Functions of Insulin-like Peptide Genes (CsILP1 and CsILP2) in Female Reproduction of the Predatory Ladybird Coccinella septempunctata (Coleoptera: Coccinellidae)
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insects
2.2. RNA Isolation and cDNA Cloning of CsILP1 and CsILP2
2.3. Bioinformatics Analysis
2.4. Expression Profiling Analysis of CsILP1 and CsILP2
2.5. RNA Interference Experiment
2.6. Statistical Analysis
3. Results
3.1. Sequence Analysis of CsILP1 and CsILP2
3.2. Expression Profiling of CsILP1 and CsILP2
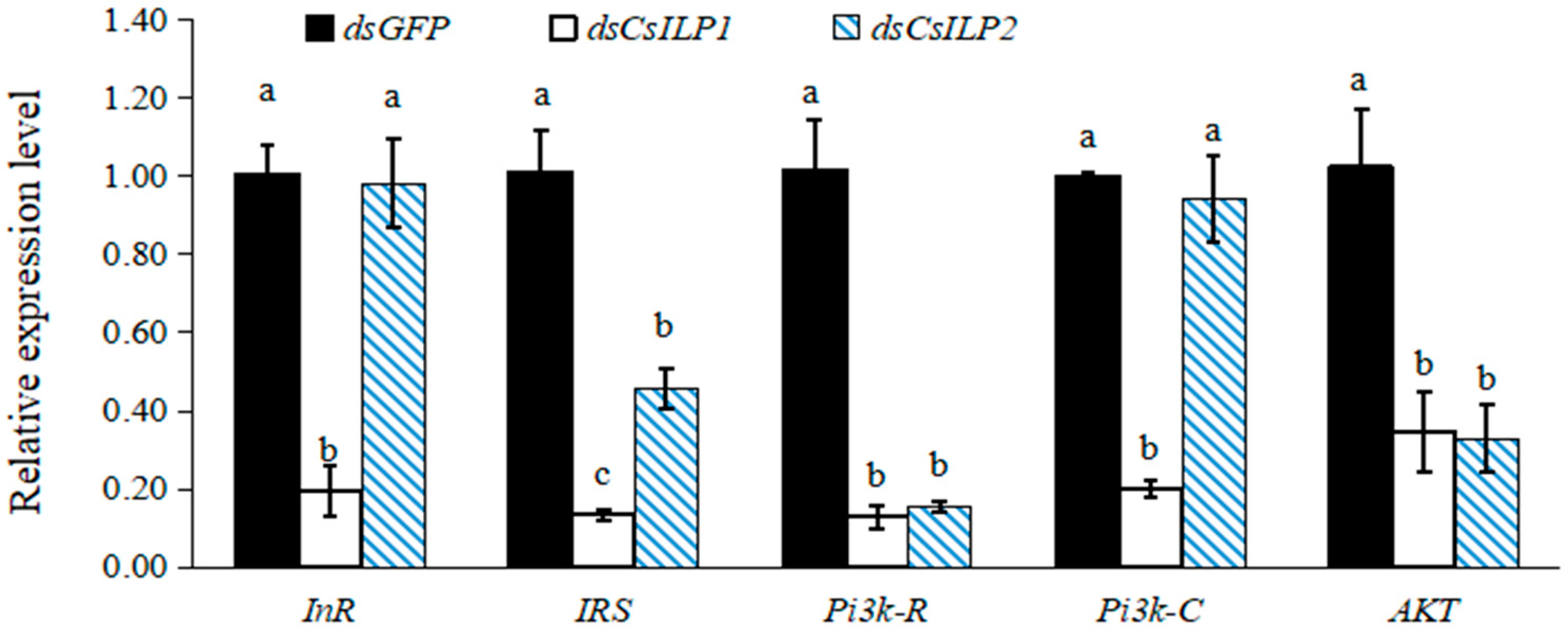
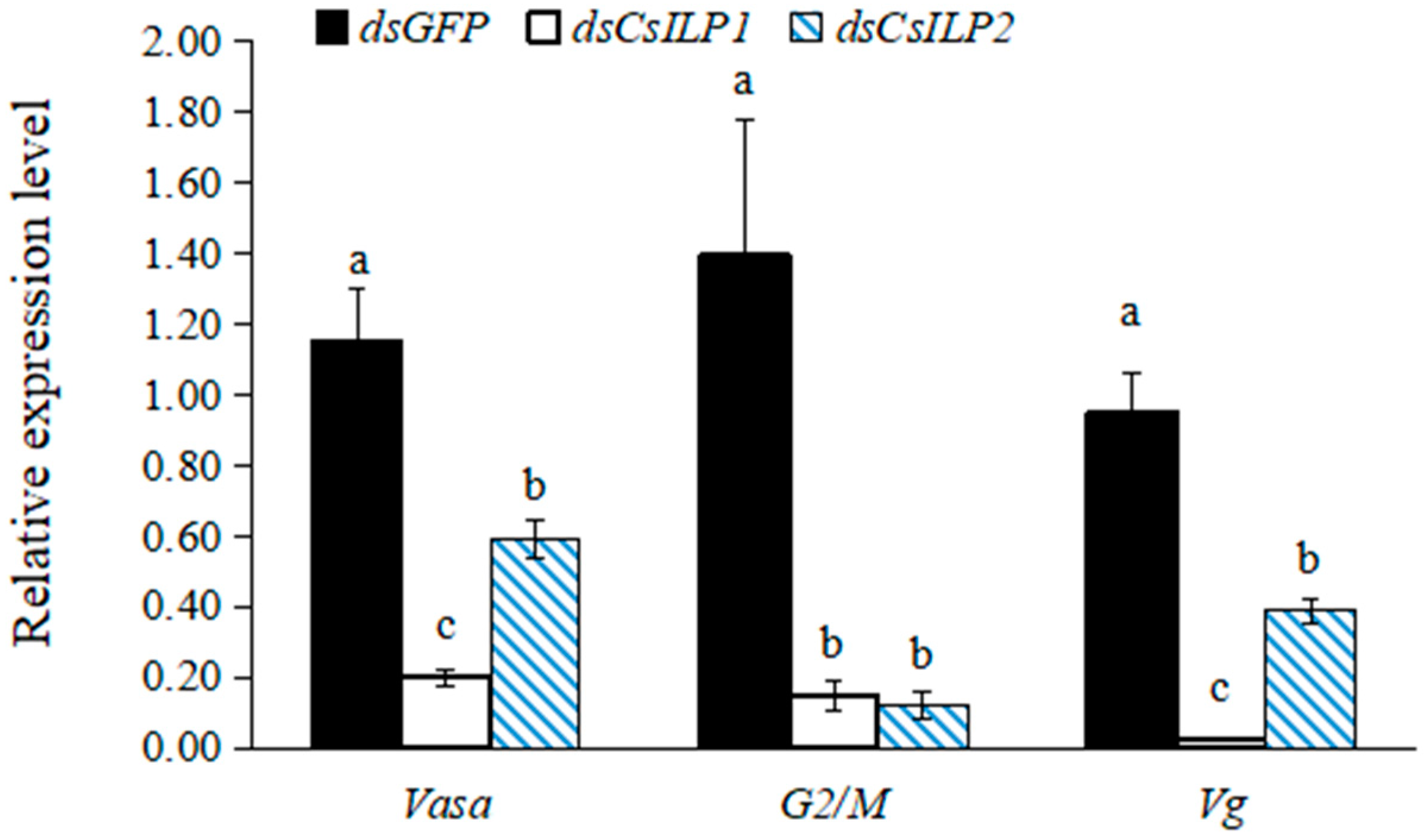
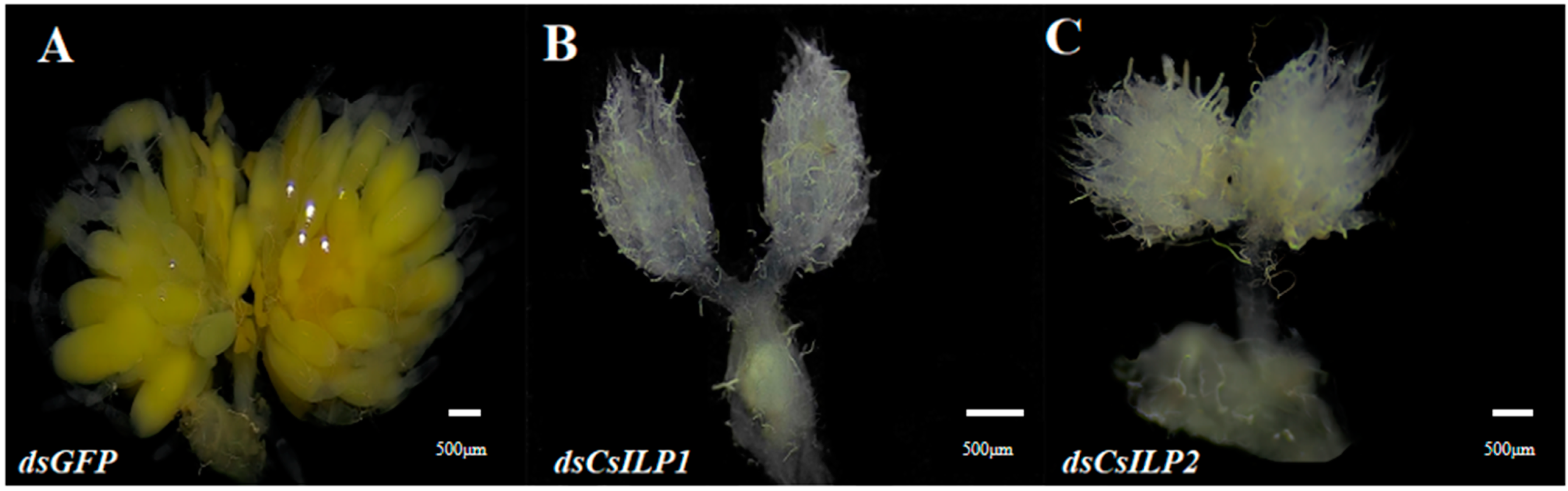
3.3. Functional Analysis of CsILP1 and CsILP2 by RNAi
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, Q.; Brown, M.R. Signaling and function of insulin-like peptides in insects. Annu. Rev. Entomol 2006, 51, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Nassel, D.R.; Liu, Y.; Luo, J. Insulin/IGF signaling and its regulation in Drosophila. Gen. Comp. Endocrinol. 2015, 221, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Nassel, D.R.; Vanden, B.J. Insulin/IGF signaling in Drosophila and other insects: Factors that regulate production, release and post-release action of the insulin-like peptides. Cell. Mol. Life Sci. 2016, 73, 271–290. [Google Scholar] [CrossRef] [PubMed]
- Chowanski, S.; Walkowiak-Nowicka, K.; Winkiel, M.; Marciniak, P.; Urbanski, A.; Pacholska-Bogalska, J. Insulin-Like Peptides and Cross-Talk With Other Factors in the Regulation of Insect Metabolism. Front. Physiol. 2021, 12, 701203. [Google Scholar] [CrossRef]
- Kawakami, A.; Iwami, M.; Nagasawa, H.; Suzuki, A.; Ishizaki, H. Structure and organization of four clustered genes that encode bombyxin, an insulin-related brain secretory peptide of the silkmoth Bombyx mori. Proc. Natl. Acad. Sci. USA 1989, 86, 6843–6847. [Google Scholar] [CrossRef]
- Nagasawa, H.; Kataoka, H.; Isogai, A.; Tamura, S.; Suzuki, A.; Ishizaki, H.; Mizoguchi, A.; Fujiwara, Y.; Suzuki, A. Amino-terminal amino Acid sequence of the silkworm prothoracicotropic hormone: Homology with insulin. Science 1984, 226, 1344–1345. [Google Scholar] [CrossRef]
- Nagasawa, H.; Kataoka, H.; Isogai, A.; Tamura, S.; Suzuki, A.; Mizoguchi, A.; Fujiwara, Y.; Suzuki, A.; Takahashi, S.Y.; Ishizaki, H. Amino acid sequence of a prothoracicotropic hormone of the silkworm Bombyx mori. Proc. Natl. Acad. Sci. USA 1986, 83, 5840–5843. [Google Scholar] [CrossRef]
