Further Evidence That Female Anoplophora glabripennis (Coleoptera: Cerambycidae) Utilizes Photo-Degradation to Produce Volatiles That Are Attractive to Adult Males
Simple Summary
Abstract
1. Introduction
2. Materials & Methods
2.1. Source of Insects
2.2. Oxidation and Analysis of Female Cuticular Hydrocarbon Extracts
2.3. Electrophysiological Analysis (GC/EAD)
2.4. Olfactometer Bioassays of Oxidized Cuticular Extracts and Synthetic Aldehydes
2.5. Field Responses of A. glabripennis to Potential Attractants
3. Results
3.1. Oxidation and Analysis of Female Cuticular Hydrocarbon Extracts
3.2. Olfactometer Bioassays of Oxidized Cuticular Extracts and Synthetic Aldehydes
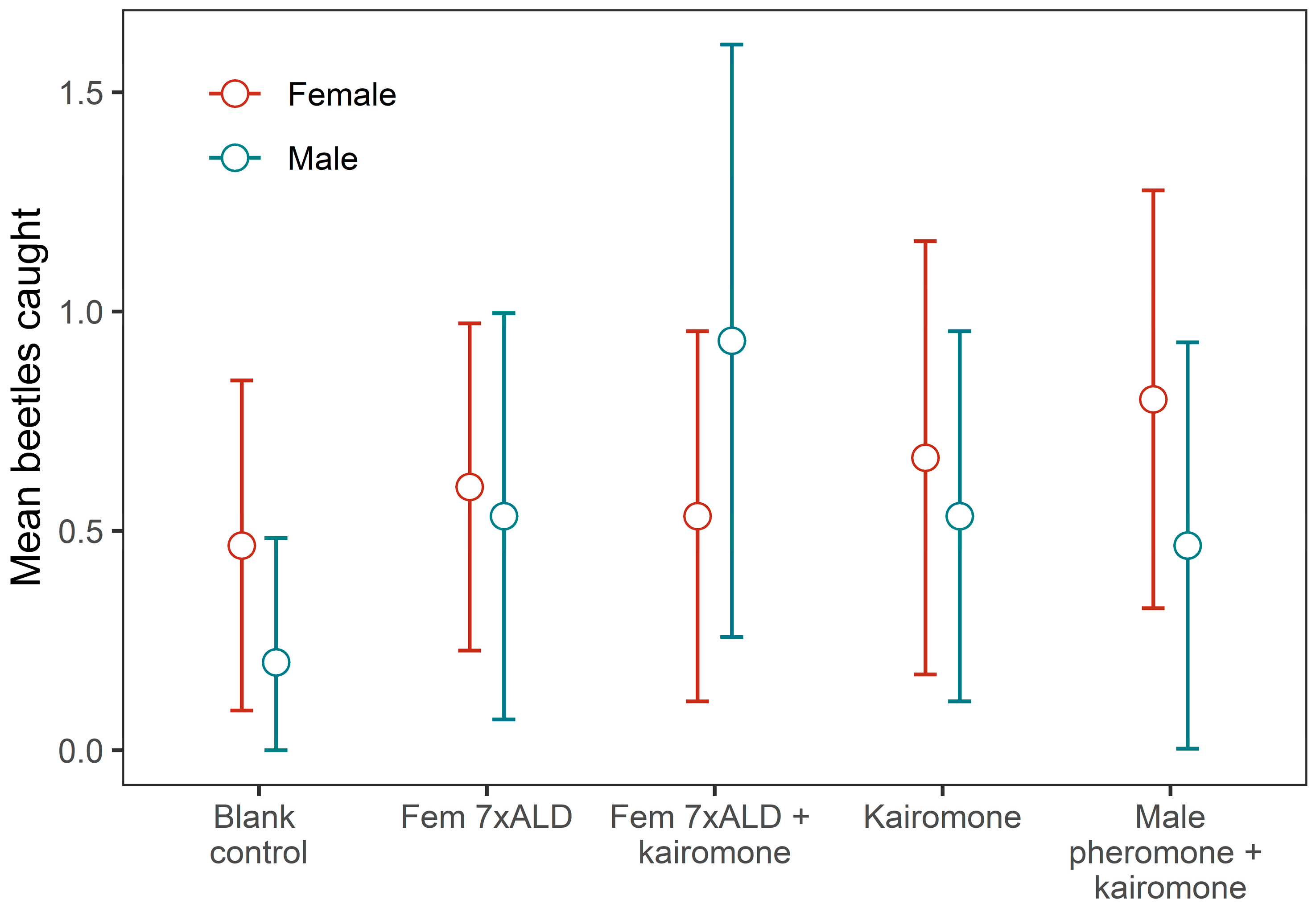
3.3. Field Responses of A. glabripennis to Potential Attractants
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wickham, J.D.; Xu, Z.; Teale, S.A. Evidence for a female-produced, long range pheromone of Anoplophora glabripennis (Coleoptera: Cerambycidae). Insect Sci. 2012, 19, 355–371. [Google Scholar] [CrossRef]
- Cavey, J.F.; Hoebeke, E.R.; Passoa, S.; Lingafelter, S.W. A new exotic threat to North American hardwood forests: An Asian longhorned beetle, Anoplophora glabripennis (Motschulsky) (Coleoptera: Cerambycidae) I. Larval description and diagnosis. Proc. Entomol. Soc. Wash. 1998, 100, 373–381. [Google Scholar]
- Poland, T.M.; Haack, R.A.; Petrice, T.R. Chicago joins New York in battle with Asian longhorned beetle. Newsl. Mich. Entomol. Soc. 1998, 43, 15–17. [Google Scholar]
- Haack, R.A.; Shaw, K.; Mastro, V.C.; Ossenbruggen, S.; Raimo, B.J. New York’s battle with the Asian longhorned beetle. J. For. 1997, 95, 11–15. [Google Scholar]
- Haack, R.A. Exotic bark- and wood-boring Coleoptera in the United States: Recent establishments and interceptions. Can. J. For. Res. 2006, 36, 269–288. [Google Scholar] [CrossRef]
- Haack, R.A.; Herard, F.; Sun, J.H.; Turgeon, J.J. Managing invasive populations of Asian longhorned beetle and citrus longhorned beetle: A worldwide perspective. Annu. Rev. Entomol. 2010, 55, 521–546. [Google Scholar] [CrossRef]
- Balser, D. Asian Longhorned Beetle. Ohio Department of Natural Resources Division of Forestry. 2011. Available online: http://forestry.ohiodnr.gov/news/post/august-is-tree-check-month-check-for-asian-longhorned-beetle (accessed on 3 August 2021).
