The Species of the mali-Group of Aphelinus (Hymenoptera: Aphelinidae), with Descriptions of Three New Species, DNA Sequence Data and One Newly-Recorded Species from China
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection of Parasitoids
2.2. Photographs and Slides of Parasitoids
2.3. DNA Sequencing
2.4. Phylogenetic Analysis
| Family/Species | GenBank Accession | Source |
|---|---|---|
| Aphelinidae | ||
| Aphelinus abdominalis | PP480262.1 | GenBank |
| A. abdominalis | PP480263.1 | GenBank |
| A. asychis | OP019281.1 | GenBank |
| A. gossypii | OP342775.1 | GenBank |
| A. varipes | OQ789199.1 | GenBank |
| A. coreae (Y18) | PQ375438.1 | This paper |
| A. hainanensis, sp.n. | PQ375436.1 | This paper |
| A. humilis (Y4) | PQ375437.1 | This paper |
| A. hyalopteraphidis | PQ375442.1 | This paper |
| A. maidis (Y29) | PQ375440.1 | This paper |
| A. ruellia, sp.n. | PQ375439.1 | This paper |
| A. tuberocephalus, sp.n. | PQ375441.1 | This paper |
| Coccophagus ceroplastae | KY606141.1 | GenBank |
| C. japonicus | KY605984.1 | GenBank |
2.5. Terminology and Abbreviations
| FAFU | College of Plant Protection, Fujian Agriculture and Forestry University, Fuzhou, Fujian, China. |
| NHMUK | Natural History Museum, London, UK. |
| TAMU | Texas A&M University, College Station, USA. |
| USNM | United States National Museum of Natural History, Washington DC, USA. |
3. Results
3.1. Modified Key Couplets to Species of the mali-Group of Aphelinus by Hopper et al. (2012) [15]
3.2. Species Accounts
3.2.1. Aphelinus tuberocephalus Wang & Huang, sp.n. (Figure 1 and Figure 2)
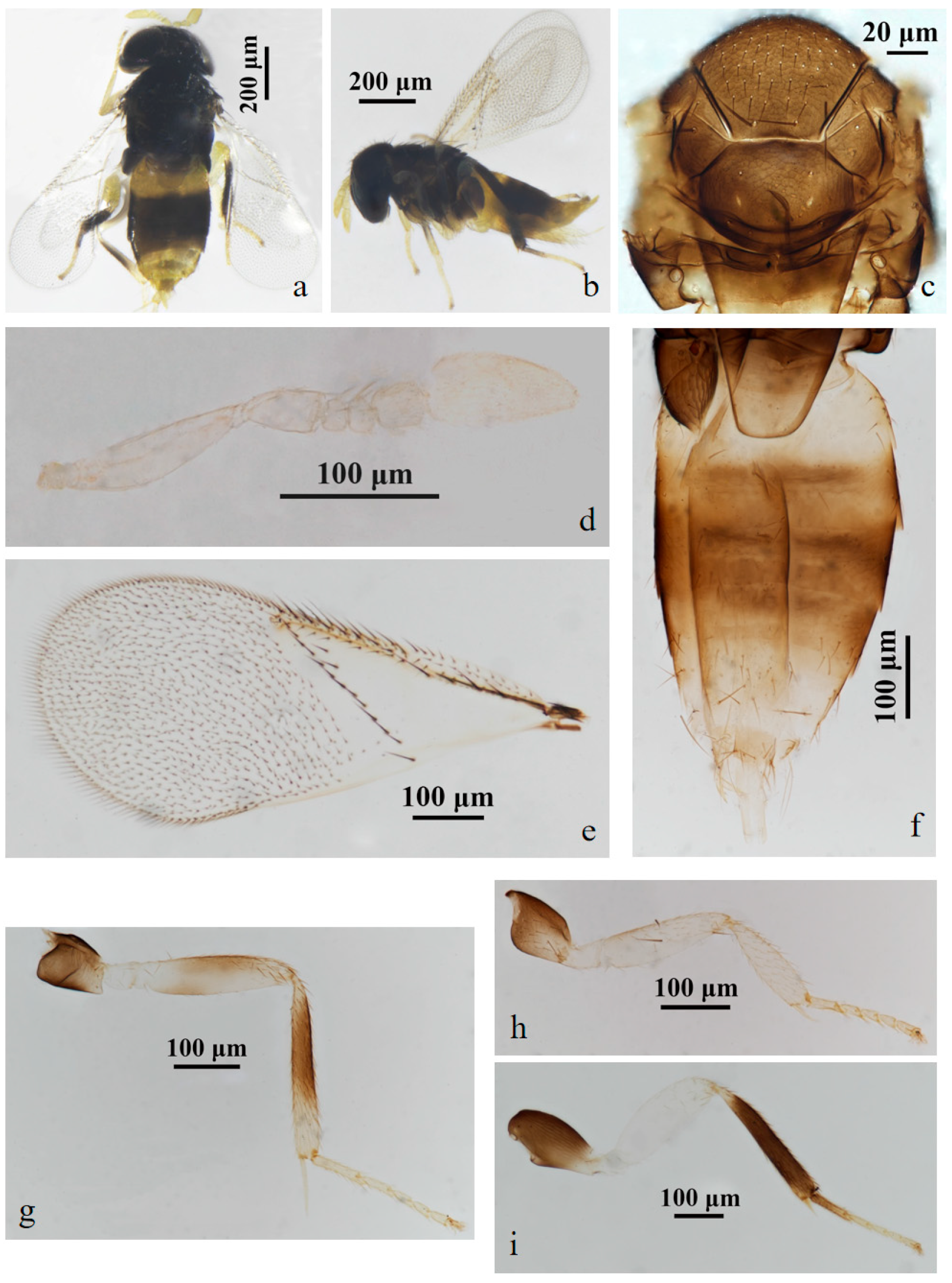

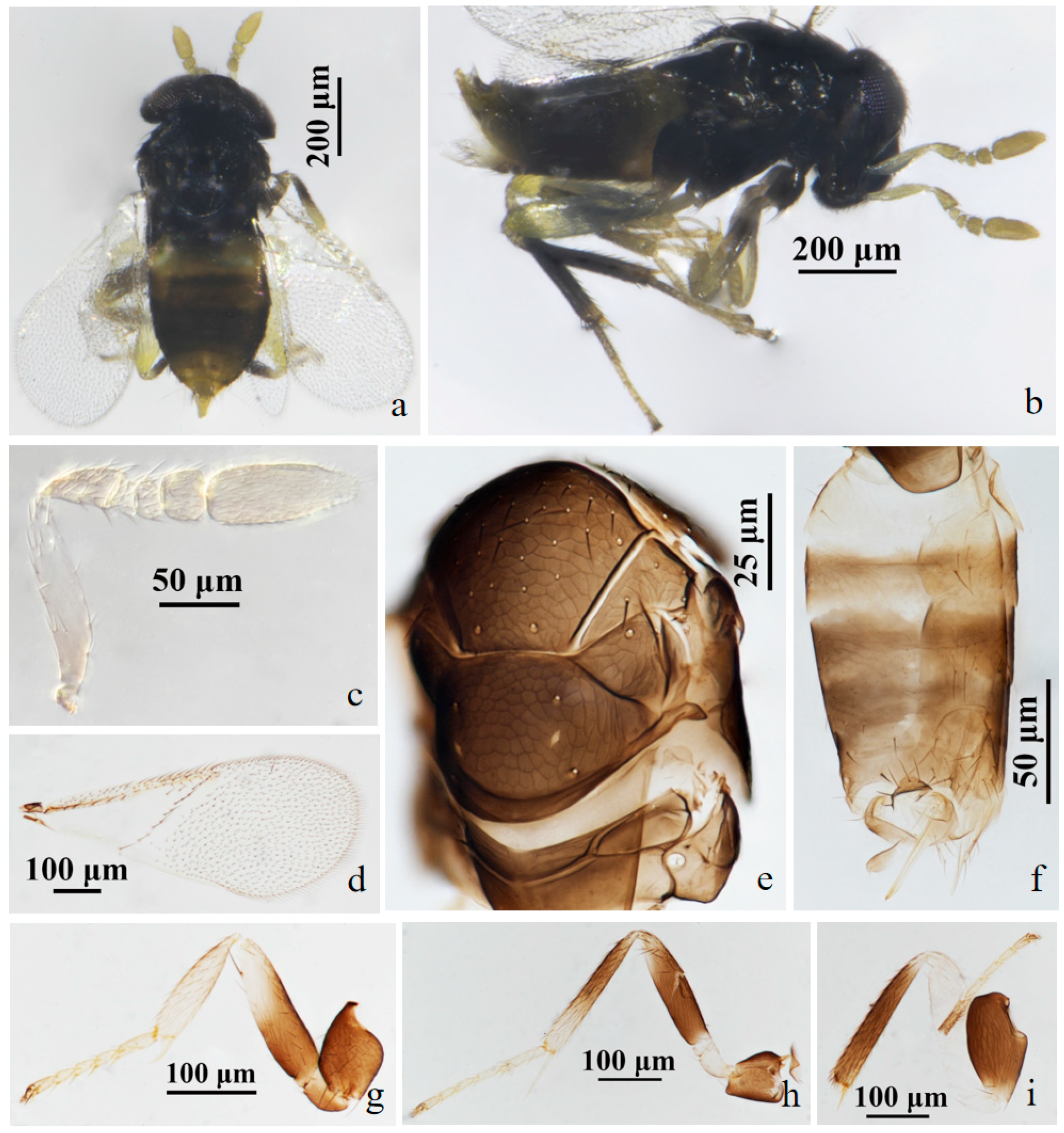
3.2.2. Aphelinus hainanensis Wang & Huang, sp.n. (Figure 3 and Figure 4)


3.2.3. Aphelinus ruellia Wang & Huang, sp.n. (Figure 5 and Figure 6)


3.2.4. Aphelinus coreae Hopper & Woolley, 2012 [15] (Figure 7 and Figure 8)

3.3. Phylogenetic Analysis
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Howard, L.O. Aphelinus mali and its travels. Ann. Entomol. Soc. Am. 1929, 22, 241–268. [Google Scholar] [CrossRef]