- Kondo, H.; Ino, M.; Suzuki, A.; Ishizaki, H.; Iwami, M. Multiple gene copies for bombyxin, an insulin-related peptide of the silkmoth Bombyx mori: Structural signs for gene rearrangement and duplication responsible for generation of multiple molecular forms of bombyxin. J. Mol. Biol. 1996, 259, 926–937. [Google Scholar] [CrossRef] [PubMed]
- Aslam, A.F.; Kiya, T.; Mita, K.; Iwami, M. Identification of novel bombyxin genes from the genome of the silkmoth Bombyx mori and analysis of their expression. Zool. Sci. 2011, 28, 609–616. [Google Scholar] [CrossRef]
- Mizoguchi, A.; Okamoto, N. Insulin-like and IGF-like peptides in the silkmoth Bombyx mori: Discovery, structure, secretion and function. Front. Physiol. 2013, 4, 217. [Google Scholar] [CrossRef]
- Marquez, A.G.; Pietri, J.E.; Smithers, H.M.; Nuss, A.; Antonova, Y.; Drexler, A.L.; Luckhart, S. Insulin-like peptides in the mosquito Anopheles stephensi: Identification and expression in response to diet and infection with Plasmodium falciparum. Gen. Comp. Endocrinol. 2011, 173, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Huygens, C.; Ribeiro, L.M.; Gaget, K.; Duport, G.; Peignier, S.; De Groef, S.; Callaerts, P. Evolutionary diversification of insulin-related peptides (IRPs) in aphids and spatiotemporal distribution in Acyrthosiphon pisum. Insect Biochem. Mol. Biol. 2022, 141, 103670. [Google Scholar] [CrossRef]
- Fu, K.Y.; Zhu, T.T.; Guo, W.C.; Ahmat, T.; Li, G.Q. Knockdown of a putative insulin-like peptide gene LdILP2 in Leptinotarsa decemlineata by RNA interference impairs pupation and adult emergence. Gene 2016, 581, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Veenstra, J.A. Arthropod IGF, relaxin and gonadulin, putative orthologs of Drosophila insulin-like peptides 6, 7 and 8, likely originated from an ancient gene triplication. PeerJ 2020, 8, e9534. [Google Scholar] [CrossRef]
- Ling, L.; Raikhel, A.S. Cross-talk of insulin-like peptides, juvenile hormone, and 20-hydroxyecdysone in regulation of metabolism in the mosquito Aedes aegypti. Proc. Natl. Acad. Sci. USA 2021, 118, e2023470118. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, N.; Yamanaka, N.; Satake, H.; Saegusa, H.; Kataoka, H.; MizoguchI, A. An ecdysteroid-inducible insulin-like growth factor-like peptide regulates adult development of the silkmoth Bombyx mori. FEBS J. 2009, 276, 1221–1232. [Google Scholar] [CrossRef]
