New Species and Records of Lemophagus Townes, 1965 (Hymenoptera, Ichneumonidae, Campopleginae), from China †
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Taxonomy
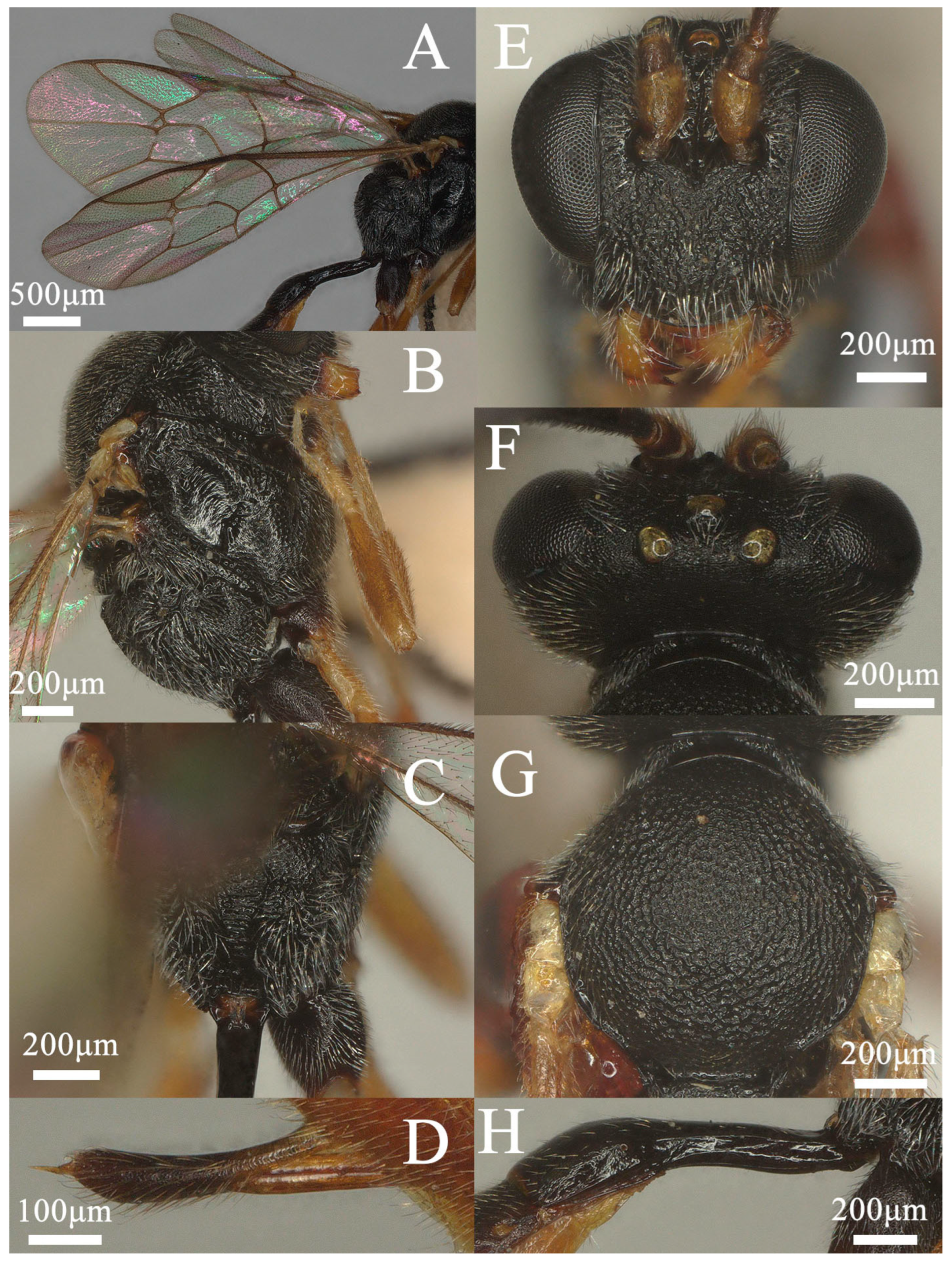
3.1.1. Lemophagus curtus Townes, 1965 (Figure 1 and Figure 2)

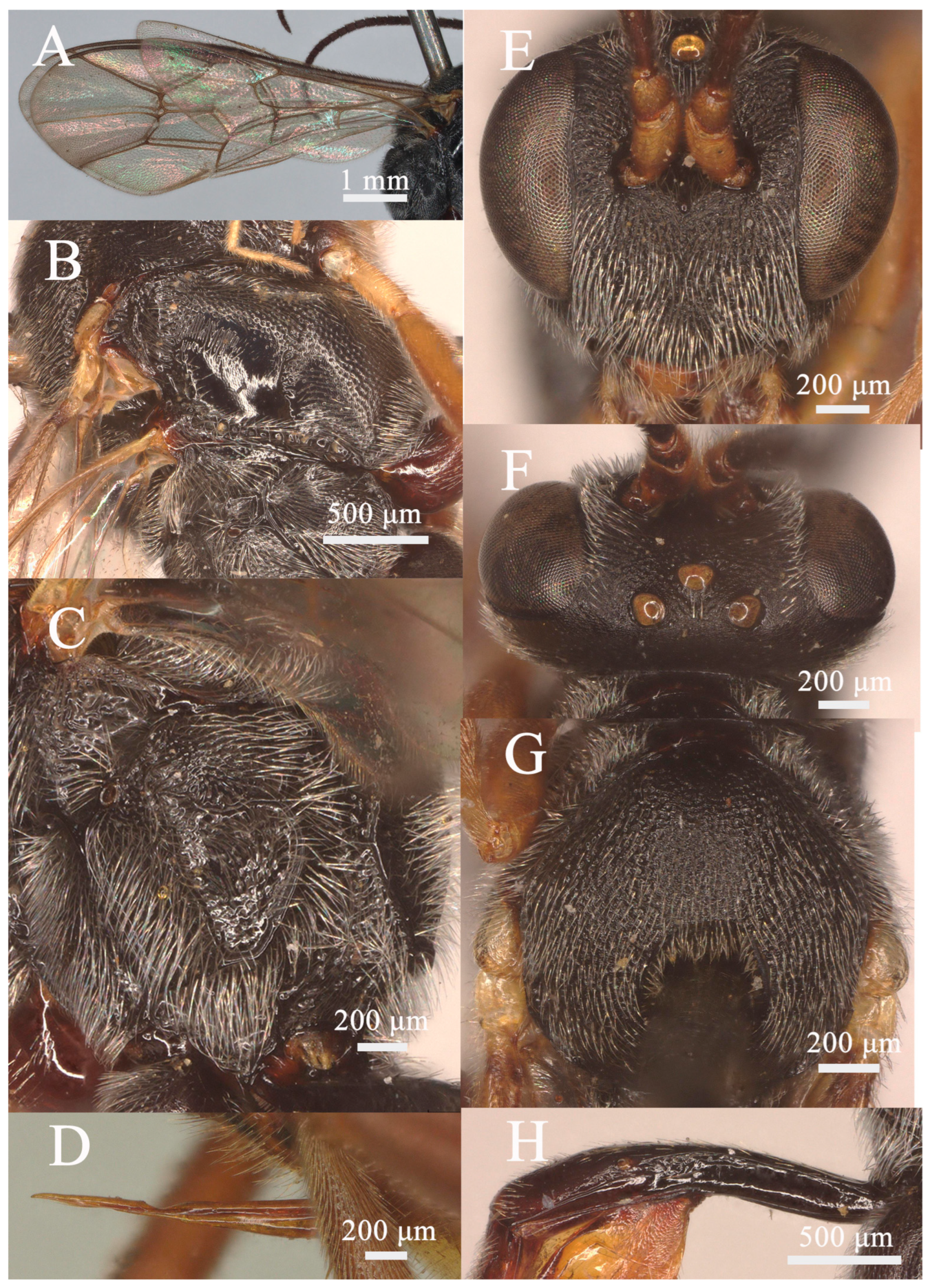
3.1.2. Lemophagus pulcher (Szépligeti, 1916) (Figure 3 and Figure 4)

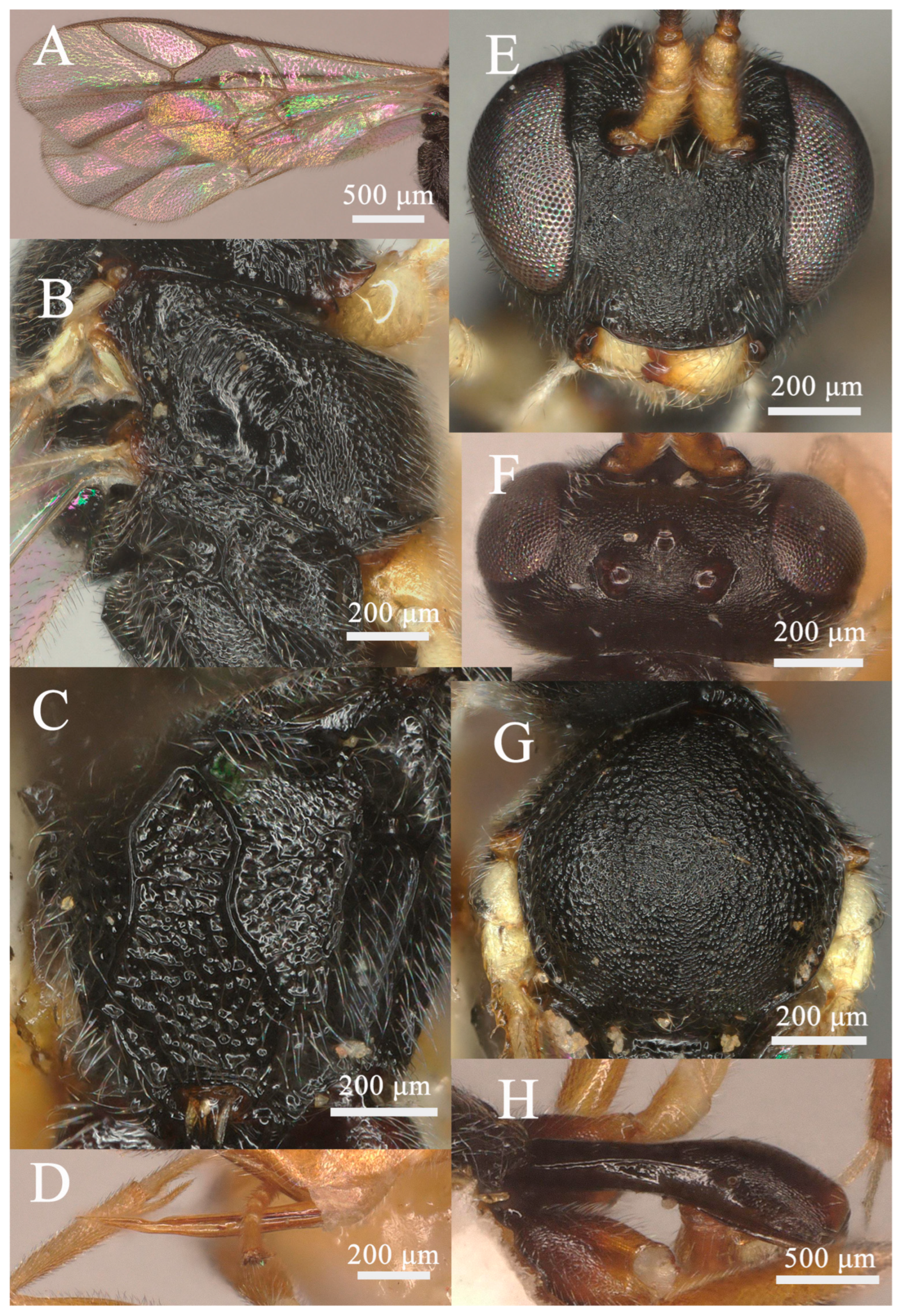
3.1.3. Lemophagus nanus sp. nov. (Figure 5 and Figure 6)

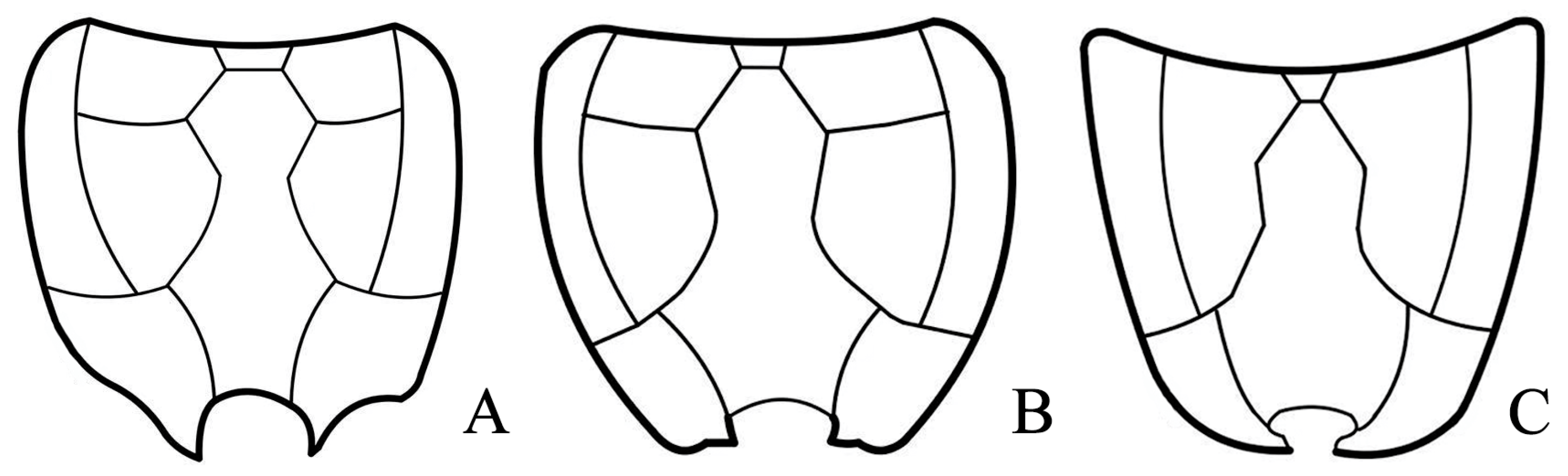
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yu, D.S.; van Achterberg, C.; Horstmann, K. Taxapad 2016. World Ichneumonoidea 2015. Taxonomy, Biology, Morphology and Distribution; Taxapad Database on Flash-Drive: Vancouver, BC, Canada, 2016. [Google Scholar]
- Kenis, M.; Herz, K.; West, R.J.; Shaw, M.R. Parasitoid assemblages reared from geometrid defoliators (Lepidoptera: Geometridae) of larch and fir in the alps. Agric. For. Entomol. 2005, 7, 307–318. [Google Scholar] [CrossRef]
- Petrice, T.R.; Strazanac, J.S.; Butler, L. A survey of hymenopteran parasitoids of forest Macrolepidoptera in the central Appalachians. J. Econ. Entomol. 2004, 97, 451–459. [Google Scholar] [CrossRef] [PubMed]
- Wahl, D.B. The final-instar larva of Venturia townesorum (Hymenoptera, Ichneumonidae). Pan-Pac. Entomol. 1985, 61, 218–220. [Google Scholar]
- Sanborne, M. A revision of the world species of Sinophorus Förster (Ichneumonidae). Mem. Am. Entomol. Inst. 1984, 38, 403. [Google Scholar]
