Niche Expansion Has Increased the Risk of Leptocybe invasa Fisher Et LaSalle Invasions at the Global Scale
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Occurrence Records
2.2. Environmental Data
2.3. Niche Comparisons Between Native and Invasive Ranges
2.4. Potential Geographical Distribution of L. invasa
3. Results
3.1. Global Occurrence of L. invasa
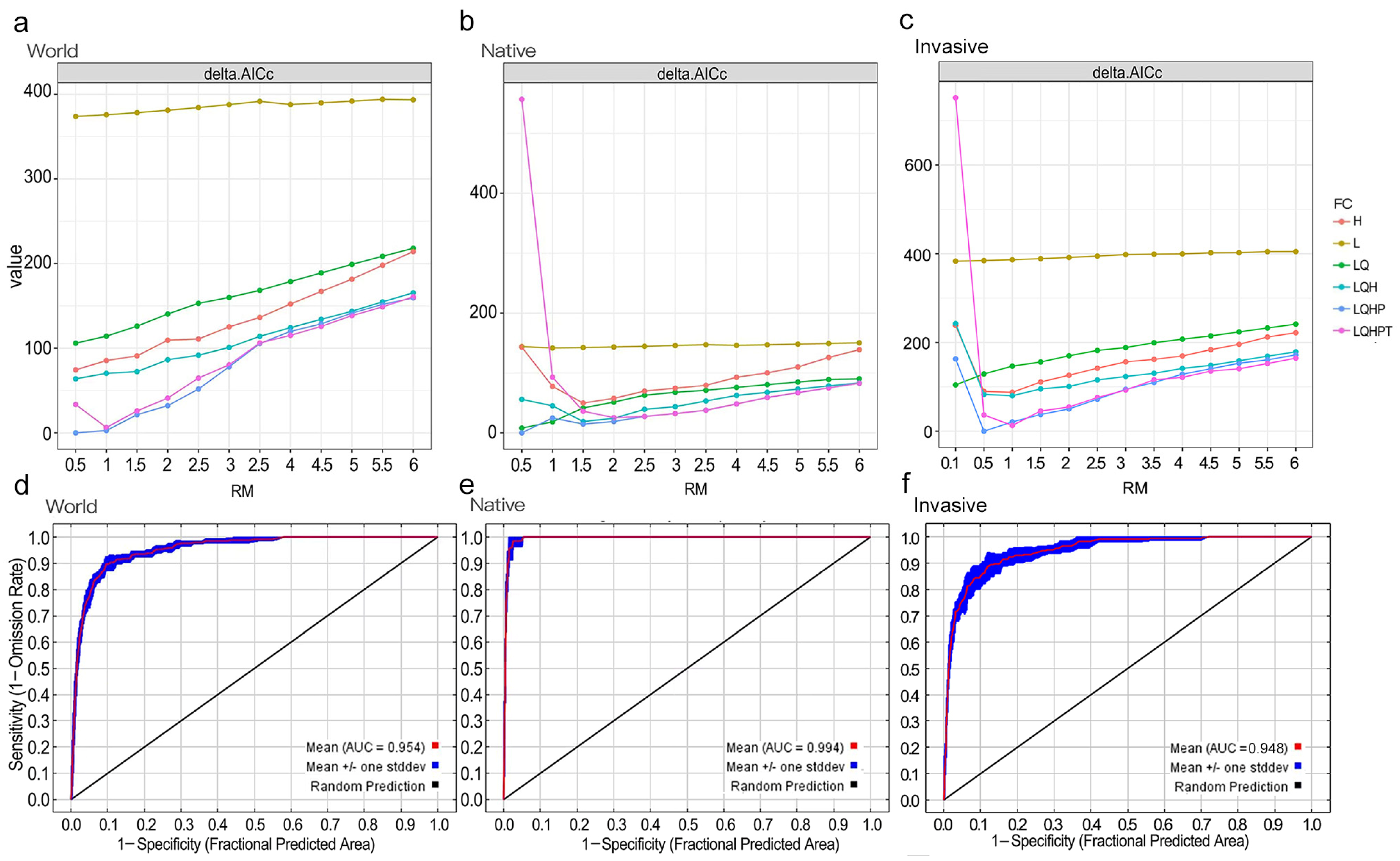
3.2. Parameters and Performance of Optimal MaxEnt Models
3.3. Importance of Environmental Variables and Response Curve
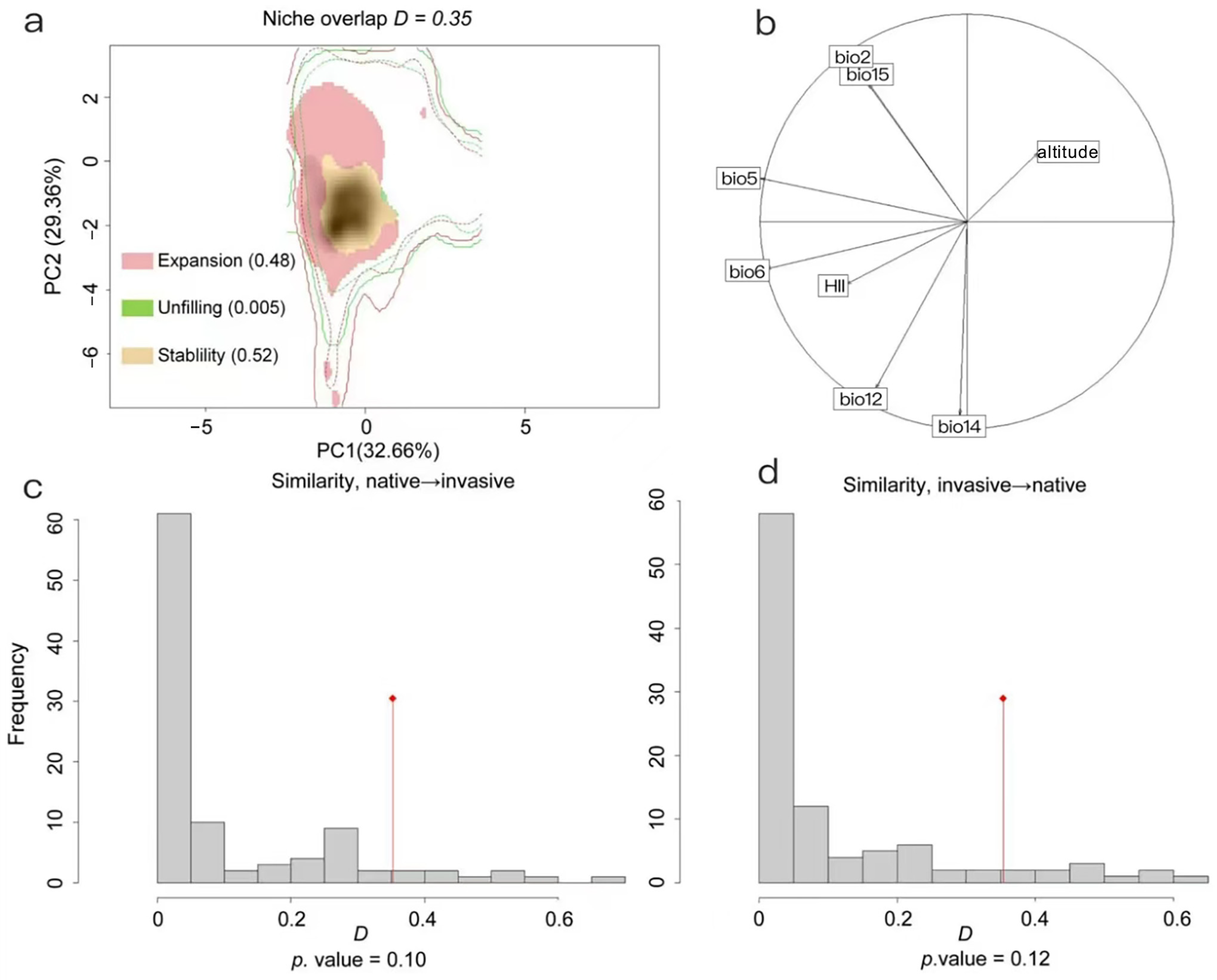
3.4. Niche Comparison and Predicted Niche Occupancy Profiles
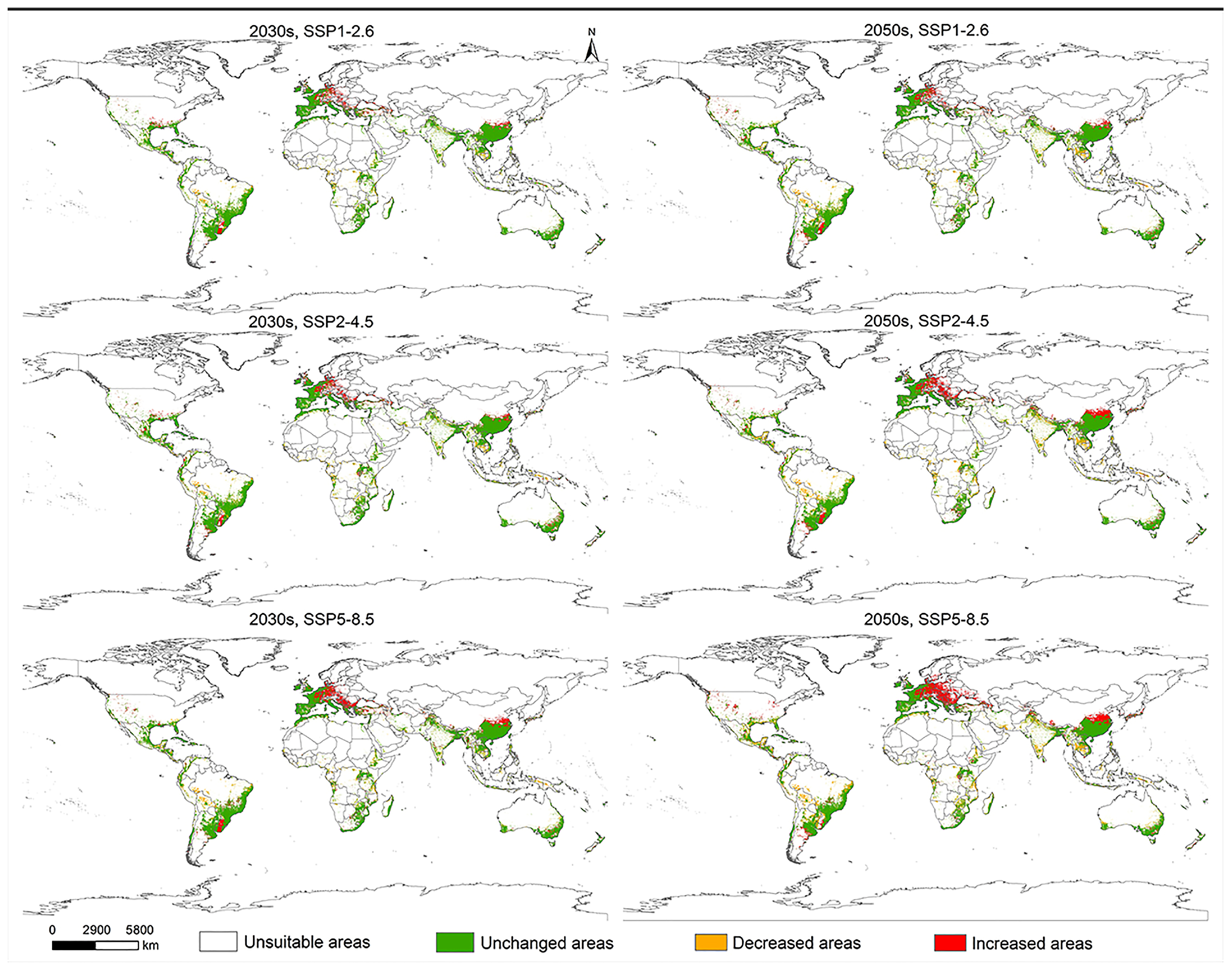
3.5. Prediction of Potentially Suitable Areas for L. invasa Under Near-Current and Future Climatic Scenarios
4. Discussion
4.1. Suitable Environmental Conditions
4.2. Niche Expansion Dynamics and Prospective Changes in Distribution
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Seebens, H.; Blackburn, T.M.; Dyer, E.E.; Genovesi, P.; Hulme, P.E.; Jeschke, J.M.; Essl, F. No saturation in the accumulation of alien species worldwide. Nat. Commun. 2017, 8, 14435. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.X.; Xian, X.Q.; Guo, J.Y.; Yang, N.W.; Zhang, Y.P.; Chen, B.X.; Liu, W.X. Monitoring the little fire ant, Wasmannia auropunctata (Roger 1863), in the early stage of its invasion in China: Predicting its geographical distribution pattern under climate change. J. Integr. Agric. 2023, 22, 2783–2795. [Google Scholar] [CrossRef]
- Vaissière, A.C.; Courtois, P.; Courchamp, F.; Kourantidou, M.; Diagne, C.; Essl, F.; Salles, J.M. The nature of economic costs of biological invasions. Biol. Invasions 2022, 24, 2081–2101. [Google Scholar] [CrossRef]
- Diagne, C.; Leroy, B.; Gozlan, R.E.; Vaissière, A.C.; Assailly, C.; Nuninger, L.; Courchamp, F. InvaCost, a public database of the economic costs of biological invasions worldwide. Sci. Data 2020, 7, 277. [Google Scholar] [CrossRef]
- Qiao, H.; Escobar, L.E.; Peterson, A.T. Accessible areas in ecological niche comparisons of invasive species: Recognized but still overlooked. Sci. Rep. 2017, 7, 121. [Google Scholar] [CrossRef]