- Huang, J. Systematic Studies on Aphelinidae of China (Hymenoptera: Chalcidoidea); Chongqing Publishing House: Chongqing, China, 1994. [Google Scholar]
- Noyes, J.S. Universal Chalcidoidea Database. Available online: https://sfg.taxonworks.org/ (accessed on 15 August 2024).
- Chen, Y.; Li, C.D. Three new species and new distributional data for five rare species of Aphelinus (Hymenoptera: Aphelinidae) from China. Zootaxa 2016, 4092, 258–272. [Google Scholar] [CrossRef] [PubMed]
- Geng, S.Y.; Li, C.D. A new record species of Aphelinus Dalman (Hymenoptera: Aphelinidae) from China. J. Northeast For. Univ. 2011, 39, 117–118. [Google Scholar]
- Li, C.D.; Langor, D.W. A new species of Aphelinus (Hymenoptera: Aphelinidae) from northeastern China. Can. Entomol. 1998, 130, 799–801. [Google Scholar] [CrossRef]
- Li, C.D.; Zhang, S. A new species and a new record of Aphelinus Dalman (Hymenoptera: Aphelinidae) from China. Entomotaxonomia 2005, 27, 69–73. [Google Scholar]
- Li, C.D.; Zhao, S.L. A new species of Aphelinus Dalman (Hymenoptera: Aphelinidae) from Northeastern China. Entomotaxonomia 1998, 20, 150–152. [Google Scholar]
- Liao, D.X.; Li, X.L.; Pang, X.F.; Chen, T.L. Economic Insect Fauna of China 34: Hymenoptera Chalcidoidea (1); Science Press: Beijing, China, 1987. [Google Scholar]
- Pan, M.X. A new species of Aphelinus Dalman from Zhejiang Province, China (Hymenoptera: Aphelinidae). Acta Zoot. Sin. 1992, 17, 75–77. [Google Scholar]
- Shih, Y.T.; Ko, C.C. Fauna Taiwan: Insecta Hymenoptera Aphelinidae; National Taiwan University: Taipei, China, 2020. [Google Scholar]
- Yang, C.K.; Chen, Y.J. Two new species of Aphelinus (Hymenoptera: Aphelinidae) parasitizing the rice root aphids. Entomotaxonomia 1995, 17, 296–302. [Google Scholar]
- Zehavi, A.; Rosen, D. A new species of Aphelinus (Hymenoptera: Aphelinidae) from Israel, with notes on the mali group. Isr. J. Entomol. 1989, 32, 101–108. [Google Scholar]