- Raffa, K.F. Some parasites of Lepidoptera larvae recently collected in Delaware. Entomol. News. 1977, 88, 81–84. [Google Scholar]
- Roberts, S.J.; Mellor, W.K.; Armbrust, E.J. Parasites of lepidopterous larvae in alfalfa and soybeans in central Illinois. Great Lakes Entomol. 1977, 10, 87–93. [Google Scholar]
- Graham, A.R. A Preliminary List of Natural Enemies of Canadian Agricultural Pests; Canada Department of Agriculture; Research Institute: Belleville, IL, USA, 1965; p. 179. [Google Scholar]
- Sheng, M.L.; Sun, S.P. Parasitic Ichneumonids on Woodborers in China (Hymenoptera: Ichneumonidae); Science Press: Beijing, China, 2010; p. 338. [Google Scholar]
- Gordon, R.; Ellington, J.; Faubion, G.F.; Graham, H. A survey of the insect parasitoids from alfalfa and associated weeds in New Mexico. Southwest. Entomol. 1987, 12, 335–350. [Google Scholar]
- Starke, H. Ichneumonidenfauna der sächsischen Oberlausitz. Nat. Lusatica 1956, 3, 17–92. [Google Scholar]
- Cheng, W.Y.; Chen, S.M.; Wang, Z.T. Survey of larval and pupal parasitoids of cane borers in spring cane. Rep. Taiwan Sugar Res. Inst. 1999, 164, 1–14. [Google Scholar]
- Aspöck, H. The biology of Raphidioptera: A review of present knowledge. Acta Zool. Acad. Sci. Hung. 2002, 48, 35–50. [Google Scholar]
- Broad, G.R.; Shaw, M.R.; Fitton, M.G. Ichneumonid wasps (Hymenoptera: Ichneumonidae): Their classification and biology. In Handbooks for the Identification of British Insects; Field Studies Council: Shrewsbury, UK, 2018; Volume 7, pp. 1–418. [Google Scholar]