- Almeida Saá, A.C.; Crivellaro, M.S.; Winter, B.B.; Pereira, G.R.; Bercovich, M.V.; Horta, P.A.; Schubert, N. Unraveling interactions: Do temperature and competition with native species affect the performance of the non-indigenous sun coral Tubastraea coccinea? Coral Reefs 2020, 39, 99–117. [Google Scholar] [CrossRef]
- Bell, D.A.; Kovach, R.P.; Muhlfeld, C.C.; Al-Chokhachy, R.; Cline, T.J.; Whited, D.C.; Whiteley, A.R. Climate change and expanding invasive species drive widespread declines of native trout in the northern Rocky Mountains, USA. Sci. Adv. 2021, 7, eabj5471. [Google Scholar] [CrossRef]
- Alves, D.F.R.; de Paiva Barros-Alves, S.; Dolabella, S.S.; de Almeida, A.C.; Martinez, P.A. Invasive shrimp Cinetorhynchus erythrostictus (Decapoda: Caridea) misidentified in the marine aquarium trade: Niche overlap with a native congeneric species. Estuar. Coast. Shelf Sci. 2021, 258, 107411. [Google Scholar] [CrossRef]
- Broennimann, O.; Fitzpatrick, M.C.; Pearman, P.B.; Petitpierre, B.; Pellissier, L.; Yoccoz, N.G.; Guisan, A. Measuring ecological niche overlap from occurrence and spatial environmental data. Glob. Ecol. Biogeogr. 2012, 21, 481–497. [Google Scholar] [CrossRef]
- Xian, X.; Zhao, H.; Humair, L.; Yang, N.; Li, J.; Weyl, P.; Liu, W.X. Niche shifts undermine the prediction performance of species distribution models: Estimating potentially suitable areas for Myriophyllum aquaticum at the global scale. Glob. Ecol. Conserv. 2023, 48, e02764. [Google Scholar] [CrossRef]
- Tu, W.; Xiong, Q.; Qiu, X.; Zhang, Y. Dynamics of invasive alien plant species in China under climate change scenarios. Ecol. Indic. 2021, 129, 107919. [Google Scholar] [CrossRef]
- Gallien, L.; Münkemüller, T.; Albert, C.H.; Boulangeat, I.; Thuiller, W. Predicting potential distributions of invasive species: Where to go from here? Divers. Distrib. 2010, 16, 331–342. [Google Scholar] [CrossRef]
