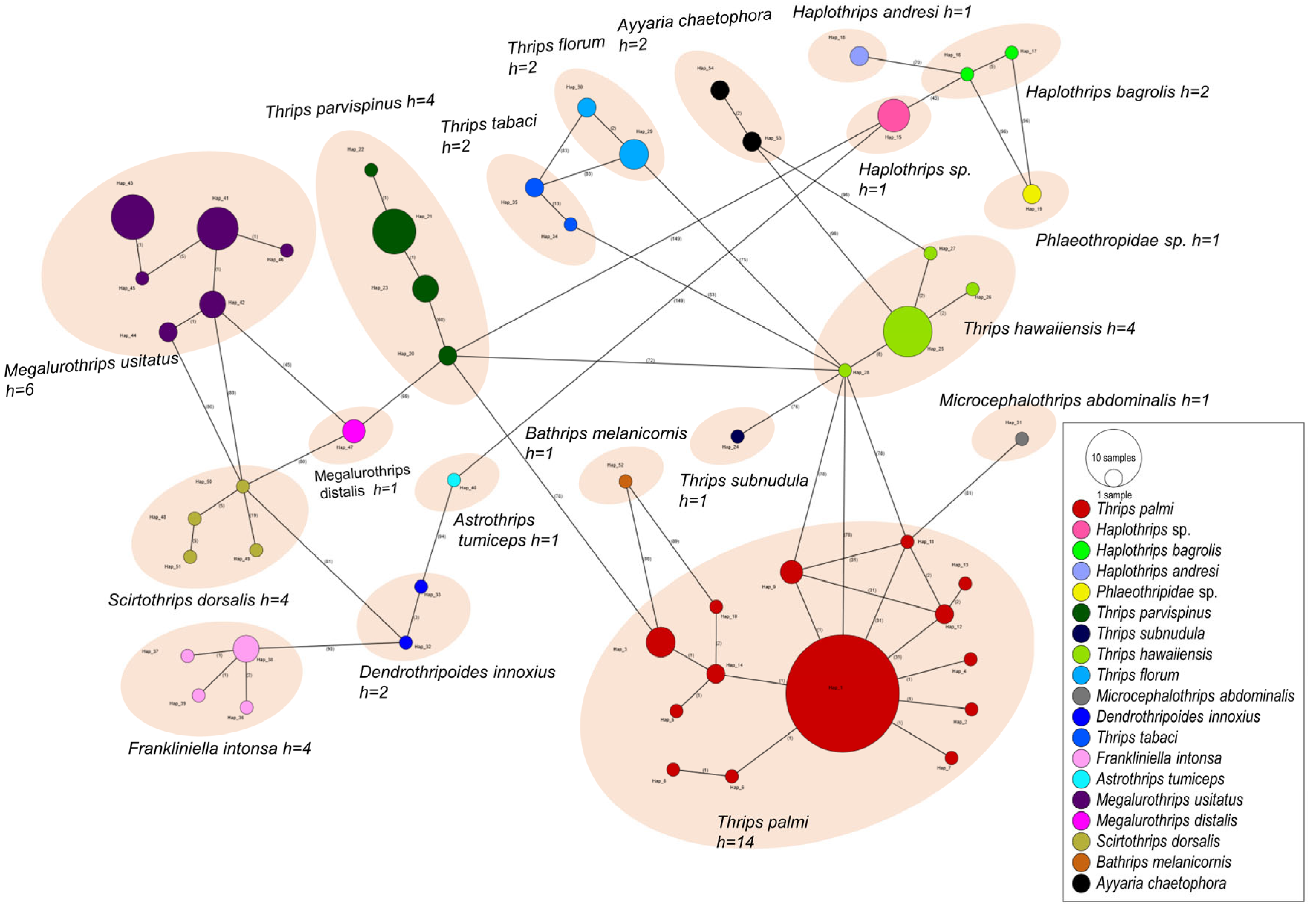
3.3. Genetic Diversity and Gene Flow Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mound, L.A. Thysanoptera: Diversity and interactions. Annu. Rev. Entomol. 2005, 50, 247–269. [Google Scholar] [CrossRef]
- Mandal, B.; Jain, R.K.; Krishnareddy, M.; Krishna Kumar, N.K.; Ravi, K.S.; Pappu, H.R. Emerging problems of Tospoviruses (Bunyaviridae) and their management in the Indian Subcontinent. Plant Dis. 2012, 96, 468–479. [Google Scholar] [CrossRef]
- Macharia, I.; Backhouse, D.; Skilton, R.; Ateka, E.; Wu, S.B.; Njahira, M.; Maina, S.; Harvey, J. Diversity of thrips species and vectors of tomato spotted wilt virus in production systems in Kenya. J. Econ. Entomol. 2015, 108, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Mound, L.A.; Morris, D.C. The insect order Thysanoptera: Classification versus systematics. Zootaxa 2007, 1668, 395–411. [Google Scholar] [CrossRef]
- Riley, D.G.; Joseph, S.V.; Srinivasan, R.; Diffie, S. Thrips vectors of tospoviruses. J. Int. Pest Manag. 2011, 2, I1–I10. [Google Scholar] [CrossRef]
- Rebijith, K.B.; Asokan, R.; Krishna Kumar, N.K.; Krishna, V.; Ramamurthy, V.V. Development of species-specific markers and Molecular differences in mtDNA of Thrips palmi Karny and Scirtothrips dorsalis Hood (Thripidae: Thysanoptera), vectors of tospoviruses (Bunyaviridae) in India. Entomol. News 2011, 122, 201–213. [Google Scholar] [CrossRef]
- Hassani-Mehraban, A.; Botermans, M.; Verhoeven, J.T.; Meekes, E.; Saaijer, J.; Peters, D.; Goldbach, R.; Kormelink, R. A distinct tospovirus causing necrotic streak on Alstro-emeria sp. in Colombia. Arch. Virol. 2010, 155, 423–428. [Google Scholar] [CrossRef] [PubMed]
- Ullman, D.E.; Sherwood, J.L.; German, T.L. Thrips as vectors of plant pathogens. In Thrips as Crop Pests; Lewis, T., Ed.; CAB International: New York, NY, USA, 1997; pp. 539–565. Available online: https://www.cabdirect.org/cabdirect/abstract/19981100131 (accessed on 1 February 2024).