- Karimi, S.; Ghassemi-Kahrizeh, A.; Hosseinzadeh, A.; Hessein, L.; Matthias, R. A new genus and two new species with some new records of the subfamily Campopleginae (Hymenoptera: Ichneumonidae) from Iran. Egypt. J. Biol. Pest. Control 2023, 33, 117. [Google Scholar] [CrossRef]
- Quicke, D.L.J. The Braconid and Ichneumonid Parasitoid Wasps: Biology, Systematics, Evolution and Ecology; Wiley Blackwell: Hoboken, NJ, USA, 2015; p. 553. [Google Scholar]
- Gonzalez, V.M.; Caballero-Grande, R.; Acevedo, B. Effects of the sampling method and of chemical control on populations of Heliothis virescens and its natural enemy Diadegma sp. on tobacco under experimental conditions. Cienc. Agric. 1980, 7, 43–49. [Google Scholar]
- Vas, Z. “Revisiting” North Korea: New species and new records of Campopleginae (Hymenoptera: Ichneumonidae). Ann. Hist. Nat. Mus. Natn. Hung. 2023, 115, 215–235. [Google Scholar] [CrossRef]
- Vas, Z.; Rezaei, S.; Fallahzadeh, M.; Mohammadi-Khoramabadi, A.; Saghaei, N.; Ljubomirov, T. Contributions to the taxonomy, identification, and biogeography of Palaearctic Campopleginae (Hymenoptera: Ichneumonidae), with the descriptions of four new species from Iran. Zootaxa 2022, 5134, 261–274. [Google Scholar] [CrossRef] [PubMed]
- He, J.H.; Chen, X.X.; Ma, Y. Hymenoptera: Ichneumonidae. Economic Insect Fauna of China; Science Press: Beijing, China, 1996; 697p. [Google Scholar]