- Koo, K.A.; Park, S.U. The effect of interplays among climate change, land-use change, and dispersal capacity on plant redistribution. Ecol. Indic. 2022, 142, 109192. [Google Scholar] [CrossRef]
- Seng Hua, L.; Wei Chen, L.; Antov, P.; Kristak, L.; Tahir, P. Engineering wood products from Eucalyptus spp. Adv. Mater. Sci. Eng. 2022, 2022, 8000780. [Google Scholar] [CrossRef]
- Mendel, Z.; Protasov, A.; Fisher, N.; La Salle, J. Taxonomy and biology of Leptocybe invasa gen. & sp. n.(Hymenoptera: Eulophidae), an invasive gall inducer on Eucalyptus. Aust. J. Entomol. 2004, 43, 101–113. [Google Scholar]
- Carvalho, M.A.F.; Pinto, I.O.; Sarmento, M.I.; Carvalho, P.H.N.; da Silva, R.S.; Rocha, J.P.L.; Sarmento, R.A. Assessment performance of Eucalyptus clones attacked by the recent invasion of Leptocybe invasa (Hymenoptera: Eulophidae): Implications to invasion pest management. J. Asia Pac. Entomol. 2022, 25, 101939. [Google Scholar] [CrossRef]
- Zhang, H.; Song, J.; Zhao, H.; Li, M.; Han, W. Predicting the distribution of the invasive species Leptocybe invasa: Combining MaxEnt and geodetector models. Insects 2021, 12, 92. [Google Scholar] [CrossRef]
- Yousuf, M.; Singh, S.; Ikram, M.; Khan, S. Biological control of eucalyptus Gall wasp, Leptocybe invasa Fisher & La Salle (Hymenoptera: Eulophidae) in Punjab, India. Arch. Agric. Environ. Sci. 2020, 5, 105–113. [Google Scholar]
- Hamdy, N.M.; Emam, A.K. Comparative Morphogenesis of Mouth Parts Sensilla Between the Leptocybe invasa and Ophelimus maskelli (Hymenoptera: Eulophidae) and Its Relationship to Their Vital Capacity. Egypt. Acad. J. Biol. Sci. 2022, 15, 23–35. [Google Scholar] [CrossRef]
- Wang, Z.; Xu, D.; Liao, W.; Xu, Y.; Zhuo, Z. Predicting the current and future distributions of Frankliniella occidentalis (Pergande) based on the MaxEnt species distribution model. Insects 2023, 14, 458. [Google Scholar] [CrossRef]
- Warren, D.L.; Glor, R.E.; Turelli, M. ENMTools: A toolbox for comparative studies of environmental niche models. Ecography 2010, 33, 607–611. [Google Scholar] [CrossRef]