- Hayat, M. Aphelinidae of India (Hymenoptera: Chalcidoidea): A Taxonomic Revision; Memoirs on Entomology; International Associated Publishers: Gainesville, FL, USA, 1998; Volume 13, pp. 1–416. [Google Scholar]
- Hopper, K.R.; Woolley, J.B.; Hoelmer, K.; Wu, K.M.; Qiao, G.X.; Lee, S.H. An identification key to species in the mali complex of Aphelinus (Hymenoptera, Chalcidoidea) with descriptions of three new species. J. Hym. Res. 2012, 26, 73–96. [Google Scholar]
- Noyes, J.S. Collecting and preserving chalcid wasps (Hymenoptera: Chalcidoidea). J. Nat. Hist. 1982, 16, 315–334. [Google Scholar] [CrossRef]
- Polaszek, A.; Ayshford, T.; Yahya, B.E.; Fusu, L. Wallaceaphytis: An unusual new genus of parasitoid wasp (Hymenoptera: Aphelinidae) from Borneo. J. Nat. Hist. 2013, 48, 1111–1123. [Google Scholar] [CrossRef]
- Folmer, O.; Black, M.; Hoeh, W.; Lutz, R.; Vrijenhoek, R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotechnol. 1994, 35, 294–299. [Google Scholar]
- Xiang, C.Y.; Gao, F.L.; Jakovlic, I.; Lei, H.P.; Hu, Y.; Zhang, H.; Zou, H.; Wang, G.T.; Zhang, D. Using PhyloSuite for molecular phylogeny and tree–based analyses. iMeta 2023, 2, e87. [Google Scholar] [CrossRef]
- Yasnosh, V.A. New species of the genus Aphelinus Dalm. (Hymenoptera, Chalcidoidea) in the fauna of the USSR. Entomol. Obozr. 1963, 42, 178–185. [Google Scholar]
- Prinsloo, G.L.; Neser, O.C. The southern African species of Aphelinus Dalman (Hymenoptera: Aphelinidae), parasitoids of aphids (Homoptera: Aphidoidea). J. African Zool. 1994, 108, 143–162. [Google Scholar]
- Timberlake, P.H. Descriptions of new chalcid-flies from Hawaii and Mexico (Hymenoptera). Proc. Hawaii. Entomol. Soc. 1924, 5, 395–417. [Google Scholar]
- Girault, A.A. Australian Hymenoptera Chalcidoidea–IV. The family Eulophidae with descriptions of new genera and species. Mem. Qld. Mus. 1913, 2, 140–296. [Google Scholar]
- Gahan, A.B. Some new parasitic Hymenoptera with notes on several described forms. Proc. US Natl. Mus. 1924, 65, 1–23. [Google Scholar] [CrossRef][Green Version]