- Morewood, W.D.; Hoover, K.; Neiner, P.; McNeil, J.; Sellmer, J.C. Host tree resistance against the polyphagous wood-boring beetle Anoplophora glabripennis. Entomol. Exp. Appl. 2004, 110, 79–86. [Google Scholar] [CrossRef]
- Zhang, F.; Jin, Y.; Chen, H.; Wu, X. Selectivity mechanism of Anoplophora glabripennis on four different species of maples. Front. Biol. China 2008, 3, 78–84. [Google Scholar] [CrossRef]
- USDA-APHIS. Available online: https://www.aphis.usda.gov/aphis/ourfocus/planthealth/plant-pest-and-disease-programs/pests-and-diseases/asian-longhorned-beetle/asian-longhorned-beetle (accessed on 2 August 2019).
- GAO Invasive Forest Pests: Lessons Learned from Three Recent Infestations May Aid in Managing Future Efforts: Report to the Chairman, Committee on Resources, House of Representatives. 2006. Available online: https://www.gao.gov/assets/250/249776.pdf (accessed on 3 August 2021).
- Nowak, D.J.; Pasek, J.E.; Sequeira, R.A.; Crane, D.E.; Mastro, V.C. Potential effect of Anoplophora glabripennis (Coleoptera: Cerambycidae) on urban trees in the United States. J. Econ. Entomol. 2001, 94, 116–122. [Google Scholar] [CrossRef]
- Zhang, A.; Oliver, J.E.; Aldrich, J.R.; Wang, B.; Mastro, V.C. Stimulatory beetle volatiles for the Asian longhorned beetle, Anoplophora glabripennis (Motschulsky). Z. Naturforsch. 2002, 57, 553–558. [Google Scholar] [CrossRef]
- Zhang, A.; Oliver, J.E.; Aldrich, J.R. Aggregation Pheromone for the Asian Longhorned Beetle, Anoplophora glabripennis (Coleoptera: Cerambycidae). U.S. Patent US6177073B1, 23 January 2001. [Google Scholar]
- Nehme, M.E.; Keena, M.A.; Zhang, A.; Baker, T.C.; Hoover, K. Attraction of Anoplophora glabripennis to male-produced pheromone and plant volatiles. Environ. Entomol. 2009, 38, 1745–1755. [Google Scholar] [CrossRef] [PubMed]
- Crook, D.J.; Lance, D.R.; Mastro, V.C. Identification of a potential third component of the male-produced pheromone of Anoplophora glabripennis and its effect on behavior. J. Chem. Ecol. 2014, 40, 1241–1250. [Google Scholar] [CrossRef] [PubMed]
- Nehme, M.; Keena, M.A.; Zhang, A.; Baker, T.C.; Xu, Z.; Hoover, K. Evaluating the use of male-produced pheromone components and plant volatiles in two trap designs to monitor Anoplophora glabripennis. Environ. Entomol. 2010, 39, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Meng, P.S.; Trotter, R.T.; Keena, M.A.; Baker, T.C.; Yan, S.; Schwartzberg, E.G.; Hoover, K. Effects of pheromone and plant volatile release rates on trapping Anoplophora glabripennis (Coleoptera: Cerambycidae) in China. Environ. Entomol. 2014, 43, 1379–1388. [Google Scholar] [CrossRef]
