Parasitism Affects Entomofauna Dynamics in Infected and Uninfected Plants: A Case Study of Orobanche anatolica Parasitizing Salvia absconditiflora
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Insects
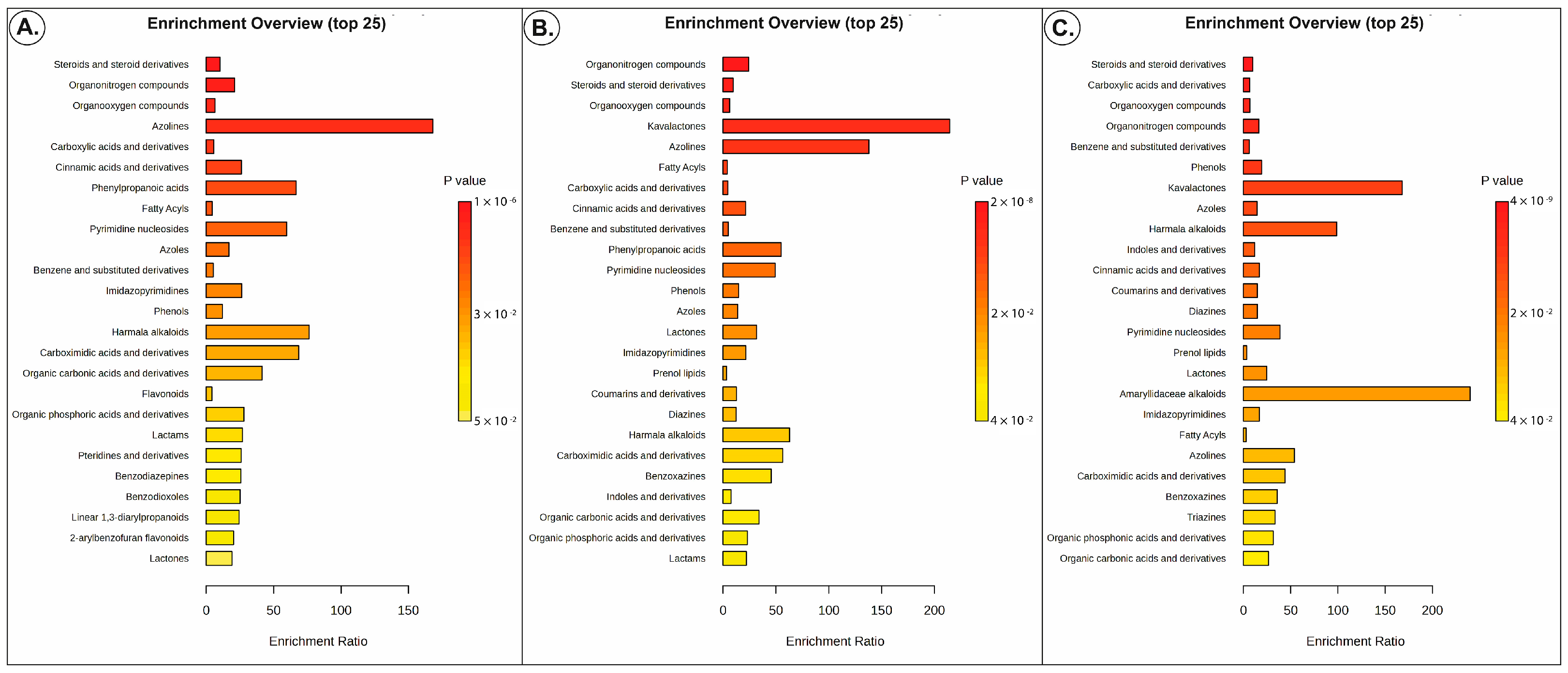
3.2. Metabolomics
4. Discussion
5. Conclusions
Supplementary Materials
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hedge, I. Flora of Turkey and the East Aegean Islands, 1st ed.; Edinburgh University Press: Edinburgh, UK, 1982; pp. 433–434. [Google Scholar]
- Uhlich, H.; Pusch, J.; Barthel, K.J. Die Sommerwurzarten Europas; Westarp Wissenschaften: Magdeburg, Germany, 1995. [Google Scholar]
- Pusch, J.; Günther, K.-F. Orobanchaceae (Sommerwurzgewächse). In Illustrierte Flora von Mitteleuropa; Hegi, G., Ed.; Weissdorn Verlag: Jena, Germany, 2009; pp. 1–99. [Google Scholar]
- Westwood, J.H.; Yoder, J.I.; Timko, M.P.; Depamphilis, C.W. The evolution of parasitism in plants. Trends Plant Sci. 2010, 15, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Thieret, J.W. The genera of Orobanchaceae in the southeastern United States. J. Arnold. Arbor. 1971, 52, 404–434. [Google Scholar] [CrossRef]
- Piwowarczyk, R.; Madeja, J.; Nobis, M. Pollen morphology of the central European broomrapes (Orobanchaceae: Orobanche, Phelipanche and Orobanchella) and its taxonomical implications. Plant Syst. Evol. 2015, 301, 795–808. [Google Scholar] [CrossRef]
- Ollerton, J.; Stott, A.; Allnutt, E.; Shove, S.; Taylor, C.; Lamborn, E. Pollination niche overlap between a parasitic plant and its host. Oecologia 2007, 151, 473–485. [Google Scholar] [CrossRef] [PubMed]
- Kuijt, J. Biology of Parasitic Flowering Plants; University of California Press: Berkeley, CA, USA, 1969. [Google Scholar]
- Jones, M. Studies into the Pollination of Orobanche Species in the British Isles. In Progress in Orobanche Research, Proceedings of the International Workshop on Orobanche Research, Obermarchtal, Tubingen, Germany, 19–22 August 1989; Eberhard-Karls-Universitat: Tubingen, Germany, 1991; pp. 6–17. [Google Scholar]
- Brandenburg, A.; Dell’olivo, A.; Bshary, R.; Kuhlemeier, C. The sweetest thing. Advances in nectar research. Curr. Opin. Plant Biol. 2009, 12, 486–490. [Google Scholar] [CrossRef]
- Heil, M. Nectar: Generation, regulation and ecological functions. Trends Plant Sci. 2011, 16, 191–200. [Google Scholar] [CrossRef]