- Pappu, H.R.; Jones, R.A.C.; Jain, R.K. Global status of tospovirus epidemics in diverse cropping systems: Successes achieved and challenges ahead. Virus Res. 2009, 141, 219–236. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.R. Plant viruses transmitted by thrips. Eur. J. Plant Pathol. 2005, 113, 119–157. [Google Scholar] [CrossRef]
- Singh, S.J.; Krishnareddy, M. Watermelon bud necrosis: A new tospovirus disease. Tospoviruses and Thrips of Floral and Vegetable Crops. Acta Hortic. 1996, 431, 68–77. [Google Scholar] [CrossRef]
- Ciuffo, M.; Mautino, G.C.; Bosco, L.; Turina, M.; Tavella, L. Identification of Dictyothrips betae as the vector of Polygonum ring spot virus. Ann. App. Biol. 2010, 157, 299–307. [Google Scholar] [CrossRef]
- Parrella, G.; Gognalons, P.; Gebre-Selassiè, K.; Vovlas, C.; Marchoux, G. An update of the host range of tomato spotted wilt virus. J. Plant Pathol. 2003, 85, 227–264. [Google Scholar]
- Oliver, J.E.; Whitfield, A.E. The genus tospovirus: Emerging Bunyaviruses that threaten food security. Annu. Rev. Virol. 2016, 3, 101–124. [Google Scholar] [CrossRef]
- Janssen, D. Tomato Spotted Wilt Ortho-Tospovirus (Tomato Spotted Wilt), CABI COMPENDIUM; CABI International: Wallingford, UK, 2022. [Google Scholar] [CrossRef]
- Brunner, P.C.; Fleming, C.; Frey, J.E. A molecular identification key for economically important thrips species (Thysanoptera: Thripidae) using direct sequencing and a PCR-RFLP-based approach. Agric. For. Entomol. 2002, 4, 127–136. [Google Scholar] [CrossRef]
- Brunner, P.C.; Chatzivassiliou, E.K.; Katis, N.I.; Frey, J.E. Host-associated genetic differentiation in Thrips tabaci (Insecta; Thysanoptera), as determined from mtDNA sequence data. Heredity 2004, 93, 364–370. [Google Scholar] [CrossRef]
- Murai, T.; Toda, S. Variation of Thrips tabaci in colour and size. In Thrips and Tospoviruses, Proceedings of the 7th International Symposium on Thysanoptera, Reggio Calabria, Italy, 2–7 July 2001; Okayama University: Okayama, Japan, 2002; pp. 377–378. Available online: https://www.ento.csiro.au/thysanoptera/Symposium/Section10/58-Murai-Toda.pdf (accessed on 2 February 2024).