- Xu, T.; Hansen, L.; Dong, C.H.; Hao, D.; Zhang, L.; Teale, S.A. Identification of a female-produced pheromone in a destructive invasive species: Asian longhorn Beetle, Anoplophora glabripennis. J. Pest Sci. 2020, 93, 1321–1332. [Google Scholar] [CrossRef]
- Hanks, L.M.; Millar, J.G. Field bioassays of cerambycid pheromones reveal widespread parsimony of pheromone structures, enhancement by host plant volatiles, and antagonism by components from heterospecifics. Chemoecology 2013, 23, 21–44. [Google Scholar] [CrossRef]
- Meier, L.R.; Zou, Y.; Millar, J.G.; Mongold-Diers, J.A.; Hanks, L.M. Synergism between enantiomers creates species-specific pheromone blends and minimizes cross-attraction for two species of cerambycid beetles. J. Chem. Ecol. 2016, 42, 1181–1192. [Google Scholar] [CrossRef]
- Crook, D.J.; Khirimian, A.; Francese, J.A.; Fraser, I.; Poland, T.M.; Sawyer, A.J.; Mastro, V.C. Development of a host-based semiochemical lure for trapping emerald ash borer, Agrilus planipennis (Coleoptera: Buprestidae). Environ. Entomol. 2008, 37, 356–365. [Google Scholar] [CrossRef]
- Kendra, P.E.; Niogret, J.; Montgomery, W.S.; Deyrup, M.A.; Epsky, N.D. Cubeb oil lures: Terpenoid emissions, trapping efficacy, and longevity for attraction of redbay ambrosia beetle (Coleoptera: Curculionidae: Scolytinae). J. Econ. Entomol. 2015, 108, 350–361. [Google Scholar] [CrossRef][Green Version]
- Ginzel, M.D.; Hanks, L.M. Role of host plant volatiles in mate location for three species of longhorned beetles. J. Chem. Ecol. 2005, 31, 213–217. [Google Scholar] [CrossRef]
- Zhang, A.; Oliver, J.E.; Chauhan, K.; Zhao, B.; Xia, L.; Xu, Z. Evidence for contact sex recognition pheromone of the Asian longhorned beetle, Anoplophora glabripennis (Coleoptera: Cerambycidae). Naturwissenschaften 2003, 90, 410–413. [Google Scholar] [CrossRef] [PubMed]
- Attygalle, A.B.; Zlatkis, A.; Middleditch, B.S. Derivitization with 1,1-dimethylhydrazine for identification of carbonyl compounds resulting from ozonolysis. J. Chromatogr. 1989, 272, 284–289. [Google Scholar] [CrossRef]