- Heywood, V.; Culham, A.; Seberg, O. Flowering Plant Families of the World; Kew Publishing: London, UK, 2007; 424p. [Google Scholar]
- Grant, V.; Grant, K.A. Flower Pollination in the Phlox Family; Columbia University Press: New York, NY, USA, 1965. [Google Scholar]
- Peris, D.; Condamine, F.L. The angiosperm radiation played a dual role in the diversification of insects and insect pollinators. Nat. Commun. 2024, 15, 552. [Google Scholar] [CrossRef]
- Benitez-Vieyra, S.; Alquicira, J.P.; Sazatornil, F.; Dominguez, C.A.; Boege, K.; Perez-Ishiwara, R.; Fornoni, J. Evolutionary transition between bee pollination and hummingbird pollination in Salvia: Comparing means, variances and covariances of corolla traits. J. Evol. Biol. 2014, 32, 783–793. [Google Scholar] [CrossRef]
- Pichersky, E.; Gershenzon, J. The formation and function of plant volatiles: Perfumes for pollinator attraction and defense. Curr. Opin. Plant Biol. 2002, 5, 237–243. [Google Scholar] [CrossRef]
- Raguso, R.A. Wake Up and Smell the Roses: The Ecology and Evolution of Floral Scent. Annu. Rev. Ecol. Evol. Syst. 2008, 39, 549–569. [Google Scholar] [CrossRef]
- Tóth, P.; Undas, A.K.; Verstappen, F.; Bouwmeester, H. Floral volatiles in parasitic plants of the Orobanchaceae. Ecol. Taxon. Implic. Front. Plant Sci. 2016, 7, 312. [Google Scholar]
- Kaiser, R. Flowers and fungi use scents to mimic each other. Science 2006, 311, 806–807. [Google Scholar] [CrossRef] [PubMed]
- Rering, C.C.; Beck, J.J.; Hall, G.W.; Mccartney, M.M.; Vannette, R.L. Nectar-inhabiting microorganisms influence nectar volatile composition and attractiveness to a generalist pollinator. New Phytol. 2018, 220, 750–759. [Google Scholar] [CrossRef] [PubMed]
- Warren, M.L.; Kram, K.E.; Theiss, K.E. Characterizing the nectar microbiome of the non-native tropical milkweed, Asclepias curassavica, in an urban environment. PLoS ONE 2020, 15, e0237561. [Google Scholar] [CrossRef] [PubMed]
- Borror, D.J.; Triplehorn, C.A.; Johnson, N.F. An Introduction to the Study of Insects, 6th ed.; Thomson Learning, Inc.: Southbank, VIC, Australia, 1989. [Google Scholar]
- Michener, C.D. The Bees of the World; Johns Hopkins University Press: Baltimore, MD, USA, 2007; pp. 1–953. [Google Scholar]