- Fujinaga, D.; Shiomi, K.; Yagi, Y.; Kataoka, H.; Mizoguchi, A. An insulin-like growth factor-like peptide promotes ovarian development in the silkmoth Bombyx mori. Sci. Rep. 2019, 9, 18446. [Google Scholar] [CrossRef]
- Veenstra, J.A. Differential expression of some termite neuropeptides and insulin/IGF-related hormones and their plausible functions in growth, reproduction and caste determination. PeerJ 2023, 11, e15259. [Google Scholar] [CrossRef]
- Yuen, A.C.; Hillion, K.H.; Wang, R.; Amoyel, M. Germ cells commit somatic stem cells to differentiation following priming by PI3K/Tor activity in the Drosophila testis. PLoS Genet. 2021, 17, e1009609. [Google Scholar] [CrossRef]
- Xue, H.; Huang, X.; Chang, G.; Ma, W.; Hull, J.J.; Chen, L. Reproductive capacity in Adelphocoris suturalis (Hemiptera: Miridae) is regulated by the insulin signaling pathway. Pestic. Biochem. Physiol. 2022, 187, 105195. [Google Scholar] [CrossRef]
- Gupta, S.; Ray, K. Somatic PI3K activity regulates transition to the spermatocyte stages in Drosophila testis. J. Biosci. 2017, 42, 285–297. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.J.; Liu, X.X.; Guo, P.H.; Teets, N.M.; Zhou, J.C.; Chen, W.B.; Luo, Q.Z.; Kanjana, N.; Li, Y.Y.; Zhang, L.S. Regulation of forkhead box O transcription factor by insulin signaling pathway controls the reproductive diapause of the lady beetle, Coccinella septempunctata. Int. J. Biol. Macromol. 2024, 258, 128104. [Google Scholar] [CrossRef] [PubMed]
- Huangfu, N.; Zhu, X.; Wang, L.; Zhang, K.; Li, D.; Chen, L.; Cui, J. Insulin receptor substrate-1 (IRS1) regulates oogenesis and vitellogenesis in Propylea japonica by mediating the FOXO transcription factor expression, independent of JH and 20E signaling pathways. J. Agric. Food Chem. 2023, 71, 300–310. [Google Scholar] [CrossRef]
- Mensah, L.B.; Goberdhan, D.; Wilson, C. mTORC1 signalling mediates PI3K-dependent large lipid droplet accumulation in Drosophila ovarian nurse cells. Biol. Open 2017, 6, 563–570. [Google Scholar] [CrossRef]
- Deng, W.M.; Althauser, C.; Ruohola-Baker, H. Notch-Delta signaling induces a transition from mitotic cell cycle to endocycle in Drosophila follicle cells. Development 2001, 128, 4737–4746. [Google Scholar] [CrossRef]
- Dehghani, M.; Lasko, P. Multiple functions of the dead-box helicase vasa in drosophila oogenesis. Results Probl. Cell Differ. 2017, 63, 127–147. [Google Scholar] [CrossRef]