- McDaniel, C.A.; Howard, R.W. Mass spectral determination of aldehydes, ketone, and carboxylic acids using 1,1-dimentylhydrazine. J. Chem. Ecol. 1985, 11, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Corey, E.J.; Suggs, J.W. Pyridinium chlorochromate. An efficient reagent for oxidation of primary and secondary alcohols to carbonyl compounds. Tetrahedron Lett. 1975, 16, 2647–2650. [Google Scholar] [CrossRef]
- Crook, D.J.; Higgins, R.A.; Ramaswamy, S.B. Antennal morphology of the soybean stemborer Dectes texanus texanus LeConte (Coleoptera: Cerambycidae). J. Kans. Entomol. Soc. 2003, 76, 397–405. [Google Scholar]
- Lacey, E.S.; Ginzel, M.D.; Millar, J.G.; Hanks, L.M. Male-produced aggregation pheromone of the cerambycid beetle Neoclytus acuminatus acuminatus. J. Chem. Ecol. 2004, 30, 1493–1507. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Heinze, G.; Schemper, M. A solution to the problem of separation in logistic regression. Stat. Med. 2002, 21, 2409–2419. [Google Scholar] [CrossRef]
- Kosmidis, I. brglm2: Bias Reduction in Generalized Linear Models; R Package Version 0.1.8.2. 2018. Available online: https://cran.r-project.org/package=brglm2/brglm2.pdf (accessed on 9 September 2024).
- Lenth, R. Emmeans: Estimated Marginal Means, Aka Least-Squares Means, R Package Version 1.10.0. 2024. Available online: https://cran.r-project.org/web/packages/emmeans/emmeans.pdf (accessed on 9 September 2024).
- Collignon, R.M.; Swift, I.P.; Zou, Y.; McElfresh, J.S.; Hanks, L.M.; Millar, J.G. The influence of host plant volatiles on the attraction of longhorn beetles to pheromones. J. Chem. Ecol. 2016, 42, 215–229. [Google Scholar] [CrossRef] [PubMed]
- Allison, J.D.; McKenna, J.L.; Millar, J.G.; McElfresh, J.S.; Mitchell, R.F.; Hanks, L.H. Response of the woodborers Monochamus carolinensis and Monochamus titillator to known cerambycid pheromones in the presence and absence of the host plant volatile α-pinene. Environ. Entomol. 2012, 41, 1587–1596. [Google Scholar] [CrossRef]
- Fierke, M.K.; Skabeikis, D.D.; Millar, J.G.; Teale, S.A.; McElfresh, J.S.; Hanks, L.M. Identification of a male-produced pheromone for Monochamus scutellatus and an attractant for the congener Monochamus notatus (Coleoptera: Cerambycidae). J. Econ. Entomol. 2012, 105, 2029–2034. [Google Scholar] [CrossRef]