- Tsugawa, H.; Cajka, T.; Kind, T.; Ma, Y.; Higgins, B.; Ikeda, K.; Kanazawa, M.; VanderGheynst, J.; Fiehn, O.; Arita, M. MS-DIAL: Data-independent MS/MS deconvolution for comprehensive metabolome analysis. Nat. Methods 2015, 12, 523–526. [Google Scholar] [CrossRef]
- Xia, J.; Psychogios, N.; Young, N.; Wishart, D.S. MetaboAnalyst: A web server for metabolomic data analysis and interpretation. Nucleic Acids Res. 2009, 37, W652–W660. [Google Scholar] [CrossRef]
- Pang, Z.; Chong, J.; Zhou, G.; Morais, D.; Chang, L.; Barrette, M.; Gauthier, C.; Jacques, P.E.; Li, S.; Xia, J. MetaboAnalyst 5.0: Narrowing the gap between raw spectra and functional insights. Nucleic Acids Res. 2021, 49, W388–W396. [Google Scholar] [CrossRef]
- Krebs, C.J. Ecological Methodology, 2nd ed.; Addison-Wesley Educational Publishers, Inc.: Menlo Park, CA, USA, 1999; pp. 1–620. [Google Scholar]
- Magurran, A.E. Measuring Biological Diversity; Blackwell Publishing: Oxford, UK, 2004; pp. 1–256. [Google Scholar]
- Kindt, R.; Coe, R. Tree Diversity Analysis: A Manual and Software for Common Statistical Methods for Ecological and Biodiversity Studies; World Agroforestry: Nairobi, Kenya, 2005; Available online: http://www.worldagroforestry.org/resources/databases/tree-diversity-analysis (accessed on 25 October 2023).
- Bani, A.; Pavlova, D.; Benizri, E.; Shallari, S.; Miho, L.; Meco, M.; Shahu, E.; Reeves, R.; Echevarria, G. Relationship between the Ni hyperaccumulator Alyssum murale and the parasitic plant Orobanche nowackiana from serpentines in Albania. Ecol. Res. 2018, 33, 549–559. [Google Scholar] [CrossRef]
- Kreutz, C.A.J. Orobanche: The European Broomrape Species. Central and Northern Europe; Natuurhistorisch Genootschap: Limburg, The Netherlands, 1995; pp. 1–159. [Google Scholar]
- Westwood, J.H.; Foy, C.L.; Cramer, C.L.; Yu, X.S. Expression of defence-related 3-hydroxy-3-methylglutaryl CoA reductase gene in response to parasitization by Orobanche spp. Mol. Plant-Microbe Interact. 1998, 11, 530–536. [Google Scholar] [CrossRef]
- Zare, G.; Dönmez, A.A. Fruit and seed morphology of the tribe Orobancheae (Orobanchaceae) genera in Turkey and its taxonomic significance. Nord. J. Bot. 2016, 34, 178–190. [Google Scholar] [CrossRef]
- Musselman, L.J.; Mann, W.F. A survey of surface characteristics of seeds of Scrophulariaceae and Orobanchaceae using scanning electron microscopy. Phytomorphology 1976, 26, 370–378. [Google Scholar]