- Tyagi, K.; Kumar, V.; Mound, L.A. Sexual dimorphism among Thysanoptera Terebrantia, with a new species from Malaysia and remarkable species from India in Aelothripidae and Thripidae. Ins. System. Evol. 2008, 39, 155–170. [Google Scholar] [CrossRef]
- Mainali, B.P.; Shrestha, S.; Lim, U.T.; Kim, Y. Molecular markers of two sympatric species of the genus Frankliniella (Thysanoptera: Thripidae). J. Asia-Pac. Entomol. 2008, 11, 45–48. [Google Scholar] [CrossRef]
- Page, R.D.M. DNA barcoding and taxonomy: Dark taxa and dark texts. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2016, 371, 20150334. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, K.; Kumar, V.; Singha, D.; Chandra, K.; Laskar, B.A.; Kundu, S.; Chakraborty, R.; Chatterjee, S. DNA Barcoding studies on Thrips in India: Cryptic species and Species complexes. Sci. Rep. 2017, 7, 4898. [Google Scholar] [CrossRef] [PubMed]
- Iftikhar, R.; Ashfaq, M.; Rasool, A.; Hebert, P.D.N. DNA Barcode Analysis of Thrips (Thysanoptera) Diversity in Pakistan Reveals Cryptic Species Complexes. PLoS ONE 2016, 11, e0146014. [Google Scholar] [CrossRef] [PubMed]
- Kadirvel, P.; Srinivasan, R.; Hsu, Y.C.; Su, F.C.; De La Pena, R. Application of cytochrome oxidase I sequence for phylogenetic analysis and identification of thrips species occurring on vegetable crops. J. Econ. Entomol. 2013, 106, 408–418. [Google Scholar] [CrossRef]
- Rebijith, K.B.; Asokan, R.; Krishna, V.; Ranjitha, H.H.; Kumar, N.K.K.; Ramamurthy, V.V. DNA barcoding and elucidation of cryptic diversity in thrips (Thysanoptera). Fla. Entomol. 2014, 97, 1328–1347. [Google Scholar] [CrossRef]
- Asokan, R.; Rebijith, K.B.; Singh, S.K.; Sidhu, A.S.; Siddharthan, S.; Karanth, P.K.; Ellango, R.; Ramamurthy, V.V. Molecular identification and phylogeny of Bactrocera species (Diptera: Tephritidae). Fla. Entomol. 2011, 94, 1026–1035. [Google Scholar] [CrossRef]
- Glover, R.H.; Collins, D.W.; Walsh, K.; Boonham, N. Assessment of loci for DNA barcoding in the genus Thrips (Thysanoptera: Thripidae). Mol. Ecol. Res. 2010, 10, 51–59. [Google Scholar] [CrossRef]
- Rebijith, K.B.; Asokan, R.; Krishna Kumar, N.K.; Krishna, V.; Chaitanya, B.N.; Ramamurthy, V.V. DNA barcoding and elucidation of cryptic aphid species (Hemiptera: Aphididae) in India. Bull. Entomol. Res. 2013, 103, 601–610. [Google Scholar] [CrossRef]
- Shufran, K.A.; Burd, J.D.; Anstead, J.A.; Lushai, G. Mitochondrial DNA sequence divergence among greenbug (Homoptera: Aphididae) biotypes: Evidence for host-adapted races. Ins. Mol. Biol. 2000, 9, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.F.; Meng, X.Q.; Min, L.; Qiao, W.N.; Wan, F.H. Rapid diagnosis of the invasive species, Frankliniella occidentalis (Pergande): A species-specific COI marker. J. App. Entomol. 2011, 136, 410–420. [Google Scholar] [CrossRef]
- Hebert, P.D.N.; Cywinska, A.; Ball, S.L.; deWaard, J.R. Biological identifications through DNA barcodes. Proc. R. Soc. Lond. Ser. B Biol. Sci. 2003, 270, 313–321. [Google Scholar] [CrossRef]
- Meyer, C.P.; Paulay, G. DNA Barcoding: Error rates based on comprehensive sampling. PLoS Biol. 2005, 3, e422. [Google Scholar] [CrossRef] [PubMed]
- Wijkamp, I.; Almarza, N.; Goldbach, R.; Peters, D. Distinct levels of specificity in thrips transmission of tospoviruses. Phytopathology 1995, 85, 1069–1074. [Google Scholar] [CrossRef]
- Chatzivassiliou, E.K.; Nagata, T.; Katis, N.I.; Peters, D. Transmission of tomato spotted wilt tospovirus by Thrips tabaci populations originating from leek. Plant Pathol. 1999, 48, 700–706. [Google Scholar] [CrossRef]
- Chatzivassiliou, E.K.; Peters, D.; Katis, N.I. The efficiency by which Thrips tabaci populations transmit tomato spotted wilt virus depends on their host preference and reproductive strategy. Phytopathology 2002, 92, 603–609. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tedeschi, R.; Ciuffo, M.; Mason, G.; Roggero, P.; Tavella, L. Transmissibility of four tospoviruses by a thelytokous population of Thrips tabaci from Liguria, Northwestern Italy. Phytoparasitica 2001, 29, 37–45. [Google Scholar] [CrossRef]
