Life History of Passaloecus pictus Ribaut, 1952 (Hymenoptera, Pemphredonidae)
Simple Summary
Abstract
1. Introduction
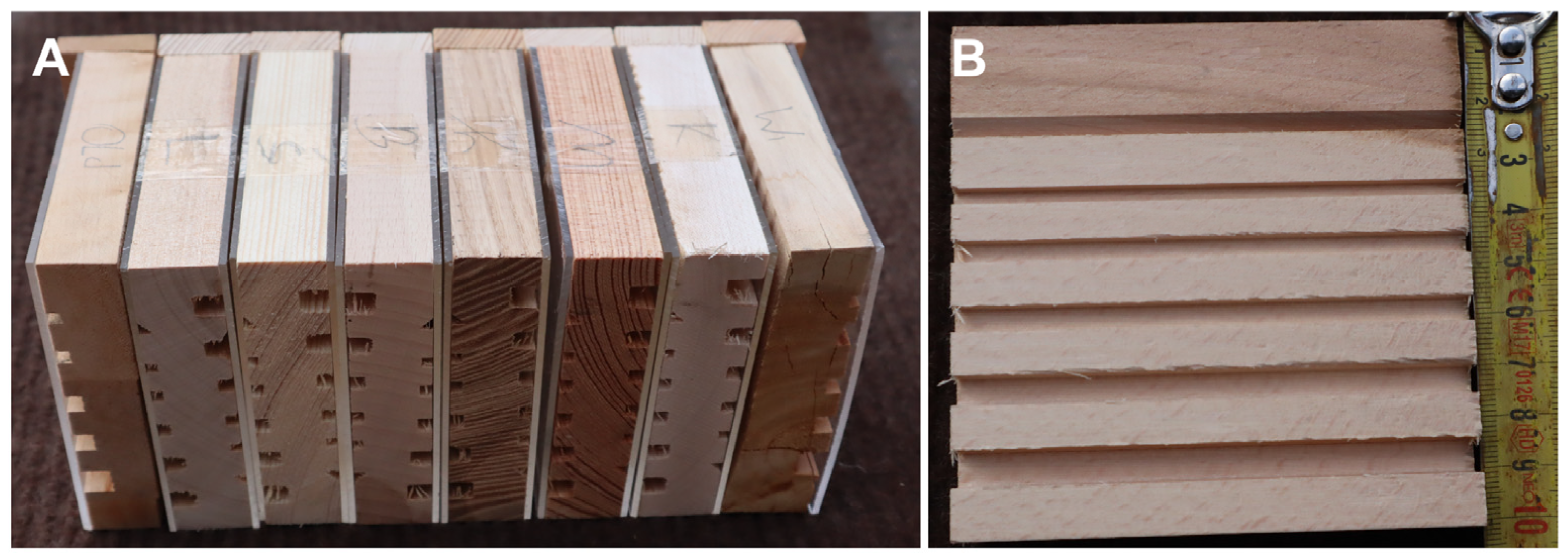
2. Materials and Methods
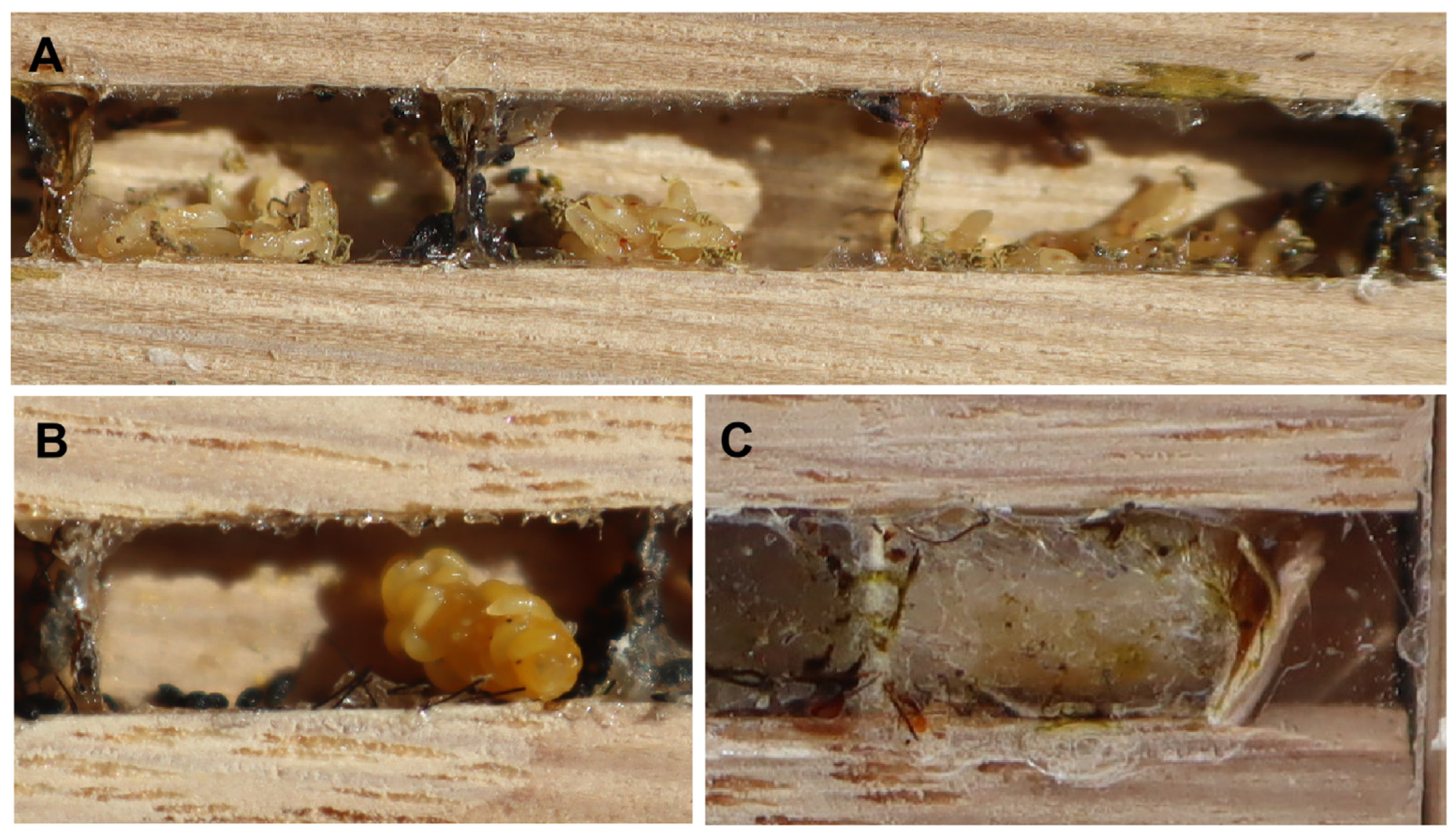