- Ryall, K.; Silk, P.; Webster, R.P.; Gutowski, J.M.; Meng, Q.; Li, Y.; Gao, W.; Fidgen, J.; Kimoto, T.; Scarr, T.; et al. Further evidence that monochamol is attractive to Monochamus (Coleoptera: Carambycidae) species, with attraction synergized by host plant volatiles and bark beetle (Coleoptera: Curculionidae) pheromones. Can. Entomol. 2014, 147, 564–579. [Google Scholar] [CrossRef]
- Teale, S.A.; Wickham, J.D.; Zhang, F.; Su, J.; Chen, Y.; Xiao, W.; Hanks, L.M.; Millar, J.G. A male-produced aggregation pheromone of Monochamus alternatus (Coleoptera: Cerambycidae), a major vector of pine wood nematode. J. Econ. Entomol. 2011, 104, 1592–1598. [Google Scholar] [CrossRef] [PubMed]
- Nehme, M.E.; Trotter, R.T.; Keena, M.A.; McFarland, C.; Coop, J.; Hull-Sanders, H.M.; Meng, P.; De Moraes, C.M.; Mescher, M.C.; Hoover, K. Development and evaluation of a trapping system for Anoplophora glabripennis (Coleoptera: Cerambycidae) in the United States. Environ. Entomol. 2014, 43, 1034–1044. [Google Scholar] [CrossRef] [PubMed]
- Wickham, J.D. Semiochemicals of the Asian Longhorn Beetle, Anoplophora glabripennis (Motschulsky) Coleoptera: Cerambycidae). Ph.D. Thesis, College of Environmental Science and Forestry, State University of New York, Syracuse, NY, USA, 2009. [Google Scholar]
- Tooker, J.F.; Koenig, W.A.; Hanks, L.M. Altered host plant volatiles are proxies for sex pheromones in the gall wasp Antistrophus rufus. Proc. Natl. Acad. Sci. USA 2002, 99, 15486–15491. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.; Tobin, P.; Wu, J.; He, W.; Xu, X.; Gries, G.; Gries, R.; Borden, J.; Turgeon, J.; de Groot, P. Detection and monitoring of the Asian longhorned beetle: Update on sentinal trees, attract and kill, and artificial lure studies. In Proceedings of the Emerald Ash Borer Research Technology Development Meeting, Cincinnati, OH, USA, 29 October–2 November 2006; USDA Forest Service: Washington, DC, USA, 2007; pp. 104–105. [Google Scholar]
- Smith, M.; Tobin, P.; Wu, J.; He, W.; Xu, X.; Gries, G.; Gries, R.; Borden, J.; Turgeon, J.; de Groot, P. Behavioral ecology of host selection in the Asian longhorned beetle: Implications for surveying, detecting, and monitoring adult beetles. In Proceedings of the 18th U.S. Department of Agriculture Interagency Research Forum on Gypsy Moth and Other Invasive Species, Annapolis, MA, USA, 9–12 January 2007; USDA Forest Service: Washington, DC, USA, 2008. [Google Scholar]