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1-km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Di Cola, V.; Broennimann, O.; Petitpierre, B.; Breiner, F.T.; D’Amen, M.; Randin, C.; Guisan, A. ecospat: An R package to support spatial analyses and modeling of species niches and distributions. Ecography 2017, 40, 774–787. [Google Scholar] [CrossRef]
- Liu, C.; Wolter, C.; Xian, W.; Jeschke, J.M. Most invasive species largely conserve their climatic niche. Proc. Natl. Acad. Sci. USA 2020, 117, 23643–23651. [Google Scholar] [CrossRef] [PubMed]
- Schoener, T.W. Nonsynchronous spatial overlap of lizards in patchy habitats. Ecology 1970, 51, 408–418. [Google Scholar] [CrossRef]
- Li, G.; Zhang, X.; Huang, J.; Wen, Z.; Du, S. Afforestation and climatic niche dynamics of black locust (Robinia pseudoacacia). For. Ecol. Manag. 2018, 407, 184–190. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum entropy modeling of species geographic distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef]
- Vaz, U.L.; Cunha, H.F.; Nabout, J.C. Trends and biases in global scientific literature about ecological niche models. Braz. J. Biol. 2015, 75, 17–24. [Google Scholar] [CrossRef]
- Muscarella, R.; Galante, P.J.; Soley-Guardia, M.; Boria, R.A.; Kass, J.M.; Uriarte, M. ENM eval: An R package for conducting spatially independent evaluations and estimating optimal model complexity for Maxent ecological niche models. Methods Ecol. Evol. 2014, 5, 1198–1205. [Google Scholar] [CrossRef]
- Kass, J.M.; Muscarella, R.; Galante, P.J.; Bohl, C.L.; Pinilla-Buitrago, G.E.; Boria, R.A.; Soley-Guardia, M.; Anderson, R.P. ENMeval 2.0: Redesigned for customizable and reproducible modeling of species’ niches and distributions. Methods Ecol. Evol. 2021, 12, 1602–1608. [Google Scholar] [CrossRef]
- Peterson, A.T.; Papeş, M.; Soberón, J. Rethinking receiver operating characteristic analysis applications in ecological niche modeling. Ecol. Model. 2008, 213, 63–72. [Google Scholar] [CrossRef]