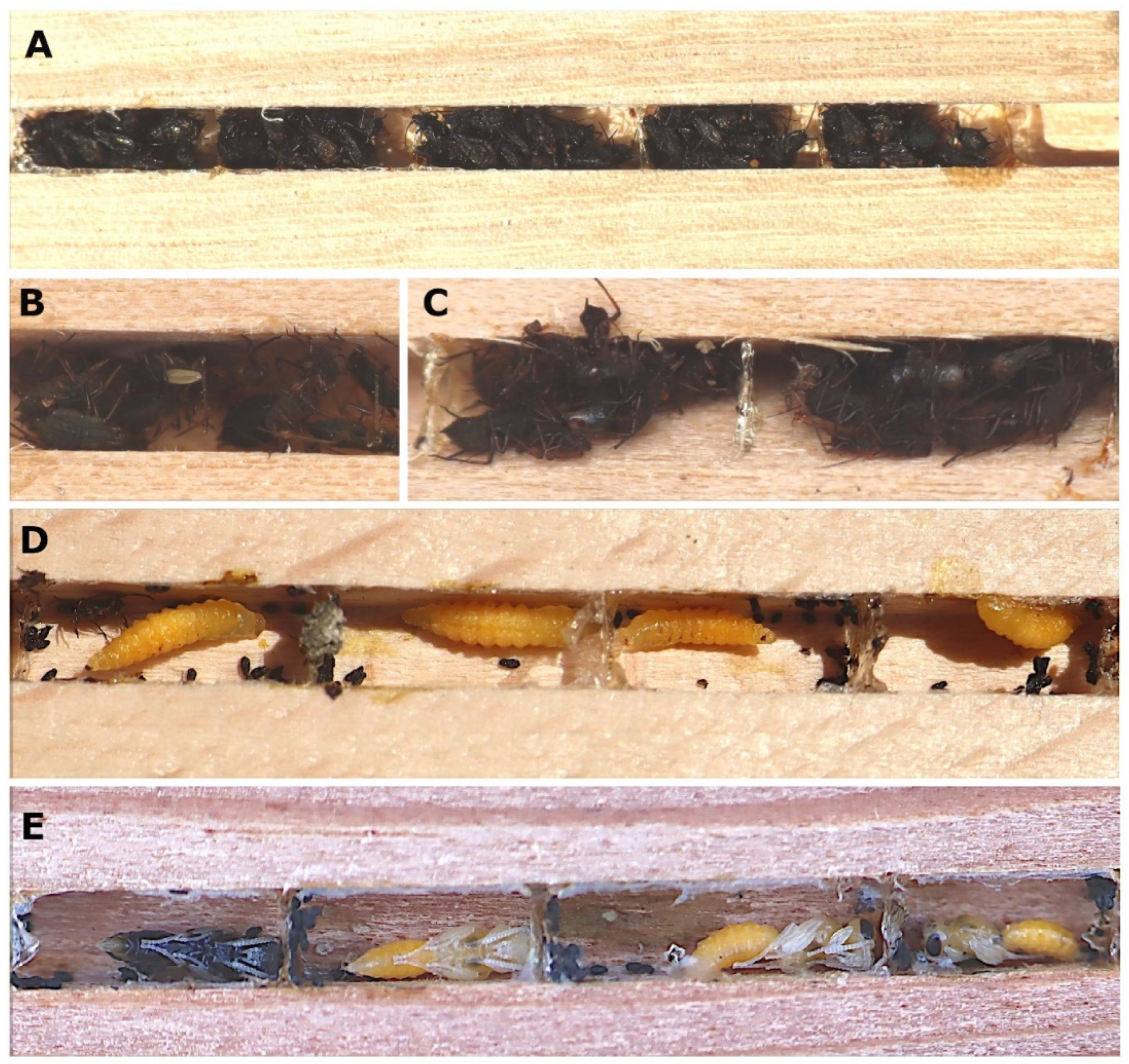
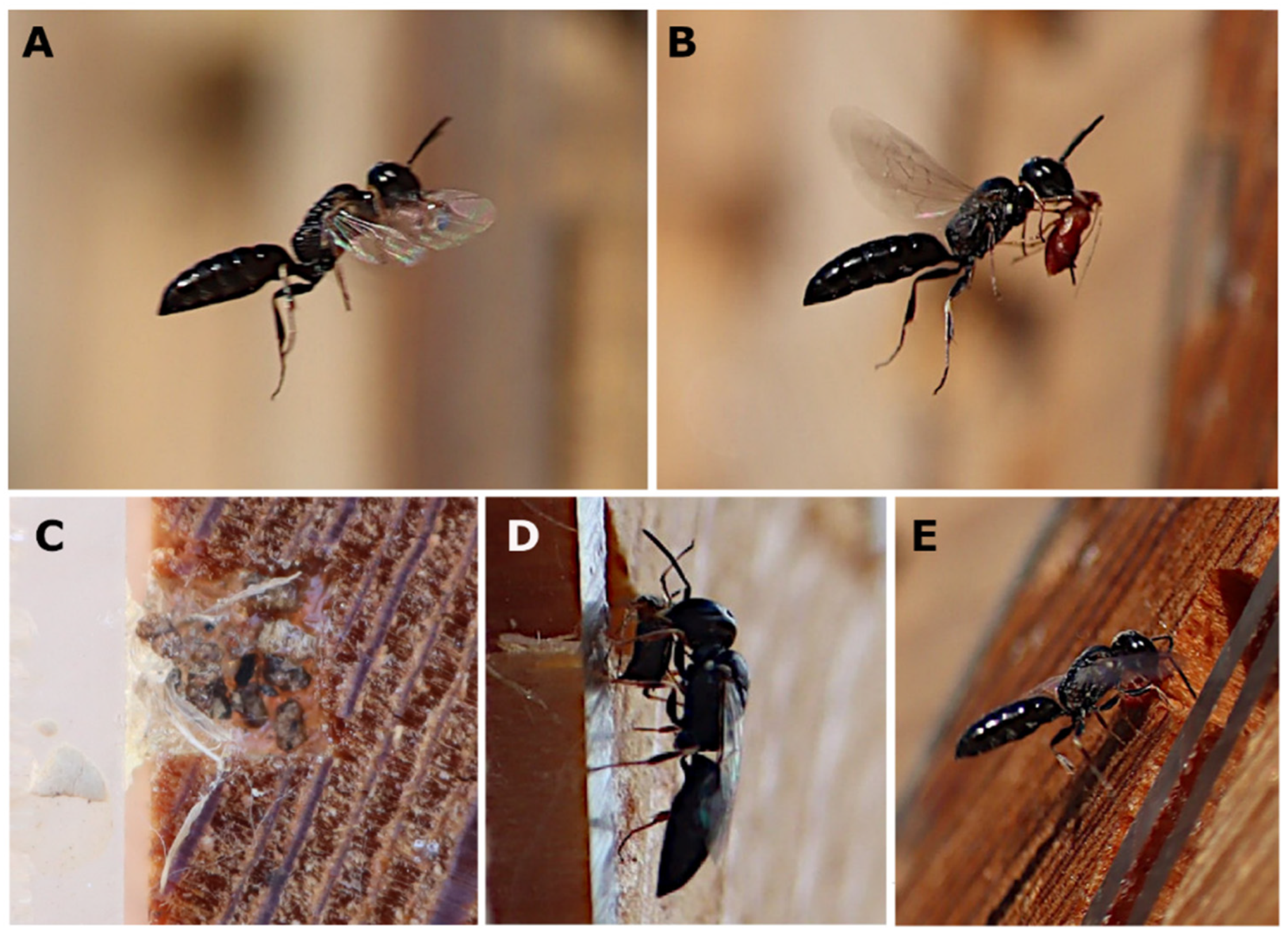
3. Results

4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sann, M.; Niehuis, O.; Peters, R.; Mayer, C.; Kozlov, A.; Podsiadlowski, L.; Bank, S.; Meusemann, K.; Misof, B.; Bleidorn, C.; et al. Phylogenomic analysis of Apoidea sheds new light on the sister group of bees. BMC Evol. Biol. 2018, 18, 71. [Google Scholar] [CrossRef] [PubMed]
- Pulawski, W. Catalog of Sphecidae sensu lato (=Apoidea Excluding Apidae). Available online: https://www.calacademy.org/scientists/projects/catalog-of-sphecidae (accessed on 20 September 2024).