- Roy, S.; Saha, T.T.; Zou, Z.; Raikhel, A.S. Regulatory pathways controlling female insect reproduction. Annu. Rev. Entomol. 2018, 63, 489–511. [Google Scholar] [CrossRef]
- Han, H.; Han, S.; Qin, Q.; Chen, J.; Wang, D.; He, Y.Z. Molecular Identification and Functional Characterization of Vitellogenin Receptor From Harmonia axyridis (Coleoptera: Coccinellidae). J. Econ. Entomol. 2022, 115, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Bahlai, C.A.; Frewin, A.; Sears, M.K.; Schaafsma, A.W.; Hallett, R.H. Predation by Coccinella septempunctata and Harmonia axyridis (Coleoptera: Coccinellidae) on Aphis glycines (Homoptera: Aphididae). Environ. Entomol. 2009, 38, 708–714. [Google Scholar] [CrossRef]
- Farooq, M.U.; Qadri, H.; Khan, M.A. Aphid species affect foraging behavior of Coccinella septempunctata (Coccinellidae: Coleoptera). Pak. J. Biol. Sci. 2017, 20, 160–164. [Google Scholar] [CrossRef]
- Lopes, P.C.; Souza, P.; Santos, J.; Borges, C.E.; Araujo, F.; Martins, J.C.; Silva, R. Spatiotemporal distribution of Schizaphis graminum (Rondani) and its natural enemy Coccinella septempunctata (Linnaeus) in graniferous sorghum crops. Braz. J. Biol. 2022, 84, e261972. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Zhi, J.R.; Li, F.L.; Li, W.H.; Zhou, Y.H. Improving the artificial diet for adult of seven spotted ladybird beetle Coccinella septempunctata L. (Coleoptera: Coccinellidae) with orthogonal design. Bull. Entomol. Res. 2018, 108, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Jalali, M.A.; Reitz, S.; Mehrnejad, M.R.; Ranjbar, F.; Ziaaddini, M. Food utilization, development, and reproductive performance of Coccinella septempunctata (Coleoptera: Coccinellidae) feeding on an aphid or psylla prey species. J. Econ. Entomol. 2019, 112, 571–576. [Google Scholar] [CrossRef]
- Turnipseed, R.K.; Ugine, T.A.; Losey, J.E. Egg predation by the introduced lady beetle, Coccinella septempunctata (Coleoptera: Coccinellidae), lowers mortality but raises relative risk for the native lady beetle, Coccinella novemnotata. PLoS ONE 2015, 10, e118493. [Google Scholar] [CrossRef]
- Khalid, M.Z.; Ahmad, S.; Ngegba, P.M.; Zhong, G. Role of endocrine system in the regulation of female insect reproduction. Biology 2021, 10, 614. [Google Scholar] [CrossRef]
- Han, B.; Zhang, T.; Feng, Y.; Liu, X.; Zhang, L.; Chen, H.; Mao, J. Two insulin receptors coordinate oogenesis and oviposition via two pathways in the green lacewing, Chrysopa pallens. J. Insect Physiol. 2020, 123, 104049. [Google Scholar] [CrossRef] [PubMed]