- Stace, C.A. Orobanchaceae. In Las Plantas con Flores; Heywood, V.H., Ed.; Reverte: Barcelona, Spain, 1985; pp. 244–245. [Google Scholar]
- Konarska, A.; Chmielewski, P. Taxonomical traits in the microstructure of flowers of parasitic Orobanche picridis with particular emphasis on secretory structures. Protoplasma 2020, 257, 299–317. [Google Scholar] [CrossRef] [PubMed]
- El-Akkad, S.S.; Hassan, E.A.; Ali, M.E. Phenolic acid changes during Orobanche parasitism on faba bean and some other hosts. Egypt. J. Biol. 2002, 4, 37–44. [Google Scholar]
- Hassan, E.A.; El-Awadi, M.E. Study on the trichomes of the parasitic weed broomrape: Morphology and histochemistry. Gen. Appl. Plant Physiol. 2009, 35, 13–21. [Google Scholar]
- Sacchetti, G.; Ballero, M.; Serafini, M.; Muzzoli, M.; Tosi, B.; Poli, F. Morphological and histochemical investigation on glandular trichomes of Orobanche ramosa subsp. nana (Orobanchaceae). Phyton 2003, 43, 207–214. [Google Scholar]
- Serafini, M.; Di Fabio, A.; Foddai, S.; Ballero, M.; Poli, F. The occurrence of phenylpropanoid glycosides in Italian Orobanche spp. Biochem. Syst. Ecol. 1995, 23, 855–858. [Google Scholar] [CrossRef]
- Baker, H.G.; Baker, I. Chemical constituents of nectar in relation to pollination mechanisms and phylogeny. In Biochemical Aspects of Evolutionary Biology; Nitecki, M.H., Ed.; University of Chicago Press: Chicago, IL, USA, 1982; pp. 131–171. [Google Scholar]
- Zúñiga, G.E.; Corcuera, L.J. Effect of gramine in the resistance of barley seedlings to the aphid Rhopalosiphum padi. Entomol. Exp. Appl. 1986, 40, 259–262. [Google Scholar] [CrossRef]
- Adler, L.S. The ecological significance of toxic nectar. Oikos 2000, 91, 409–420. [Google Scholar] [CrossRef]
- Machado, C.A.; Robbins, N.; Gilbert, T.P.; Herre, E.A. Critical review of host specificity and its coevolutionary implications in the fig/fig-wasp mutualism. Proc. Natl. Acad. Sci. USA 2005, 102, 6558–6565. [Google Scholar] [CrossRef]
- Santolamazza-Carbone, S.; Sotelo, T.; Velasco, P.; Cartea, M.E. Antibiotic properties of the glucosinolates of Brassica oleracea var. acephala similarly affect generalist and specialist larvae of two lepidopteran pests. J. Pest Sci. 2016, 89, 195–206. [Google Scholar] [CrossRef]
- Guerrant, E.O.; Fiedler, P.L. Flower defenses against nectar-pilferage by ants. Biotropica 1981, 13, 25–33. [Google Scholar] [CrossRef]