- Ma, G.; Ma, C.S. Potential distribution of invasive crop pests under climate change: Incorporating mitigation responses of insects into prediction models. Curr. Opin. Insect Sci. 2022, 49, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.L.; Li, J.; Yang, Z.D.; Xian, Z.H.; Wei, J.G.; Lu, W.; Wang, X.P. A review of invasive biology, prevalence and management of Leptocybe invasa Fisher & La Salle (Hymenoptera: Eulophidae: Tetrastichinae). Afr. Entomol. 2014, 22, 68–79. [Google Scholar]
- Otieno, B.A.; Nahrung, H.F.; Steinbauer, M.J. Where did you come from? Where did you go? Investigating the origin of invasive Leptocybe species using distribution modelling. Forests 2019, 10, 115. [Google Scholar] [CrossRef]
- Yan, C.; Li, X.; Chen, Y.S.; Luo, Z.D. Disaster Characteristics, Cause Analysis, and Prevention and Control Strategies of Leptocybe invasa in Eucalyptus Forests in Southern Jiangxi. J. Anhui Agric. Sci. 2020, 48, 3. [Google Scholar]
- Porqueddu, C.; Ates, S.; Louhaichi, M.; Kyriazopoulos, A.P.; Moreno, G.; del Pozo, A.; Ovalle, C.; Ewing, M.A.; Nichols, P.G.H. Grasslands in ‘Old World’and ‘New World’Mediterranean-climate zones: Past trends, current status and future research priorities. Grass Forage Sci. 2016, 71, 1–35. [Google Scholar] [CrossRef]
- De Souza, A.R.; Barbosa, L.R.; de Souza Passos, J.R.; de Castro e Castro, B.M.; Zanuncio, J.C.; Wilcken, C.F. Longevity and survival of Leptocybe invasa (Hymenoptera: Eulophidae), an invasive gall inducer on Eucalyptus, with different diets and temperatures. PeerJ. 2018, 6, e5265. [Google Scholar] [CrossRef]
- Fenn-Moltu, G.; Ollier, S.; Caton, B.; Liebhold, A.M.; Nahrung, H.; Pureswaran, D.S.; Bertelsmeier, C. Alien insect dispersal mediated by the global movement of commodities. Ecol. Appl. 2023, 33, e2721. [Google Scholar] [CrossRef]
- Ciesla, W.M. The role of human activities on forest insect outbreaks worldwide. Int. Forest Rev. 2015, 17, 269–281. [Google Scholar] [CrossRef]
- Nyeko, P.; Mutitu, E.K.; Day, R.K. Eucalyptus infestation by Leptocybe invasa in Uganda. Afr. J. Ecol. 2009, 47, 299–307. [Google Scholar] [CrossRef]