- Han, H.; Feng, Z.Y.; Han, S.P.; Chen, J.; Wang, D.; He, Y.Z. Molecular identification and functional characterization of Methoprene-Tolerant (Met) and Kruppel-Homolog 1 (Kr-h1) in Harmonia axyridis (Coleoptera: Coccinellidae). J. Econ. Entomol. 2022, 115, 334–343. [Google Scholar] [CrossRef]
- Zhang, T.T.; Zhang, G.C.; Zeng, F.F.; Liu, C.Y.; Mao, J.J. Insulin-like peptides regulate vitellogenesis and oviposition in the green lacewing, Chrysopa septempunctata. Bull. Entomol. Res. 2017, 107, 148–154. [Google Scholar] [CrossRef]
- Fu, Y.L.; Chen, Z.H. The concentration of juvenile hormone in female adults of Coccinella septempunctata during ovarian development. Acta Entomol. Sin. 1984, 27, 268–274. [Google Scholar]
- Cheng, Z.; Qin, Q.J.; Wang, D.; Han, S.P.; Zhang, S.; He, Y.Z. Sublethal and transgenerational effects of exposures to the thiamethoxam on the seven-spotted lady beetle, Coccinella septempunctata L. (Coleoptera: Coccinellidae). Ecotoxicol. Environ. Saf. 2022, 243, 114002. [Google Scholar] [CrossRef]
- Lu, J.; Yang, C.; Zhang, Y.; Pan, H. Selection of reference genes for the normalization of RT-qPCR data in gene expression studies in insects: A systematic review. Front. Physiol. 2018, 9, 1560. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Preisser, E.L.; Zhang, H.; Liu, Y.; Dai, L.; Pan, H.; Zhou, X. Corrigendum: Selection of reference genes for RT-qPCR analysis in Coccinella septempunctata to assess un-intended effects of RNAi transgenic plants. Front. Plant. Sci. 2016, 7, 1835. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Yu, Y.; Li, W.; Li, F. Influence of artificial diets on biological characteristics and digestive enzymes of Coccinella septempunctata L. J. Insect Sci. 2023, 23, 7. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.S.; Kundapura, S.V.; Dey, D.; Palaniappan, C.; Sekar, K.; Kulal, A.; Ramagopal, U.A. Cumulative phylogenetic, sequence and structural analysis of Insulin superfamily proteins provide unique structure-function insights. Mol. Inform. 2024, 43, e202300160. [Google Scholar] [CrossRef]
- Dai, Y.; Li, X.; Ding, J.; Liang, Z.; Guo, R.; Yi, T.; Liu, W. Molecular and expression characterization of insulin-like signaling in development and metabolism of Aedes albopictus. Parasit. Vectors 2023, 16, 134. [Google Scholar] [CrossRef]