- Jacobson, A.L.; Booth, W.; Vargo, E.L.; Kennedy, G.G. Thrips tabaci population genetic structure and polyploidy in relation to competency as a vector of tomato spotted wilt virus. PLoS ONE 2013, 8, e54484. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, A.L.; Kennedy, G.G. Specific insect-virus interactions are responsible for variation in competency of different Thrips tabaci isolines to transmit different tomato spotted wilt virus isolates. PLoS ONE 2013, 8, e54567. [Google Scholar] [CrossRef] [PubMed]
- Fekrat, L.; Shishehbor, P.; Manzari, S.; Nejadian, E.S. Comparative development, reproduction and life table parameters of three populations of Thrips tabaci (Thysanoptera: Thripidae) on onion and tobacco. J. Entomol. Soc. Iran. 2009, 29, 11–23. [Google Scholar]
- Li, X.W.; Fail, J.; Wang, P.; Feng, J.N.; Shelton, A.M. Performance of arrhenotokous and thelytokous Thrips tabaci (Thysanoptera: Thripidae) on onion and cabbage and its implications on evolution and pest management. J. Econ. Entomol. 2014, 107, 1526–1534. [Google Scholar] [CrossRef]
- Toda, S.; Morishita, M. Identification of three-point mutations on the sodium channel gene in pyrethroid-resistant Thrips tabaci (Thysanoptera: Thripidae). J. Econ. Entomol. 2009, 102, 2296–2300. [Google Scholar] [CrossRef]
- Allen, B.; Lippner, G.; Chen, Y.T.; Fotouhi, B.; Momeni, N. Evolutionary dynamics on any population structure. Nature 2017, 544, 227–230. [Google Scholar] [CrossRef]
- Peixoto, M.G.C.D.; Carvalho, M.R.S.; Egito, A.A.; Steinberg, R.S.; Bruneli, F.Â.T.; Machado, M.A.; Santos, F.C.; Rosse, I.C.; Fonseca, P.A.S. Genetic Diversity and Population Genetic Structure of a Guzerá (Bos indicus) meta-population. Animals 2021, 11, 1125. [Google Scholar] [CrossRef]
- Sharma, D.; Rao, D.V. A field survey of pest of cauliflower, cabbage and okra in some areas of Jaipur. Int. J. Life Sci. Biotechnol. Pharm. Res. 2012, 2, 122–127. [Google Scholar]
- Reddy, M.R.S.; Reddy, G.S. An ecofriendly method to combat Helicoverpa armigera (Hub) on sweet orange (Citrus sinensis L.). Insect Environ. 1999, 4, 143–144. [Google Scholar]
- Hoque, M.; Mollik, S.R.; Nazimuddin, M.; Alam, S.N.; Khorsheduzzaman, M.; Jasmine, H.S.; Sultana, N.A.; Rhaman, M.; Ahmed, B.; Rajotte, E.; et al. Survey of bean pod borer (Maruca testulalis), whitefly and aphids and their natural enemies on country bean (Lablab purpureus). In Tenth Annual Report of the Integrated Pest Management Collaborative Research Support Program; IPM CRSP: Blacksburg, VA, USA, 2002; Volume 10, pp. 123–124. [Google Scholar]
- Khatun, M.F.; Jahan, M.; Das, K.R.; Lee, K.Y.; Kil, E.J. Population dynamics and biorational management of sucking insect vectors on chili (Capsicum annuum L.) in Bangladesh. Arch. Insect Biochem. Physiol. 2022, 112, e21980. [Google Scholar] [CrossRef] [PubMed]
- Douglas, M.R.; Chang, J.; Begum, K.; Subramanian, S.; Tooker, J.F.; Alam, S.N.; Ramasamy, S. Evaluation of biorational insecticides and DNA barcoding as tools to improve insect pest management in lablab bean (Lablab purpureus) in Bangladesh. J. Asia-Pac. Entomol. 2018, 21, 1326–1336. [Google Scholar] [CrossRef]
- Folmer, O.; Black, M.; Hoeh, W.; Lutz, R.; Vrijenhoek, R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotechnol. 1994, 3, 294–299. [Google Scholar]
- Schaffer, A.A.; Aravind, L.; Madden, T.L.; Shavirin, S.; Spouge, J.L.; Wolf, Y.I.; Koonin, E.V.; Altschul, S.F. Improving the accuracy of PSI-BLAST protein database searches with composition-based statistics and other refinements. Nucleic Acids Res. 2001, 29, 2994–3005. [Google Scholar] [CrossRef]
- Thompson, J.D.; Gibson, T.J.; Plewniak, F.; Jeanmougin, F.; Higgins, D.G. The Clustal_X windows interface: Flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997, 25, 4876–4882. [Google Scholar] [CrossRef]