- Chapman, J.W.; Reynolds, D.R.; Wilson, K. Long-range seasonal migration in insects: Mechanisms, evolutionary drivers and ecological consequences. Ecol. Lett. 2015, 18, 287–302. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhao, H.; Xian, X.; Zhu, J.; Chen, B.; Jia, T.; Wang, R.; Liu, W. Geographical Distribution Pattern and Ecological Niche of Solenopsis invicta Buren in China under Climate Change. Diversity 2023, 15, 607. [Google Scholar] [CrossRef]
- Peng, X.; Wang, H.; Yang, Z. Differences in Male-Killing Rickettsia Bacteria between Lineages of the Invasive Gall-Causing Pest Leptocybe invasa. Insects 2023, 14, 757. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Li, M.; Zheng, H.; Dong, T.; Zhang, X. A phylogeographical analysis of the beetle pest species Callosobruchus chinensis (Linnaeus, 1758) in China. Insects 2022, 13, 145. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.I.; Choi, Y.; Yoo, Y.; Engler, R.; Lee, K.; Jeon, S.W. Integrated spatial model based evaluation methodology for optimal invasive species management: Common ragweed in the Republic of Korea. Env. Res. Lett. 2022, 17, 034047. [Google Scholar] [CrossRef]
- Drake, J.E.; Vårhammar, A.; Aspinwall, M.J.; Pfautsch, S.; Ghannoum, O.; Tissue, D.T.; Tjoelker, M.G. Pushing the envelope: Do narrowly and widely distributed Eucalyptus species differ in response to climate warming? New Phytol. 2024, 243, 82–97. [Google Scholar] [CrossRef]
- Pairo, P.E.; Rodriguez, E.E.; Bellocq, M.I.; Aceñolaza, P.G. Changes in taxonomic and functional diversity of plants in a chronosequence of Eucalyptus grandis plantations. Sci. Rep. 2021, 11, 10768. [Google Scholar] [CrossRef]
- Messal, M.; Vivas, M.; Kemler, M.; Begerow, D.; Brachmann, A.; Witfeld, F.; Slippers, B. Fungal communities of eucalyptus grandis leaves are influenced by the insect pest leptocybe invasa. Front. Microbiol. 2022, 13, 841621. [Google Scholar] [CrossRef]
- Calazans, C.C.; Pereira, G.S.; Souza, J.L.; Nunes, V.V.; Álvares-Carvalho, S.V.; Dantas, J.O.; Silva-Mann, R. Insights into Leptocybe invasa resistance in Eucalyptus: Phenotying, genotying and in silico approaches. Braz. J. Biol. 2024, 84, e279850. [Google Scholar]