- Yasui, H.; Akino, T.; Fukaya, M.; Wakamura, S.; Ono, H. Sesquiterpene hydrocarbons: Kairomones with a releaser effect in the sexual communication of the white-spotted longicorn beetle, Anoplophora malasiaca (Thomson) (Coleoptera: Cerambycidae). Chemoecology 2008, 18, 233–242. [Google Scholar] [CrossRef]
- Yasui, H. Chemical communication in mate location and recognition in the white-spotted longicorn beetle, Anoplophora malasiaca (Coleoptera: Cerambycidae). Appl. Entomol. Zool. 2009, 44, 183–194. [Google Scholar] [CrossRef]
- Wei, J.; Zhou, Q.; Hall, L.; Myrick, A.; Hoover, K.; Shields, K.; Baker, T.C. Olfactory sensory neurons of the Asian longhorned beetle Anoplophora glabripennis, specifically responsive to its two aggregation-sex pheromone components. J. Chem. Ecol. 2018, 44, 637–649. [Google Scholar] [CrossRef]
- Blomquist, G.J.; Ginzel, M.D. Chemical ecology, biochemistry, and molecular biology of insect hydrocarbons. Annu. Rev. Entomol. 2021, 66, 45–60. [Google Scholar] [CrossRef]
- Baeckstrom, P.; Bjorkling, F.; Hogberg, H.E.; Norin, T. Cross-coupling of vinyl cuprates and allylic halides and synthesis of the Comstock mealybug pheromone via photo-oxidation of 2,6-dimethyl-2, 5-heptadiene. Acta Chem. Scand. B 1984, 38, 779–782. [Google Scholar] [CrossRef]
- Faal, H.; Canlas, I.J.; Cossé, A.; Jones, T.H.; Carrillo, D.; Cooperband, M.F. Investigating photo-degradation as a potential pheromone production pathway in spotted lanternfly, Lycorma delicatula. Insects 2023, 14, 551. [Google Scholar] [CrossRef]
- Bartelt, R.J.; Cossé, A.A.; Petroski, R.J.; Weaver, D.K. Cuticular hydrocarbons and novel alkenediol diacetates from wheat stem sawfly (Cephus cinctus): Natural oxidation to pheromone components. J. Chem. Ecol. 2002, 28, 385–405. [Google Scholar] [CrossRef] [PubMed]
- Faal, H.; Silk, P.J.; LeClair, G.; Teale, S.A. Biologically active cuticular compounds of female Sirex noctilio. Entomol. Exp. Appl. 2022, 170, 327–338. [Google Scholar] [CrossRef]
- Staples, J.K.; Bartelt, R.J.; Cossé, A.A.; Whitman, D.W. Sex pheromone of the pine false webworm Acantholyda erythrocephala. J. Chem. Ecol. 2009, 35, 1448–1460. [Google Scholar] [CrossRef] [PubMed]