- Townes, H.K. Nomenclatural notes on European Ichneumonidae (Hymenoptera). Pol. Pismo Entomol. 1965, 35, 409–417. [Google Scholar]
- Sonan, J. A few host known Ichneumonidae found in Japan and Formosa. Trans. Nat. Hist. Soc. Formosa 1930, 20, 268–273. [Google Scholar]
- Townes, H.K. The genera of Ichneumonidae, Part 3. Mem. Am. Entomol. Inst. 1970, 13, 1–307. [Google Scholar]
- Horstmann, K. Bemerkungen zur Systematik einiger Gattungen der Campopleginae IV (Hymenoptera, Ichneumonidae). Z. Arbeitsgem. Oesterr. Entomol. 2004, 56, 13–35. [Google Scholar]
- Klopfstein, S.; Broad, G.R.; Urfer, K.; Vårdal, H.; Haraldseide, H. An interactive key to the European genera of Campopleginae (Hymenoptera, Ichneumonidae) and 20 new species for Sweden. Entomol. Tidskr. 2022, 143, 121–156. [Google Scholar]
- Haye, T.; Kenis, M. Biology of Lilioceris spp. (Coleoptera: Chrysomelidae) and their parasitoids in Europe. Biol. Control 2004, 29, 399–408. [Google Scholar] [CrossRef]
- Dysart, R.J.; Maltby, H.L.; Brunson, M.H. Larval parasites of Oulema melanopus in Europe and their colonization in the United States. Entomophaga 1973, 18, 122–167. [Google Scholar] [CrossRef]
- Shaw, M.R.; Horstmann, K.; Whiffin, A. Two hundred and twenty-five species of reared western Palaearctic Campopleginae (Hymenoptera: Ichneumonidae) in the National Museums of Scotland, with descriptions of new species of Campoplex and Diadegma, and records of fifty-five species new to Britain. Entomol. Gaz. 2016, 67, 177–222. [Google Scholar]