- Daude, M.M.; Ságio, S.A.; Rodrigues, J.N.; Lima, N.M.P.; Lima, A.A.; Sarmento, M.I.; Barreto, H.G. Reference genes for Eucalyptus spp. under Beauveria bassiana inoculation and subsequently infestation by the galling wasp Leptocybe invasa. Sci. Rep. 2024, 14, 2556. [Google Scholar] [CrossRef]
- Puretz, B.O.; Gonzalez, C.J.; Mota, T.A.; Dallacort, S.; Carvalho, V.R.; Silva, R.M.L.; Wilcken, C.F. Quadrastichus mendeli (Hymenoptera: Eulophidae): Parasitism on Leptocybe invasa (Hymenoptera: Eulophidae) and first record in Brazil. Braz. J. Biol. 2022, 82, e264771. [Google Scholar] [CrossRef] [PubMed]
- Chiles, C.R.; Melo, R.S.; Otto, M.S.G.; Zanini, A.M.; Godoy, W.A.C.; De Barros Ferraz, S.F. How can structure and composition of Eucalyptus plantation landscape reduce leaf-cutting ants? Forest Ecol. Manag. 2022, 518, 120250. [Google Scholar] [CrossRef]
- Demetriou, J.; Koutsoukos, E.; Radea, C.; Roy, H.E.; Arianoutsou, M.; Martinou, A.F. Uninvited pests of an unwelcomed tree: A survey on alien chalcidoid wasps (Hymenoptera: Chalcidoidea) associated with Eucalyptus trees in Cyprus. BioInvasions Rec. 2022, 11, 390–400. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ouyang, X.; Pan, J.; Rao, H.; Sun, Q. Niche Expansion Has Increased the Risk of Leptocybe invasa Fisher Et LaSalle Invasions at the Global Scale. Insects 2024, 15, 985. https://doi.org/10.3390/insects15120985
Ouyang X, Pan J, Rao H, Sun Q. Niche Expansion Has Increased the Risk of Leptocybe invasa Fisher Et LaSalle Invasions at the Global Scale. Insects. 2024; 15(12):985. https://doi.org/10.3390/insects15120985
Chicago/Turabian StyleOuyang, Xianheng, Jiangling Pan, Hui Rao, and Qiaoyun Sun. 2024. "Niche Expansion Has Increased the Risk of Leptocybe invasa Fisher Et LaSalle Invasions at the Global Scale" Insects 15, no. 12: 985. https://doi.org/10.3390/insects15120985
APA StyleOuyang, X., Pan, J., Rao, H., & Sun, Q. (2024). Niche Expansion Has Increased the Risk of Leptocybe invasa Fisher Et LaSalle Invasions at the Global Scale. Insects, 15(12), 985. https://doi.org/10.3390/insects15120985