- Nei, M.; Kumar, S. Molecular Evolution and Phylogenetics; Oxford University Press: New York, NY, USA, 2000; Volume 333, ISBN 0195135849. [Google Scholar]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef]
- Kimura, M. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 1980, 16, 111–120. [Google Scholar] [CrossRef]
- Letunic, I.; Bork, P. Interactive Tree of Life (iTOL) v5: An online tool for phylogenetic tree display and annotation. Nucleic Acids Res. 2021, 49, 293–296. [Google Scholar] [CrossRef]
- Felsenstein, J. Confidence limits on phylogenies: An approach using the bootstrap. Evolution 1985, 39, 783–791. [Google Scholar] [CrossRef]
- Librado, P.; Rozas, J. DnaSP v5: A software for comprehensive analysis of DNA polymorphism data. Bioinformatics 2009, 25, 1451–1452. [Google Scholar] [CrossRef] [PubMed]
- Tajima, F. Statistical method for testing the neutral mutation hypothesis by DNA polymorphism. Genetics 1989, 123, 585–595. [Google Scholar] [CrossRef]
- Jensen, J.L.; Bohonak, A.J.; Kelley, S.T. Isolation by distance, web service. BMC Genet. 2005, 6, 13. [Google Scholar] [CrossRef]
- Leigh, J.W.; Bryant, D. PopART: Full-feature software for haplotype network construction. Methods. Ecol. Evol. 2015, 6, 1110–1116. [Google Scholar] [CrossRef]
- Excoffier, L.; Smouse, P.E.; Quattro, J.M. Analysis of molecular variance inferred from metric distances among DNA haplotypes: Application to human mitochondrial DNA restriction data. Genetics 1992, 131, 479–491. [Google Scholar] [CrossRef] [PubMed]
- Excoffier, L.; Laval, G.; Schneider, S. ARLEQUIN version 3.0: An integrated software package for population genetics data analysis. Evol. Bioinform. Online 2005, 1, 47–50. [Google Scholar] [CrossRef]
- Crawford, D.L. Thysanoptera of Mexico and the South, I. Pomona College J. Entomol. 1910, 2, 153–170. [Google Scholar]
- Reyes, C. Thysanoptera (Hexapoda) of The Philippine Islands. Raffles Bull. Zool. 1994, 42, 107–507. [Google Scholar]
- Tyagi, K.; Kumar, V. First report of Western Flower Thrips, Frankliniella occidentalis (Pergande) (Thripidae: Thysanoptera) from India—A potential havoc to Indian agriculture. Halters 2015, 6, 1–3. [Google Scholar]
- Ghosh, A.; Jagdale, S.S.; Basavaraj; Dietzgen, R.G. Genetics of Thrips palmi (Thysanoptera: Thripidae). J. Pest Sci. 2020, 93, 27–39. [Google Scholar] [CrossRef]
- Cannon, R.J.C.; Matthews, L.; Collins, D.W. A review of the pest status and control options for Thrips palmi. Crop Prot. 2007, 26, 1089–1098. [Google Scholar] [CrossRef]
- Mound, L.A.; Collins, D.W. A South Asian pest species newly recorded from Europe: Thrips parvispinus (Thysanoptera: Thripidae), its confused identity and potential quarantine significance. J. Eur. Entomol. 2000, 97, 197–200. [Google Scholar] [CrossRef]
- Tyagi, K.; Kumar, V.; Singha, D.; Chakraborty, R. Morphological and DNA barcoding evidence for invasive pest thrips, Thrips parvispinus (Thripidae: Thysanoptera), newly recorded from India. J. Ins. Sci. 2015, 15, 105. [Google Scholar] [CrossRef] [PubMed]
- Nakahara, S. The genus Thrips Linnaeus (Thysanoptera: Thripidae) of the New world. Tech. Bull. United States Dep. Agric. 1994, 1822, 1–183. [Google Scholar]
- Moritz, G.; Mound, L.A.; Morris, D.C.; Goldarazena, A. Pest Thrips of the World—An Identification System Using Molecular and Microscopial Methods; CDROM; Centre for Biological Information Technology, University of Queensland: Brisbane, QLD, Australia, 2004; ISBN 1864997818. [Google Scholar]
- Palmer, J. Megalurothrips in the flowers of tropical legumes: A morphometric study. In Population Structure, Genetics, and Taxonomy of Aphids and Thysanoptera; Holman, J., Pelikan, J., Dixon, A.F.G., Weisman, L., Eds.; SPB Publishin: The Hague, The Netherlands, 1987; pp. 480–495. Available online: https://www.cabidigitallibrary.org/doi/10.1079/cabicompendium.52634 (accessed on 1 February 2024).