- Okamoto, N.; Yamanaka, N.; Endo, Y.; Kataoka, H.; Mizoguchi, A. Spatiotemporal patterns of IGF-like peptide expression in the silkmoth Bombyx mori predict its pleiotropic actions. Gen. Comp. Endocrinol. 2011, 173, 171–182. [Google Scholar] [CrossRef]
- Gontijo, A.M.; Garelli, A. The biology and evolution of the Dilp8-Lgr3 pathway: A relaxin-like pathway coupling tissue growth and developmental timing control. Mech. Dev. 2018, 154, 44–50. [Google Scholar] [CrossRef]
- Semaniuk, U.; Strilbytska, O.; Malinovska, K.; Storey, K.B.; Vaiserman, A.; Lushchak, V.; Lushchak, O. Factors that regulate expression patterns of insulin-like peptides and their association with physiological and metabolic traits in Drosophila. Insect Biochem. Mol. Biol. 2021, 135, 103609. [Google Scholar] [CrossRef]
- Liao, S.; Nassel, D.R. Drosophila insulin-like peptide 8 (DILP8) in ovarian follicle cells regulates ovulation and metabolism. Front. Endocrinol. 2020, 11, 461. [Google Scholar] [CrossRef]
- Li, H.; Luo, X.; Li, N.; Liu, T.; Zhang, J. Insulin-like peptide 8 (Ilp8) regulates female fecundity in flies. Front. Cell Dev. Biol. 2023, 11, 1103923. [Google Scholar] [CrossRef]
- Yamaguchi, S.T.; Kobayashi, R.; Tomita, J.; Kume, K. The regulation of circadian rhythm by insulin signaling in Drosophila. Neurosci. Res. 2022, 183, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Wang, Z.; Gong, S.; Zhang, Y.; Xue, C. Sublethal concentration of λ-cyhalothrin inhibits insulin-like peptides and leads to reproductive toxicity in Chrysoperla sinica. Insect Sci. 2024; Online ahead of print. [Google Scholar] [CrossRef]
- Umbers, K.D.; Fabricant, S.A.; Gawryszewski, F.M.; Seago, A.E.; Herberstein, M.E. Reversible colour change in Arthropoda. Biol. Rev. Camb. Philos. Soc. 2014, 89, 820–848. [Google Scholar] [CrossRef]
- Li, J.X.; Tian, Z.; Liu, X.F.; Li, B.; An, H.M.; Brent, C.S.; Wang, J.L.; Wang, X.P.; Liu, W. Juvenile hormone regulates the photoperiodic plasticity of elytra coloration in the ladybird Harmonia axyridis. Mol. Ecol. 2023, 32, 2884–2897. [Google Scholar] [CrossRef]
- Riehle, M.A.; Fan, Y.; Cao, C.; Brown, M.R. Molecular characterization of insulin-like peptides in the yellow fever mosquito, Aedes aegypti: Expression, cellular localization, and phylogeny. Peptides 2006, 27, 2547–2560. [Google Scholar] [CrossRef]
- Sheng, Z.; Xu, J.; Bai, H.; Zhu, F.; Palli, S.R. Juvenile hormone regulates vitellogenin gene expression through insulin-like peptide signaling pathway in the red flour beetle, Tribolium castaneum. J. Biol. Chem. 2011, 286, 41924–41936. [Google Scholar] [CrossRef]