- Gold, M.S.; Casagrande, R.A.; Tewksbury, L.A.; Livingston, S.B.; Kenis, M. European parasitoids of Lilioceris lilii (Coleoptera: Chrysomelidae). Can. Entomol. 2001, 133, 671–674. [Google Scholar] [CrossRef]
- Aubert, J.F.; Halperin, J.; Gerling, D. Les Ichneumonides d’Israel. Entomophaga 1984, 29, 211–235. [Google Scholar] [CrossRef]
- Tewksbury, L.; Casagrande, R.A.; Cappuccino, N.; Kenis, M. Establishment of Parasitoids of the Lily Leaf Beetle (Coleoptera: Chrysomelidae) in North America. Environ. Entomol. 2017, 46, 226–236. [Google Scholar] [CrossRef] [PubMed]
- Coulson, J.R. Releases of Beneficial Organisms in the United States and Territories–1983; U.S. Dept. of Agriculture, Agricultural Research Service: Beltsville, MD, USA, 1994; p. 113.
- Haynes, D.L.; Gage, S.H.; Fulton, W. Management of the cereal leaf beetle pest ecosystem. Quaest. Entomol. 1974, 101, 165–176. [Google Scholar]
- Hendrickson, R.M.; Gruber, F.; Mailloux, G.; Drea, J.J. Parasite colonizations against Crioceris asparagi (L.) and C. duodecimpunctata (L.) (Coleptera: Chrysomelidae) in North America from 1983 to 1988. Proc. Entomol. Soc. Wash. 1991, 93, 67–69. [Google Scholar]
- Tewksbury, E.A. Introduction and Establishment of Three Parasitoids of the Lily Leaf Beetle, Lilioceris lilii (Coleoptera: Chrysomelidae) in North of America. Ph.D. Thesis, University of the Rhoda Island, Kingston, RI, USA, 2014; 61p. [Google Scholar]