- Lebreton, S.; Borrero-Echeverry, F.; Gonzalez, F.; Solum, M.; Wallin, E.A.; Hedenström, E.; Hansson, B.S.; Gustavsson, A.L.; Bengtsson, M.; Birgersson, G.A. Drosophila female pheromone elicits species-specific long-range attraction via an olfactory channel with dual specificity for sex and food. BMC Biol. 2017, 15, 88. [Google Scholar] [CrossRef] [PubMed]
- Cossé, A.A.; Bartelt, R.J.; Weaver, D.K.; Zilkowski, B.W. Pheromone components of the wheat stem sawfly: Identification, electrophysiology, and field bioassay. J. Chem. Ecol. 2002, 28, 407–423. [Google Scholar] [CrossRef]
- Bartelt, R.J.; Jones, R.L. (Z)-10-nonadecenal: A pheromonally active air oxidation product of (Z,Z)-9, 19 dienes in yellowheaded spruce sawfly. J. Chem. Ecol. 1983, 9, 1333–1341. [Google Scholar] [CrossRef]
- Swedenborg, P.D.; Jones, R.L. (Z)-4-tridecenal, a pheromonally active air oxidation product from a series of (Z,Z)-9-13 dienes in Macrocentrus grandii Goidanich (Hymenoptera: Braconidae). J. Chem. Ecol. 1992, 18, 1913–1931. [Google Scholar] [CrossRef]
- Hoover, K.; Keena, M.; Nehme, M.; Wang, S.; Meng, P.; Zhang, A. Sex-specific trail pheromone mediates complex mate finding behavior in Anoplophora glabripennis. J. Chem. Ecol. 2014, 40, 169–180. [Google Scholar] [CrossRef]
- Lacey, E.S.; Ray, A.M.; Hanks, L.M. Calling behavior of the cerambycid beetle Neoclytus acuminatus acuminatus (F.). J. Insect Behav. 2007, 20, 117–128. [Google Scholar] [CrossRef]
- Graves, F.; Baker, T.C.; Zhang, A.; Keena, M.; Hoover, K. Sensory aspects of trail-following behaviors in the Asian longhorned beetle Anoplophora glabripennis. J. Insect Behav. 2016, 29, 615–628. [Google Scholar] [CrossRef]
- Carlson, D.A.; Mayer, M.S.; Silhacek, D.L.; James, J.D.; Beroza, M.; Bierl, B.A. Sex attractant pheromone of the house fly: Isolation, identification and synthesis. Science 1971, 174, 76–78. [Google Scholar] [CrossRef] [PubMed]
- Lima, I.; Tadeo, E.; Remedios-Mendoza, M.; Martinez-Hernández, M.; Ruiz-Montiel, C. Evidence of a pheromone involved in the behaviour of Drosophila suzukii Matsumura (Diptera: Drosophilidae). J. Appl. Entomol. 2023, 147, 990–1000. [Google Scholar] [CrossRef]
- Frankel, E.N. Lipid Oxidation; Oily Press: Dundee, UK, 1998. [Google Scholar]
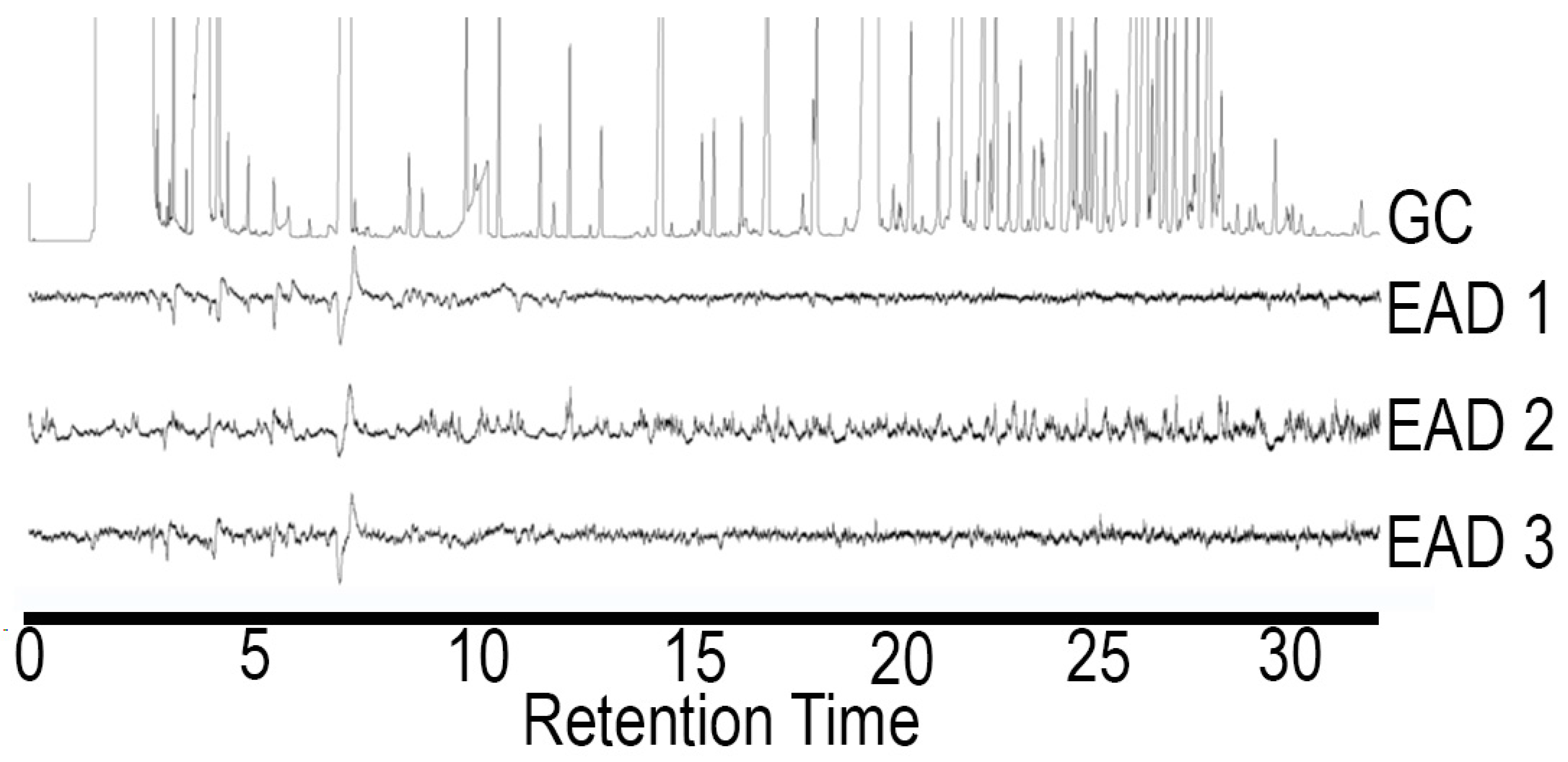
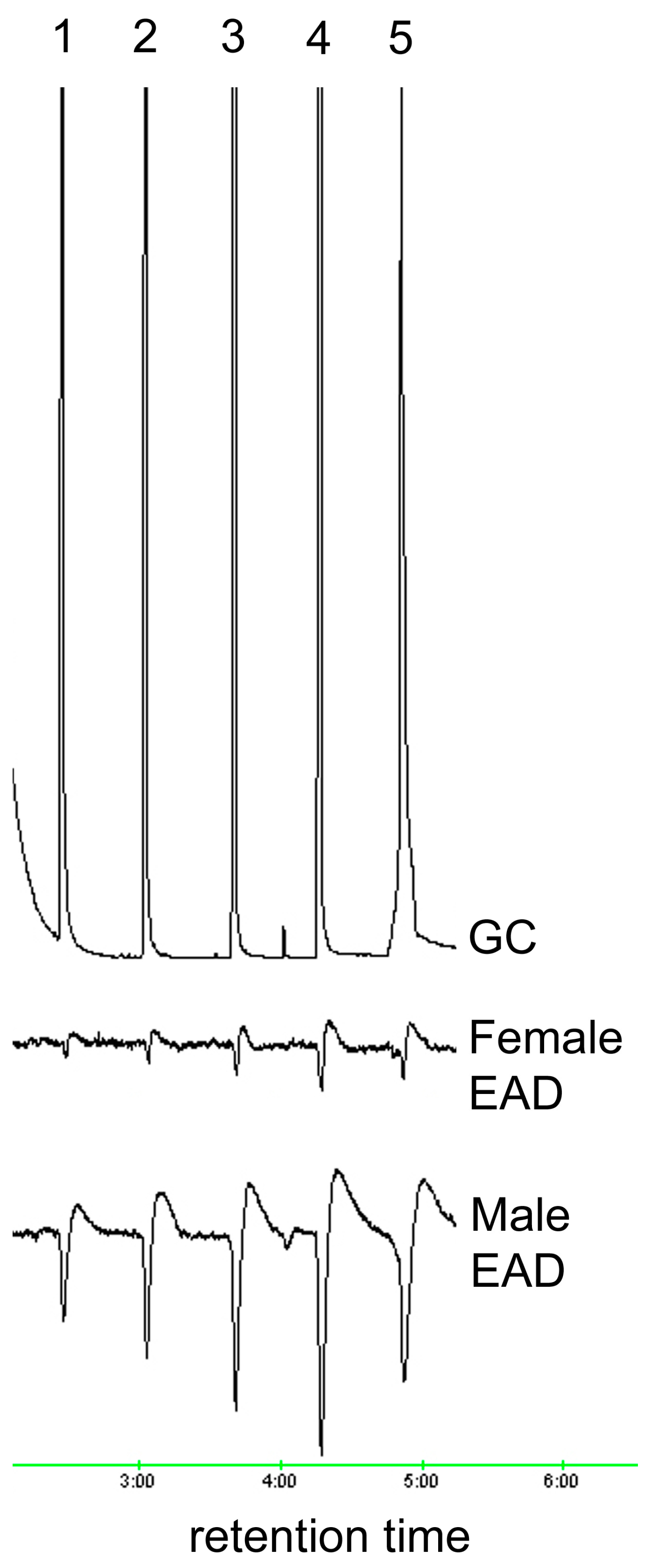
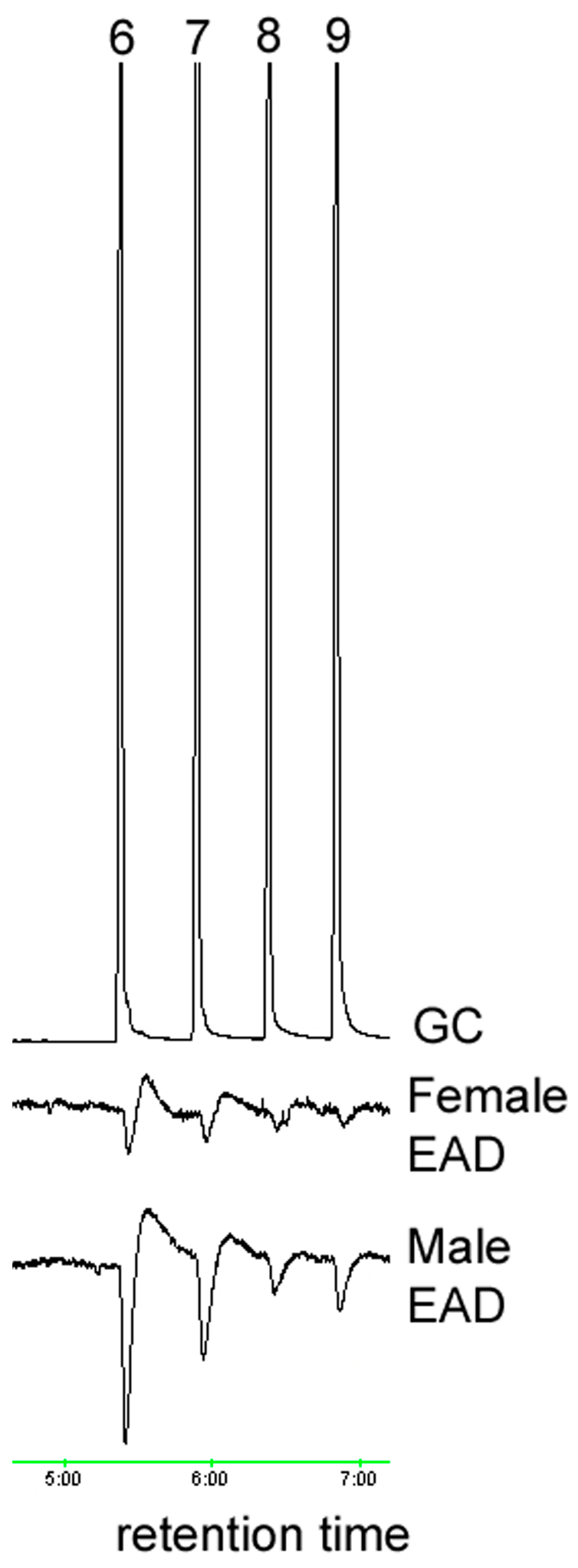

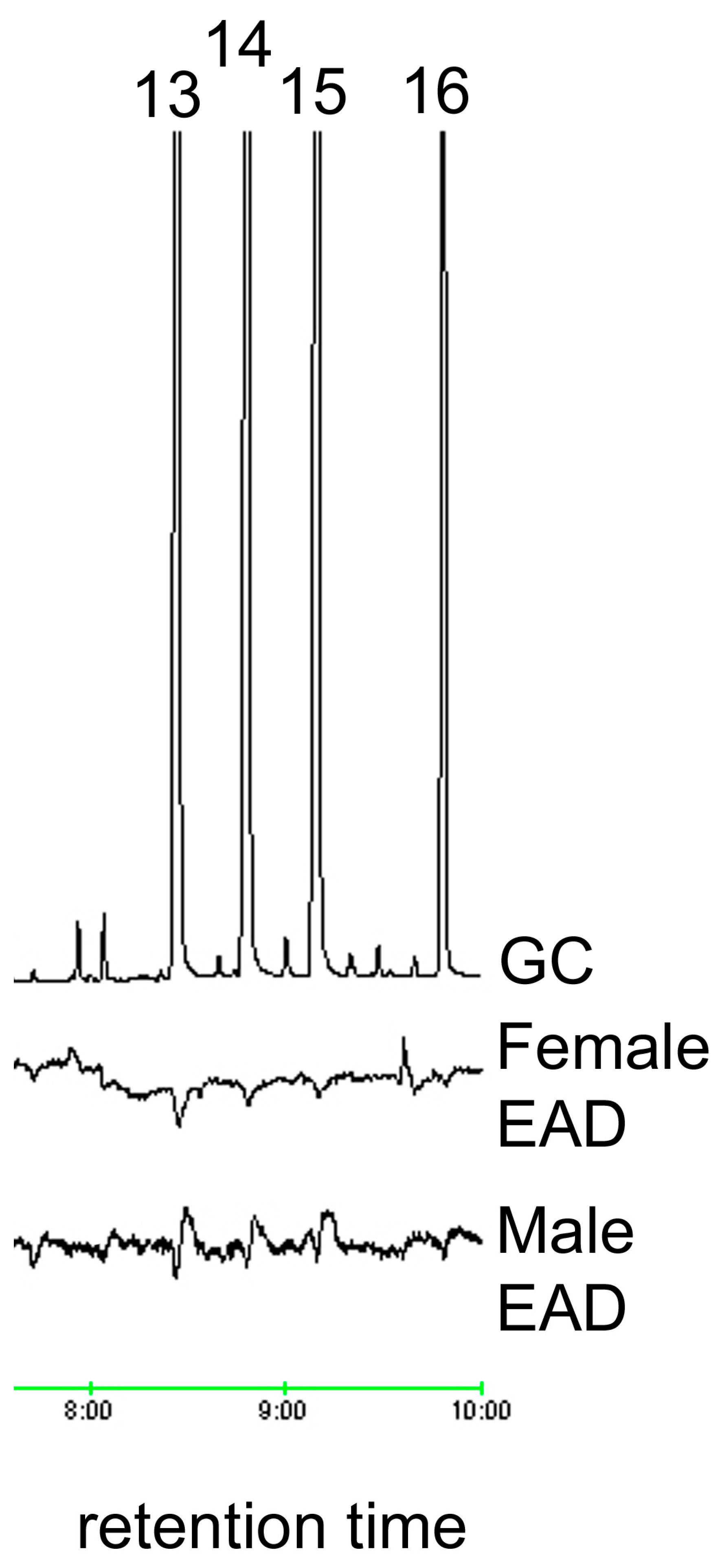
| Aldehyde | Retention Time (Min) | Relative Peak Area | Percent Ratio of Total Aldehydes |