- Sartiami, D.; Mound, L. Identification of the terebrantian thrips (Insecta, Thysanoptera) associated with cultivated plants in Java, Indonesia. ZooKeys 2013, 306, 1–21. [Google Scholar] [CrossRef]
- Ananthakrishnan, T.N.; Varatharajan, R.; Gopinathan, K. Seasonal periodicity of thrips infesting some Compositae in relation to pollination. Proc. Indian Natl. Sci. Acad. Calcutta 1981, B47, 811–815. [Google Scholar]
- Gopinathan, K.; Varatharajan, R.; Ananthakrishnan, T.N. Incidence of Microcephalothrips abdominalis (Crawford) (Thysanoptera: Insecta) in relation to the pollination bio1ogy of the weed Ageratum conyzoides Linn. (Compositae). Proc. Indian Natl. Sci. Acad. B47 1981, 4, 505–509. [Google Scholar]
- Prasada-Rao, R.D.V.J.; Reddy, A.S.; Reddy, S.V.; Thirumala-Devi, K.; Rao, S.C.; Kumar, M.V.; Subramaniam, K.; Reddy, Y.T.; Nigam, S.N.; Reddy, D.V.R. The host range of Tobacco streak virus in India and transmission by thrips. Ann. Appl. Biol. 2003, 142, 365–368. [Google Scholar] [CrossRef]
- Sharman, M.; Persley, D.M.; Thomas, J.E. Distribution in Australia and seed transmission of Tobacco streak virus in Parthenium hysterophorus. Plant Dis. 2009, 93, 708–712. [Google Scholar] [CrossRef]
- Greber, R.S.; Klose, M.J.; Teakle, D.S.; Milne, J.R. High incidence of tobacco streak virus in tobacco and its transmission by Microcephalothrips abdominalis and pollen from Ageratum houstonianum. Plant Dis. 1991, 75, 450–452. [Google Scholar] [CrossRef]
- Hebert, P.D.N.; Penton, E.H.; Burns, J.M.; Janzen, D.H.; Hallwachs, W. Ten species in one: DNA barcoding reveals cryptic species in the Neotropical skipper butterfly Astraptes fulgerator. Proc. Natl. Acad. Sci. USA 2004, 101, 14812–14817. [Google Scholar] [CrossRef] [PubMed]
- Dellicour, S.; Flot, J.F. Delimiting species-poor data sets using single molecular markers: A study of barcode gaps, haplowebs and GMYC. Syst. Biol. 2015, 64, 900–908. [Google Scholar] [CrossRef] [PubMed]
- Prevot, V.; Jordaens, K.; Sonet, G.; Backeljau, T. Exploring species level taxonomy and species delimitation methods in the facultatively self-fertilizing land snail genus Rumina (Gastropoda: Pulmonata). PLoS ONE 2013, 8, e60736. [Google Scholar] [CrossRef]
- Torriani, M.V.G.; Mazzi, D.; Hein, S.; Dorn, S. Structured populations of the oriental fruit moth in an agricultural ecosystem. Mol. Ecol. 2010, 19, 2651–2660. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.J.; Wang, Z.H.; Gong, Y.J.; Zhu, L.; Hoffmann, A.A.; Wei, S.J. Low genetic diversity but strong population structure reflects multiple introductions of western flower thrips (Thysanoptera: Thripidae) into China followed by human-mediated spread. Evol. Appl. 2017, 10, 391–401. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Z.; Zhang, J.; Huang, J.; Wang, L.; Li, Y.; Hafeez, M.; Lu, Y. Population genetic diversity and structure of Thrips tabaci (Thysanoptera: Thripidae) on Allium hosts in China, inferred from mitochondrial COI gene sequences. J. Econ. Entomol. 2020, 113, 1426–1435. [Google Scholar] [CrossRef]

| Family | Identified Species | Common Name | Status | Host Plants |
|---|---|---|---|---|
| Thripidae | Thrips palmi (47%) | Melon thrips | Polyphagous | Ash Gourd |
| Bean | ||||
| Bitter gourd | ||||