- Olszewski, P.; Wiśniowski, B.; Ljubomirov, T. Current list of the Polish digger wasps (Hymenoptera: Spheciformes). Spixiana 2021, 44, 81–107. [Google Scholar]
- Bohart, R.M.; Menke, A.S. Digger Wasps of the World. A Generic Revision; University of California Press: Berkeley, CA, USA, 1976; 696p. [Google Scholar]
- Kazenas, V.L. Fauna and Biology of Sphecid Wasps (Hymenoptera, Sphecidae) of Kazakhstan and Central Asia; Kazgos INTI: Almaty, Kazakhstan, 2001; 333p. [Google Scholar]
- Jacobs, H.J. Die Grabwespen Deutschlands. Ampulicidae, Sphecidae, Crabronidae; Goecke & Evers: Keltern, Germany, 2007; 207p. [Google Scholar]
- Olszewski, P.; Pawlikowski, T. Passaloecus pictus Ribaut, 1952 (Hymenoptera: Crabronidae)—Pierwsze stwierdzenie w Polsce. Wiadomości Entomologiczne 2013, 32, 266–269. [Google Scholar]
- Janvier, H. Recherches sur les Hymenopteres nidifiants aphidivores. III. Le genre Passaloecus (Shuckhard). Ann. De Sci. Nat. Zool. 1961, 12, 847–883. [Google Scholar]
- Raemakers, I. De graafwesp Passaloecus pictus nieuw voor Nederland (Hymenoptera: Crabronidae). Ned. Faun. Meded. 2008, 29, 21–26. [Google Scholar]
- Blösch, M. Die Grabwespen Deutschlands—Lebensweise, Verhalten, Verbreitung; Goecke & Evers: Keltern, Germany, 2000; 480p. [Google Scholar]
- Blackman, R.L.; Eastop, V.F. Aphids on the World’s Trees. An Identification and Information Guide; CAB International: Wallingford, UK, 1994; 987p. [Google Scholar]
- Blackman, R.L.; Eastop, V.F. Aphids on the World’s Crops. An Identification and Information Guide; The Natural History Museum: London, UK, 2000; 466p. [Google Scholar]
- Szelegiewicz, H. Klucze do Oznaczania Owadów Polski, cz. XVII. Pluskwiaki Równoskrzydłe—Homoptera, Zeszyt 5a, Mszyce—Aphidodea; PWN: Warszawa, Poland, 1978; p. 107ss. [Google Scholar]
- Available online: https://idtools.org/id/AphID/polycosmo10.html (accessed on 18 October 2024).
- Szelegiewicz, H. Mszyce—Aphidoidea. Katalog Fauny Polski; PWN: Warszawa, Poland, 1968; Volume 4, p. 316ss. [Google Scholar]
- Holman, J. Host Plant Catalog of Aphids. Palearctic Region; Springer: Dordrecht, The Netherlands, 2009; 1140p. [Google Scholar]
- Mokrousov, M.V.; Popov, I.B. Digger wasps (Hymenoptera, Apoidea: Ampulicidae, Sphecidae, Crabronidae) of the Black Sea coast of Krasnodar Territory, Abkhazia, and adjacent areas. Entomol. Rev. 2016, 96, 559–599. [Google Scholar] [CrossRef]
- Schneider, N. Abeilles et guêpes anthropophiles du Luxembourg (Insecta, Hymenoptera, Aculeata). Bull. De La Société Des Nat. Luxemb. 2006, 107, 131–145. [Google Scholar]
- Tsuneki, K. The Genus Passaloecus Shuckard of Japan, with Ethological Observations on Some Species (Hymenoptera, Sphecidae, Pemphredoninae); Series II, Natural Science; Memoirs of the Faculty of Liberal Arts, Fukui University: Bunkyo, Fukui, 1955; pp. 1–21. [Google Scholar]
- Ribaut, H. Espèces françaises du genre Passalœcus (Hym. Sphegidae). Bull. De La Société Entomol. De Fr. 1952, 57, 23–28. [Google Scholar] [CrossRef]