- Lake, E.C.; Tewksbury, L.; Smith, M.C.; Dray, F.A.; Russell, A.D.; Madeira, P.T.; Rayamajhi, M.B.; Casagrande, R.A. Potential for negative interactions between successful arthropod and weed biological control programs: A case study with Lilioceris species. Biol. Control 2020, 144, 1–8. [Google Scholar] [CrossRef]
- Khalaim, A.I.; Kasparyan, D.R. Cryptinae, Campopleginae. In Key to the Insects of Russia Far East. Vol. IV. Neuropteroidea, Mecoptera, Hymenoptera. Pt 5; Lelej, A.S., Ed.; Dalnauka: Vladivostok, Russia, 2007; 1052p. (In Russain) [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, Y.; Wei, C.; Liu, C.; Dong, Y. New Species and Records of Lemophagus Townes, 1965 (Hymenoptera, Ichneumonidae, Campopleginae), from China. Insects 2024, 15, 932. https://doi.org/10.3390/insects15120932
Han Y, Wei C, Liu C, Dong Y. New Species and Records of Lemophagus Townes, 1965 (Hymenoptera, Ichneumonidae, Campopleginae), from China. Insects. 2024; 15(12):932. https://doi.org/10.3390/insects15120932
Chicago/Turabian StyleHan, Yuanyuan, Chengxue Wei, Chong Liu, and Yan Dong. 2024. "New Species and Records of Lemophagus Townes, 1965 (Hymenoptera, Ichneumonidae, Campopleginae), from China" Insects 15, no. 12: 932. https://doi.org/10.3390/insects15120932
APA StyleHan, Y., Wei, C., Liu, C., & Dong, Y. (2024). New Species and Records of Lemophagus Townes, 1965 (Hymenoptera, Ichneumonidae, Campopleginae), from China. Insects, 15(12), 932. https://doi.org/10.3390/insects15120932