| Brinjal | ||||
| Cucumber | ||||
| Marigold | ||||
| Okra | ||||
| Pumpkin | ||||
| Ridge gourd | ||||
| Rose | ||||
| Tomato | ||||
| Thrips hawaiiensis (9%) | The Hawaiian flower thrips | Highly polyphagous | Bean | |
| Cotton | ||||
| Marigold | ||||
| Mustard | ||||
| Rapeseed | ||||
| Thrips parvispinus (9%) | Taiwanese thrips | Polyphagous | Brinjal | |
| Chili | ||||
| Cucumber | ||||
| Marigold | ||||
| Thrips florum (3%) | Banana thrips | Highly polyphagous | Bean | |
| Cotton | ||||
| Lemon | ||||
| Rose | ||||
| Thrips tabaci (1%) | Onion thrips | Polyphagous | Garlic | |
| Thrips subnudula (one sample) | Flower thrips | Polyphagous | Chili | |
| Ayyaria chaetophora (2%) | - | Probably polyphagous | Marigold | |
| Astrothrips tumiceps (one sample) | - | Apparently polyphagous | Rose | |
| Bathrips melanicornis (one sample) | - | Polyphagous | Sweet potato | |
| Dendrothripoides innoxius (1%) | - | Monophagous | Sweet potato | |
| Frankliniella intonsa (3%) | Flower thrips | Polyphagous | Brinjal | |
| Pumpkin | ||||
| Rose | ||||
| Megalurothrips usitatus (14%) | Bean flower thrips | Oligophagous | Bean | |
| Bitter gourd | ||||
| Brinjal | ||||
| Rose | ||||
| Sponge gourd | ||||
| Yard long bean | ||||
| Megalurothrips distalis (1%) | Background bean thrips | - | Mustard | |
| Rose | ||||
| Microcephalothrips abdominalis (one sample) | Composite thrips | Important pollinator | Marigold | |
| Scirtothrips dorsalis (2%) | Chilli thrips | Highly polyphagous | Chili | |
| Phlaeothripidae | Haplothrips sp. (3%) | - | Polyphagous | Chili |
| Cotton | ||||
| Haplothrips andresi (1%) | - | Polyphagous | Rose | |
| Haplothrips bagrolis (1%) | - | Polyphagous | Rooster flower | |
| Phlaeothripidae (1%) | - | Polyphagous | Nag Chapa |
| Species | Number of Sequences | Intraspecific Distance | No. of Haplotype | Haplotype Diversity | Nucleotide Diversity | No. of Segregating Sites | Tajima D | Fu’s Fs Statistic | |
|---|---|---|---|---|---|---|---|---|---|
| p-Distance | K2P Distance | ||||||||
| Thrips palmi | 97 | 0.0062 | 0.0065 | 14 | 0.38466 | 0.00579 | 43 | −2.0927 | −1.319 |
| Thrips parvispinus | 18 | 0.0257 | 0.0281 | 4 | 0.59477 | 0.02511 | 62 | −1.1411 | 13.813 |
| Thrips hawaiiensis | 17 | 0.0024 | 0.0027 | 4 | 0.33088 | 0.00270 | 12 | −2.2602 | 0.613 |
| Thrips florum | 7 | 0.0018 | 0.0022 | 2 | 0.47619 | 0.00182 | 2 | 0.0503 | 0.406 |
| Thrips tabaci | 3 | 0.0164 | 0.0169 | 2 | 0.66667 | 0.01657 | 13 | 0 | 4.053 |
| Frankliniella intonsa | 7 | 0.0019 | 0.0021 | 4 | 0.71429 | 0.00219 | 4 | −1.4341 | −1.217 |
| Megalurothrips usitatus | 29 | 0.0063 | 0.0068 | 6 | 0.73645 | 0.00677 | 9 | 1.7084 | 2.328 |
| Megalurothrips distalis | 3 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| Scirtothrips dorsalis | 4 | 0.0247 | 0.0255 | 4 | 1.0000 | 0.02422 | 24 | −0.4541 | 0.880 |
| Ayyaria chaetophora | 4 | 0.0022 | 0.0025 | 2 | 0.66667 | 0.00255 | 2 | 1.8930 | 1.530 |
| Haplothrips sp. | 6 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| Haplothrips bagrolis | 2 | 0.0077 | 0.0078 | 2 | 1.00000 | 0.00956 | 5 | 0 | 1.609 |
| Haplothrips andresi | 2 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| Phlaeothripidae sp. | 2 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| Dendrothripoides innoxius | 2 | 0.0064 | 0.0065 | 2 | 1.00000 | 0.00574 | 3 | 0 | 1.386 |