- Ashmead, W.H. Descriptions of some new North American Chalcididae. III: Euderinae (Hymenoptera: Chalcidoidea). Can. Entomol. 1888, 20, 101–107. [Google Scholar] [CrossRef]
- Evans, G.A.; Schauff, M.E.; Kok-Yokomi, M.L.; Yokomi, R.K. A new species of Aphelinus (Hymenoptera: Aphelinidae) that parasitizes the spirea aphid, Aphis spiraecola Patch (Homoptera: Aphididae). Proc. Entomol. Soc. Wash. 1995, 97, 17–21. [Google Scholar]
- Heraty, J.M.; Woolley, J.B.; Hopper, K.R.; Hawks, D.L.; Kim, J.W.; Buffington, M. Molecular phylogenetics and reproductive incompatibility in a complex of cryptic species of aphid parasitoids. Mol. Phylogenet. Evol. 2007, 45, 480–493. [Google Scholar] [CrossRef] [PubMed]
- Gokhman, V.E.; Kuhn, K.L.; Woolley, J.B.; Hopper, K.R. Variation in genome size and karyotype among closely related aphid parasitoids (Hymenoptera, Aphelinidae). Comp. Cytogenet. 2017, 11, 97–117. [Google Scholar] [CrossRef] [PubMed]

| Primer | Sequence | Cycling Conditions | |||
|---|---|---|---|---|---|
| LCO1490 | 5′-GGTCAACAAAATCATAAAGATATTGG-3′ | Denaturation | Annealing | Extension | Cycles |
| HCO2198 | 5′-TAAACTTCAGGGTGACCAAAAAATCA-3′ | 94 °C (30 s) | 47 °C (30 s) | 72 °C (1 min) | 35 |
| Species | Distribution | Hosts | References |
|---|---|---|---|
| A. basilicus | India, Sri Lanka, Bangladesh | aphids on Ocimum basilicum, Lantana camara and okra | [14] |
| A. campestris | Russia | aphid on Polygonum sp. | [13,20] |
| A. coreae | Korea, China (Fujian) | Aphis glycines | [15], Note in this paper |
| A. engaeus | South Africa | Rhodesaclerda sp. on Loranthus dregei | [15,21] |
| A. ficusae | South Africa | aphid on Ficus sycomorus | [15,21] |
| A. glycinis | China (Liaoning) | Aphis glycines | [15] |
| A. gossypii | USA (Honolulu); cosmopolitan in Old World tropics | aphid on Ficus sycomorus, Aphis sp. on Hibiscus | [13,14,15,22] |
| A. hainanensis, sp.n. | China (Hainan) | an unidentified aphid on Hibiscus rosa-sinensis | Described in this paper |