- Pan, X.; Pei, Y.; Zhang, C.; Huang, Y.; Chen, L.; Wei, L.; Chen, X. Effect of insulin receptor on juvenile hormone signal and fecundity in Spodoptera litura (F.). Insects 2022, 13, 701. [Google Scholar] [CrossRef]
- Tian, Z.; Guo, S.; Zhu, F.; Liu, W.; Wang, X.P. Targeting coat protein II complex genes via RNA interference inhibits female adult feeding and reproductive development in the cabbage beetle Colaphellus bowringi. Pest. Manag. Sci. 2022, 78, 2141–2150. [Google Scholar] [CrossRef]
- Wu, S.; Tang, Y.; Su, S.; Ding, W.; He, H.; Xue, J.; Li, Y. RNA interference knockdown of insulin receptor inhibits ovarian development in Chilo suppressalis. Mol. Biol. Rep. 2022, 49, 11765–11773. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Tan, D.; Wang, F.; Guo, S.; Liu, J.; Cuthbertson, A.; Sang, W. Insulin peptides and their receptors regulate ovarian development and oviposition behavior in Diaphorina citri. Insect Sci. 2023, 30, 95–108. [Google Scholar] [CrossRef] [PubMed]
- Mouawad, R.; Himadewi, P.; Kadiyala, D.; Arnosti, D.N. Selective repression of the Drosophila cyclin B promoter by retinoblastoma and E2F proteins. Biochim. Biophys. Acta Gene Regul. Mech. 2020, 1863, 194549. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.Y.; Chen, J.J.; Liu, M.Y.; He, W.W.; Reynolds, J.A.; Wang, Y.N.; Zhang, L.S. Enhanced degradation of juvenile hormone promotes reproductive diapause in the predatory ladybeetle Coccinella Septempunctata. Front. Physiol. 2022, 13, 877153. [Google Scholar] [CrossRef] [PubMed]
- Castro-Arnau, J.; Marin, A.; Castells, M.; Ferrer, I.; Maestro, J.L. The expression of cockroach insulin-like peptides is differentially regulated by physiological conditions and affected by compensatory regulation. J. Insect Physiol. 2019, 114, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Sang, M.; Li, C.; Wu, W.; Li, B. Identification and evolution of two insulin receptor genes involved in Tribolium castaneum development and reproduction. Gene 2016, 585, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Xue, W.H.; Liu, Y.L.; Jiang, Y.Q.; He, S.F.; Wang, Q.Q.; Yang, Z.N.; Xu, H.J. Molecular characterization of insulin-like peptides in the brown planthopper, Nilaparvata lugens (Hemiptera: Delphacidae). Insect Mol. Biol. 2020, 29, 309–319. [Google Scholar] [CrossRef]