| Species | Thrips palmi | Thrips parvispinus | Thrips hawaiiensis | Thrips florum | Thrips tabaci | Frankliniella intonsa | Megalurothrips usitatus | Megalurothrips distalis | Scirtothrips dorsalis | Ayyaria chaetophora | Haplothrips sp. | Haplothrips bagrolis | Haplothrips andresi | Phlaeothripidae sp. | Dendrothripoides innoxius |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Thrips palmi | - | ||||||||||||||
| Thrips parvispinus | 0.9101 | - | |||||||||||||
| Thrips hawaiiensis | 0.9724 | 0.9048 | - | ||||||||||||
| Thrips florum | 0.9756 | 0.9196 | 0.9847 | - | |||||||||||
| Thrips tabaci | 0.9331 | 0.8936 | 0.9413 | 0.9427 | - | ||||||||||
| Frankliniella intonsa | 0.9800 | 0.9314 | 0.9869 | 0.9900 | 0.9503 | - | |||||||||
| Megalurothrips usitatus | 0.9645 | 0.9133 | 0.9715 | 0.9775 | 0.9398 | 0.9769 | - | ||||||||
| Megalurothrips distalis | 0.9847 | 0.9250 | 0.9922 | 0.9948 | 0.9583 | 0.9941 | 0.9621 | - | |||||||
| Scirtothrips dorsalis | 0.9227 | 0.8739 | 0.9270 | 0.9351 | 0.8888 | 0.9334 | 0.9037 | 0.9230 | - | ||||||
| Ayyaria chaetophora | 0.9803 | 0.9290 | 0.9859 | 0.9897 | 0.9534 | 0.9895 | 0.9758 | 0.9944 | 0.9386 | - | |||||
| Haplothrips sp. | 0.9900 | 0.9584 | 0.9956 | 0.9971 | 0.9732 | 0.9967 | 0.9894 | 1.0000 | 0.9612 | 0.9962 | - | ||||
| Haplothrips bagrolis | 0.9737 | 0.9441 | 0.9799 | 0.9818 | 0.9592 | 0.9822 | 0.9746 | 0.9841 | 0.9471 | 0.9818 | 0.9425 | - | |||
| Haplothrips andresi | 0.9908 | 0.9595 | 0.9958 | 0.9971 | 0.9729 | 0.9967 | 0.9889 | 1.0000 | 0.9602 | 0.9964 | 1.0000 | 0.9645 | - | ||
| Phlaeothripidae sp. | 0.9905 | 0.9592 | 0.9956 | 0.9972 | 0.9745 | 0.9966 | 0.9895 | 1.0000 | 0.9591 | 0.9962 | 1.0000 | 0.9740 | 1.0000 | - | |
| Dendrothripoides innoxius | 0.9671 | 0.9205 | 0.9769 | 0.9779 | 0.9406 | 0.9775 | 0.9672 | 0.9840 | 0.9070 | 0.9793 | 0.9907 | 0.9750 | 0.9909 | 0.9902 | - |
| Source of Variation | df | Sum of Squares | Variance Components | Percentage of Variation | Fixation Indices (F-Statistics) |
|---|---|---|---|---|---|
| Among groups | 18 | 10,885.224 | 68.317 Va | 96.90 | FCT = 0.969 |
| Among populations within groups | 31 | 142.789 | 0.602 Vb | 0.85 | FSC = 0.275 |
| Within populations | 161 | 255.075 | 1.584 Vc | 2.25 | FST = 0.977 |
| Total | 210 | 11,283.088 | 70.503 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khatun, M.F.; Hwang, H.-S.; Kang, J.-H.; Lee, K.-Y.; Kil, E.-J. Genetic Diversity and DNA Barcoding of Thrips in Bangladesh. Insects 2024, 15, 107. https://doi.org/10.3390/insects15020107
Khatun MF, Hwang H-S, Kang J-H, Lee K-Y, Kil E-J. Genetic Diversity and DNA Barcoding of Thrips in Bangladesh. Insects. 2024; 15(2):107. https://doi.org/10.3390/insects15020107
Chicago/Turabian StyleKhatun, Mst. Fatema, Hwal-Su Hwang, Jeong-Hun Kang, Kyeong-Yeoll Lee, and Eui-Joon Kil. 2024. "Genetic Diversity and DNA Barcoding of Thrips in Bangladesh" Insects 15, no. 2: 107. https://doi.org/10.3390/insects15020107
APA StyleKhatun, M. F., Hwang, H.-S., Kang, J.-H., Lee, K.-Y., & Kil, E.-J. (2024). Genetic Diversity and DNA Barcoding of Thrips in Bangladesh. Insects, 15(2), 107. https://doi.org/10.3390/insects15020107