| A. mali | China, India, Cosmopolitan | Aphis gossypii, A. spiraecola, Eriosoma lanigerum on apple, Myzus persicae, ? Aspidiotus destructor | [2,13,14,15] |
| A. niger | Australia (Brisbane, Queensland) | unknown | [15,23] |
| A. paramali | Israel | melon aphid, Aphis gossypii, pomegranate aphid, Aphis punicae | [13,15] |
| A. rhamni | China (Beijing) | Aphis glycines | [15] |
| A. ruellia, sp.n. | China (Fujian) | an unidentified aphid on Ruellia simplex | Described in this paper |
| A. sanborniae | USA (Pennsylvania) | Sanbornia juniperi | [15,24] |
| A. siphonophorae | North America | Siphonophora sp. | [15,25] |
| A. spiraecolae | China (Guangdong) | Aphis spiraecola | [15,26] |
| A. tuberocephalus, sp.n. | China (Fujian) | Tuberocephalus momonis | Described in this paper |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, Z.; Luo, Y.; Ge, J.; Huang, J.; Polaszek, A.; Wang, Z. The Species of the mali-Group of Aphelinus (Hymenoptera: Aphelinidae), with Descriptions of Three New Species, DNA Sequence Data and One Newly-Recorded Species from China. Insects 2024, 15, 945. https://doi.org/10.3390/insects15120945
Dong Z, Luo Y, Ge J, Huang J, Polaszek A, Wang Z. The Species of the mali-Group of Aphelinus (Hymenoptera: Aphelinidae), with Descriptions of Three New Species, DNA Sequence Data and One Newly-Recorded Species from China. Insects. 2024; 15(12):945. https://doi.org/10.3390/insects15120945
Chicago/Turabian StyleDong, Zhigang, Ye Luo, Junqing Ge, Jian Huang, Andrew Polaszek, and Zhuhong Wang. 2024. "The Species of the mali-Group of Aphelinus (Hymenoptera: Aphelinidae), with Descriptions of Three New Species, DNA Sequence Data and One Newly-Recorded Species from China" Insects 15, no. 12: 945. https://doi.org/10.3390/insects15120945
APA StyleDong, Z., Luo, Y., Ge, J., Huang, J., Polaszek, A., & Wang, Z. (2024). The Species of the mali-Group of Aphelinus (Hymenoptera: Aphelinidae), with Descriptions of Three New Species, DNA Sequence Data and One Newly-Recorded Species from China. Insects, 15(12), 945. https://doi.org/10.3390/insects15120945