- Steiner, A. Les Hyménoptères prédateurs du Périgord Noir. I. Hyménoptères Sphecoidea de la région des Eyzies (Dordogne). Ann. De La Société Entomol. De Fr. 1955, 123, 127–147. [Google Scholar] [CrossRef]
- Corbet, S.A.; Backhouse, M. Aphid hunting wasps: A field study of Passaloecus. Trans. Entomol. Soc. Lond. 1975, 127, 11–30. [Google Scholar] [CrossRef]
- Van Breugel, P.; van Bijenhotels, G. EIS Kenniscentrum Insecten en Andere Ongewervelden; Naturalis Biodiversity Center: Leiden, The Netherlands, 2023; 486p. [Google Scholar]
- Peeters, T.M.J.; van Achterberg, C.; Heitmans, W.R.B.; Klein, W.F.; Lefeber, V.; van Loon, A.J.; Mabelis, A.A.; Nieuwen-huijsen, H.; Reemer, M.; de Rond, J.; et al. De wespen en mieren van Nederland (Hymenoptera: Aculeata). Nederlandse Fauna 6; Nationaal Natuurhistorisch Museum Naturalis, Leiden, knnv Uitgeverij, Utrecht & European Invertebrate Survey: Leiden, The Netherlands, 2004. [Google Scholar]
- Lomholdt, O. The Sphecidae (Hymenoptera) of Fennoscandia and Denmark. In Fauna Entomologica Scandinavica (Klampenbork); Scandinavian Science Press: Klampenborg, Denmark, 1984; Volume 4, pp. 1–452. [Google Scholar]
- Ruchin, A.; Antropov, A. Wasp fauna (Hymenoptera: Bethylidae, Chrysididae, Dryinidae, Tiphiidae, Mutillidae, Scoliidae, Pompilidae, Vespidae, Sphecidae, Crabronidae & Trigonalyidae) of Mordovia State Nature Reserve and its surroundings in Russia. J. Threat. Taxa 2019, 11, 13195–13250. [Google Scholar] [CrossRef]
- Woydak, H. Hymenoptera Aculeata Westfalica Familia: Sphecidae (Grabwespen); Westfälisches Museum für Naturkunde Landschaftsverband Westfalen-Lippe: Münster, Germany, 1996; pp. 3–135. [Google Scholar]
| Species | Number of Aphids | Main Host Plant of Aphids |
|---|---|---|
| Macrosiphoniella millefolii (De Geer, 1773) Dysaphis crataegi (Kaltenbach, 1843) Macrosiphoniella persequens (Walker, 1852) Uroleucon achilleae (Koch, 1855) Total | 124 20 1 282 427 | Anthemis arvensis L., Achillea millefolium L., Achillea pannonica Scheele Anthemis arvensis L., Crataegus monogyna Jacq., Crataegus laevigata (Poir.) DC., Apiaceae Tanacetum vulgare L. Achillea millefolium L. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olszewski, P.; Strażyński, P. Life History of Passaloecus pictus Ribaut, 1952 (Hymenoptera, Pemphredonidae). Insects 2024, 15, 928. https://doi.org/10.3390/insects15120928
Olszewski P, Strażyński P. Life History of Passaloecus pictus Ribaut, 1952 (Hymenoptera, Pemphredonidae). Insects. 2024; 15(12):928. https://doi.org/10.3390/insects15120928
Chicago/Turabian StyleOlszewski, Piotr, and Przemysław Strażyński. 2024. "Life History of Passaloecus pictus Ribaut, 1952 (Hymenoptera, Pemphredonidae)" Insects 15, no. 12: 928. https://doi.org/10.3390/insects15120928
APA StyleOlszewski, P., & Strażyński, P. (2024). Life History of Passaloecus pictus Ribaut, 1952 (Hymenoptera, Pemphredonidae). Insects, 15(12), 928. https://doi.org/10.3390/insects15120928