- Detzel, A.; Wink, M. Attraction, deterrence or intoxication of bees (Apis mellifera) by plant allelochemicals. Chemoecology 1993, 4, 8–18. [Google Scholar] [CrossRef]
- Yotavong, P.; Boonsoong, B.; Pluempanupat, W.; Koul, O.; Bullangpoti, V. Effects of the botanical insecticide thymol on biology of a braconid, Cotesia plutellae (Kurdjumov), parasitizing the diamondback moth, Plutella xylostella L. Int. J. Pest Manag. 2015, 61, 171–178. [Google Scholar] [CrossRef]
- Jung, H.H.; Floreancig, P.E. Gold-catalyzed synthesis of oxygen- and nitrogen-containing heterocycles from alkynyl ethers: Application to the total synthesis of andrachcinidine. J. Org. Chem. 2007, 72, 7359–7366. [Google Scholar] [CrossRef] [PubMed]
- Morgan, E.D. Azadirachtin, a scientific gold mine. Bioorg. Med. Chem. 2009, 17, 4096–4105. [Google Scholar] [CrossRef]
- Kilani-Morakchi, S.; Morakchi-Goudjil, H.; Sifi, K. Azadirachtin-Based Insecticide: Overview, Risk Assessments, and Future Directions. Front. Agron. 2021, 3, 676208. [Google Scholar] [CrossRef]
- Farder-Gomes, C.F.; Saravanan, M.; Martinez, L.C.; Plata-Rueda, A.; Zanuncio, J.C.; Serrao, J.E. Azadirachtin-based biopesticide affects the respiration and digestion in Anticarsia gemmatalis caterpillars. Toxins Rev. 2022, 41, 466–475. [Google Scholar] [CrossRef]
- Maliszewska, J.; Rogalska, J. Effect of capsaicin on behaviour of cockroaches under dissimilar long-term temperature adaptation conditions. Bull. Insectology 2022, 75, 187–195. [Google Scholar]
- Nicolson, S.W.; Nepi, M.; Pacini, E. (Eds.) Nectaries and Nectar; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2007. [Google Scholar]
- Li, Y.; Qin, Y.; Liu, S.; Xing, R.; Yu, H.; Li, K.; Li, P. Preparation, characterization, and insecticidal activity of avermectin-grafted-carboxymethyl chitosan. Biomed. Res. Int. 2016, 9805675. [Google Scholar] [CrossRef]
- Sicker, D.; Frey, M.; Schulz, M.; Gierl, A. Role of natural benzoxazinones in the survival strategy of plants. Int. Rev. Cytol. 2000, 198, 319–346. [Google Scholar]
- Bravo, H.R.; Copaja, S.V. Contents and morphological distribution of 2,4-dihydroxy-1,4-benzoxazin-3-one and 2-benzoxazolinone in Acanthus mollis in relation to protection from larvae of Pseudaletia impuncta. Ann. Appl. Biol. 2002, 140, 129–132. [Google Scholar] [CrossRef]