- Lu, K.; Chen, X.; Liu, W.T.; Zhou, Q. TOR pathway-mediated juvenile hormone synthesis regulates nutrient-dependent female reproduction in Nilaparvata lugens (Stal). Int. J. Mol. Sci. 2016, 17, 438. [Google Scholar] [CrossRef]
- Perez-Hedo, M.; Rivera-Perez, C.; Noriega, F.G. The insulin/TOR signal transduction pathway is involved in the nutritional regulation of juvenile hormone synthesis in Aedes aegypti. Insect Biochem. Mol. Biol. 2013, 43, 495–500. [Google Scholar] [CrossRef]
- Zhai, Y.; Sun, Z.; Zhang, J.; Kang, K.; Chen, J.; Zhang, W. Activation of the TOR signalling pathway by glutamine regulates insect fecundity. Sci. Rep. 2015, 5, 10694. [Google Scholar] [CrossRef]
- Zheng, W.; Wu, F.; Ye, Y.; Li, T.; Zhang, Z.; Zhang, H. Small GTPase Rab40C is up regulated by 20-hydroxyecdysone and insulin pathways to regulate ovarian development and fecundity. Insect Sci. 2022, 29, 1583–1600. [Google Scholar] [CrossRef]





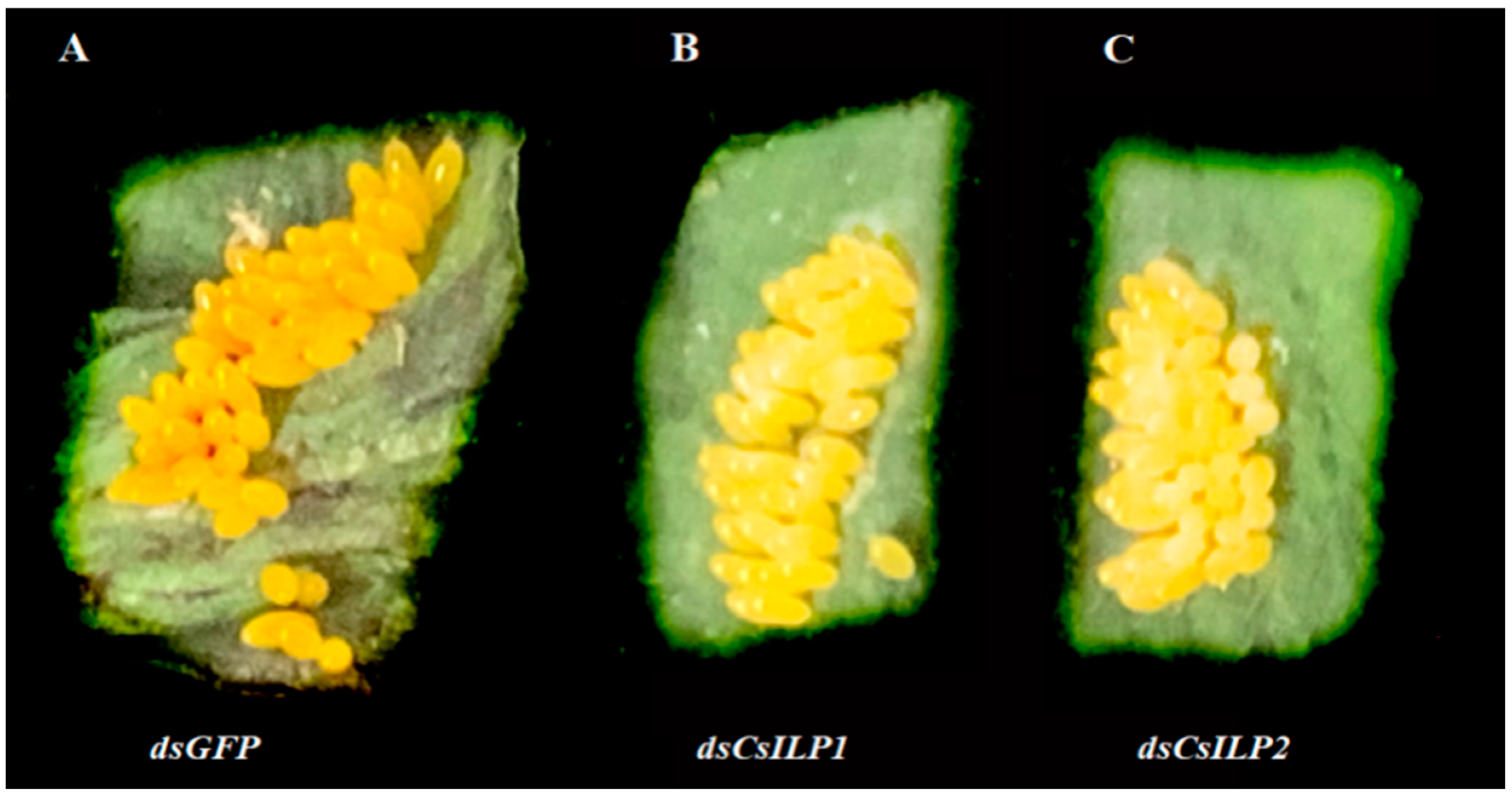
| Treatments | Pre-Oviposition (Days) | 14 Days Fecundity (Eggs per Female) | Hatching Rate (%) |
|---|---|---|---|
| dsGFP | 10.17 ± 0.29 c | 296.79 ± 22.54 a | 90.11 ± 0.93 a |
| dsCsILP1 | 15.75 ± 0.36 a | 29.71 ± 8.83 b | 83.11 ± 1.23 b |
| dsCsILP2 | 14.71 ± 0.26 b | 49.13 ± 13.03 b | 82.44 ± 1.50 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, S.; Wang, D.; Qin, Q.; Chen, K.; Zhang, W.; He, Y. Functions of Insulin-like Peptide Genes (CsILP1 and CsILP2) in Female Reproduction of the Predatory Ladybird Coccinella septempunctata (Coleoptera: Coccinellidae). Insects 2024, 15, 981. https://doi.org/10.3390/insects15120981
Feng S, Wang D, Qin Q, Chen K, Zhang W, He Y. Functions of Insulin-like Peptide Genes (CsILP1 and CsILP2) in Female Reproduction of the Predatory Ladybird Coccinella septempunctata (Coleoptera: Coccinellidae). Insects. 2024; 15(12):981. https://doi.org/10.3390/insects15120981
Chicago/Turabian StyleFeng, Shanshan, Da Wang, Qiuju Qin, Ke Chen, Wenjing Zhang, and Yunzhuan He. 2024. "Functions of Insulin-like Peptide Genes (CsILP1 and CsILP2) in Female Reproduction of the Predatory Ladybird Coccinella septempunctata (Coleoptera: Coccinellidae)" Insects 15, no. 12: 981. https://doi.org/10.3390/insects15120981
APA StyleFeng, S., Wang, D., Qin, Q., Chen, K., Zhang, W., & He, Y. (2024). Functions of Insulin-like Peptide Genes (CsILP1 and CsILP2) in Female Reproduction of the Predatory Ladybird Coccinella septempunctata (Coleoptera: Coccinellidae). Insects, 15(12), 981. https://doi.org/10.3390/insects15120981