- Schuler, M.A. P450s in plant–insect interactions. Biochim. Biophys. Acta (BBA) Proteins Proteom. 2011, 1814, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Niculaes, C.; Abramov, A.; Hannemann, L.; Frey, M. Plant protection by benzoxazinoids—Recent insights into biosynthesis and function. Agronomy 2018, 8, 143. [Google Scholar] [CrossRef]
- Liggri, P.G.; Tsitsanou, K.E.; Stamati, E.C.; Saitta, F.; Drakou, C.E.; Leonidas, D.D.; Fessas, D.; Zographos, S.E. The structure of AgamOBP5 in complex with the natural insect repellents carvacrol and thymol: Crystallographic, fluorescence and thermodynamic binding studies. Int. J. Biol. Macromol. 2023, 237, 124009. [Google Scholar] [CrossRef]
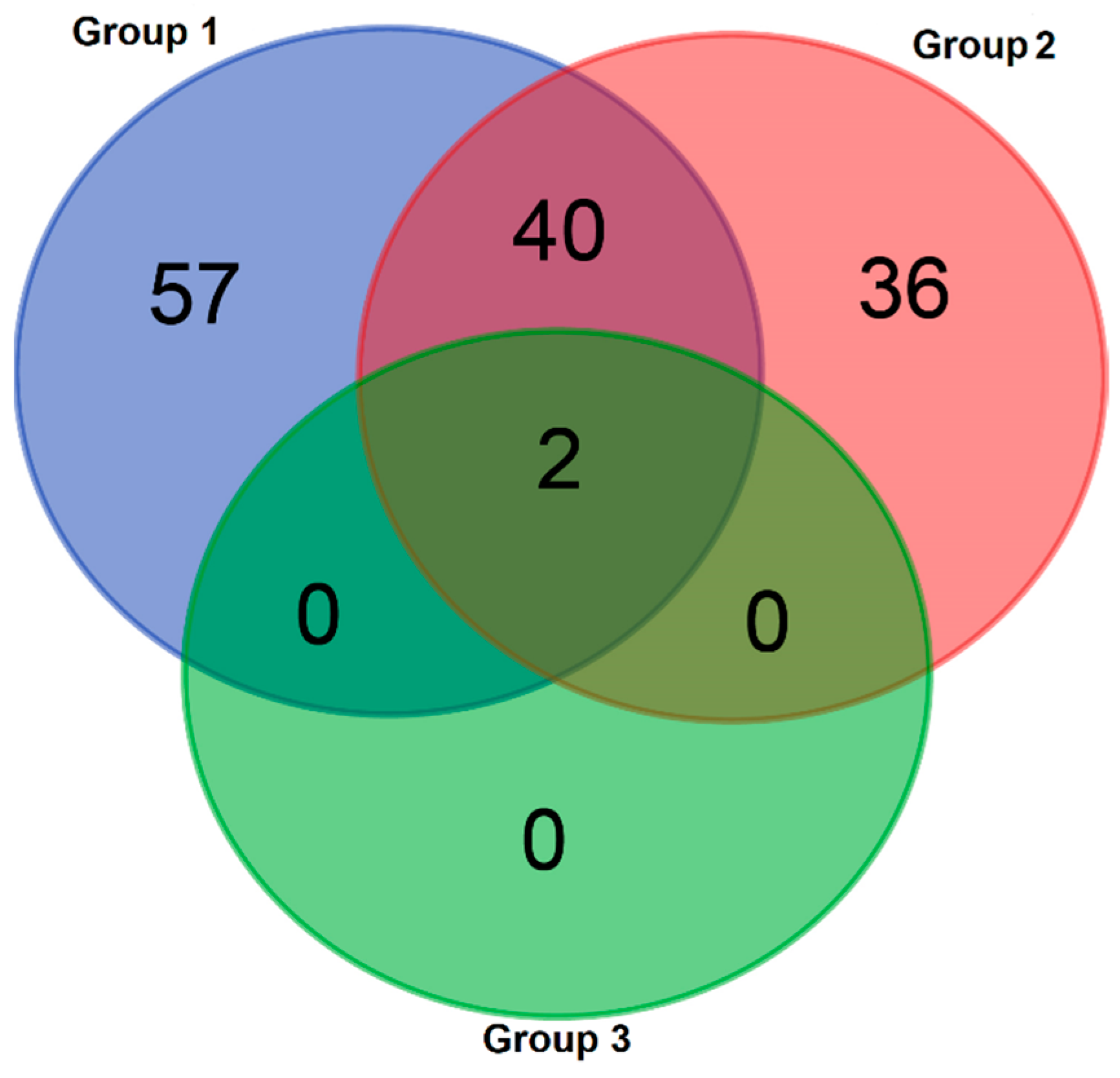

| Group 1 | Group 2 | Group 3 | |
|---|---|---|---|
| Taxa_S | 95 | 75 | 2 |
| Individuals | 297 | 161 | 32 |
| Dominance_D | 0.1139 | 0.04325 | 0.5488 |
| Simpson_1-D | 0.8861 | 0.9568 | 0.4512 |
| Shannon_H | 3.387 | 3.803 | 0.6435 |
| Evenness_e^H/S | 0.3112 | 0.5981 | 0.9516 |
| Brillouin | 3.014 | 3.273 | 0.5836 |
| Menhinick | 5.512 | 5.911 | 0.3536 |
| Margalef | 16.51 | 14.56 | 0.2885 |
| Equitability_J | 0.7437 | 0.8809 | 0.9284 |
| Fisher_alpha | 48.3 | 54.62 | 0.4729 |
| Berger–Parker | 0.3131 | 0.1491 | 0.6563 |
| Chao-1 | 303 | 141.4 | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Özenirler, Ç. Parasitism Affects Entomofauna Dynamics in Infected and Uninfected Plants: A Case Study of Orobanche anatolica Parasitizing Salvia absconditiflora. Insects 2024, 15, 929. https://doi.org/10.3390/insects15120929
Özenirler Ç. Parasitism Affects Entomofauna Dynamics in Infected and Uninfected Plants: A Case Study of Orobanche anatolica Parasitizing Salvia absconditiflora. Insects. 2024; 15(12):929. https://doi.org/10.3390/insects15120929
Chicago/Turabian StyleÖzenirler, Çiğdem. 2024. "Parasitism Affects Entomofauna Dynamics in Infected and Uninfected Plants: A Case Study of Orobanche anatolica Parasitizing Salvia absconditiflora" Insects 15, no. 12: 929. https://doi.org/10.3390/insects15120929
APA StyleÖzenirler, Ç. (2024). Parasitism Affects Entomofauna Dynamics in Infected and Uninfected Plants: A Case Study of Orobanche anatolica Parasitizing Salvia absconditiflora. Insects, 15(12), 929. https://doi.org/10.3390/insects15120929