|---|---|---|---|
| Hexanal | 9.08 | t | |
| Heptanal | 9.98 | 3.0 | 6.9 |
| Octanal | 10.78 | t | |
| Nonanal | 11.51 | 15.5 | 35.9 |
| Decanal | 12.20 | t | |
| Undecanal | 12.80 | t | |
| Dodecanal | 13.45 | t | |
| Tridecanal | 14.02 | t | |
| Tetradecanal | 14.58 | 2.3 | 5.3 |
| Pentadecanal | 15.10 | t | |
| Hexadecanal | 15.60 | 1.5 | 3.5 |
| Heptadecanal | 16.20 | 1.4 | 3.2 |
| Octadecanal | 16.87 | 15.1 | 34.9 |
| Nonadecanal | 17.62 | t | |
| Eicosanal | 18.52 | 4.4 | 10.2 |
| Docosanal | 20.90 | t |
| Sex | Odor Source 1 | Odor Source 2 | Choice 1 | Choice 2 | % Response to Choice 1 | χ2 | p |
|---|---|---|---|---|---|---|---|
| Male | 7 × ALD (10 µg) | hexane (10 µL) | 20 | 5 | 80 | 7.84 | 0.003 |
| Male | 7 × ALD (1 µg) | hexane (1 µL) | 19 | 6 | 76 | 5.76 | 0.001 |
| Male | 7 × ALD (1 mg) in 200 µL Min Oil | Min Oil & Hexane | 19 | 6 | 76 | 5.76 | 0.001 |
| Male | 1FE Body Wash UV treated | 10 µL hexane (10 µL) | 22 | 3 | 88 | 12.96 | 0.001 |
| Male | 7 × ALD Fem Ratio (10 µg) | hexane (10 µL) | 21 | 4 | 84 | 10.24 | 0.001 |
| Female | 7 × ALD (10 µg) | hexane (10 µL) | 8 | 17 | 32 | 2.56 | NS |
| Female | 7 × ALD (1 µg) | hexane (1 µL) | 12 | 13 | 48 | 0 | NS |
| Sex | Odor Source 1 | Odor Source 2 | Choice 1 | Choice 2 | % Response to Choice 1 | χ2 | p |
|---|---|---|---|---|---|---|---|
| Male | 3 Host Blend (1 µg) | hexane (10 µL) | 11 | 14 | 44 | 0.16 | NS |
| Male | 7 × ALD (1 µg) + 3 Host Blend (1 µg) | hexane (20 µL) | 20 | 5 | 80 | 7.84 | 0.003 |
| Male | 7 × ALD (1 µg) + 3 Host Blend (1 µg) | 3 Host Blend (1 µg) | 12 | 13 | 48 | 0 | NS |
| Male | 7 × ALD (10 µg) + 3 Host Blend (10 µg) | 3 Host Blend (10 µg) | 19 | 6 | 76 | 5.76 | 0.001 |
| Male | Non Trace 7 × ALD (10 µg) + 3 Host Blend (10 µg) | 3 Host Blend (10 µg) | 15 | 10 | 60 | 0.64 | NS |
| Male | Non Trace 7 × ALD (10 µg) | Hexane (10 µL) | 15 | 10 | 60 | 0.64 | NS |
| Contrast | Rate Ratio | SE | z | p |
|---|---|---|---|---|
| Fem 7 × ALD/blank control | 1.72 | 0.72 | 1.30 | 0.28 |
| Fem 7 × ALD + kairomone/blank control | 2.21 | 0.89 | 1.98 | 0.06 |
| Fem 7 × ALD + kairomone/Fem 7 × ALD | 1.29 | 0.45 | 0.74 | 0.47 |
| Contrast | Rate Ratio | SE | z | p |
|---|---|---|---|---|
| Fem 7 × ALD/blank control | 2.67 | 1.81 | 1.45 | 0.17 |
| Fem 7 × ALD + kairomone/blank control | 4.67 | 2.97 | 2.42 | 0.02 |
| Fem 7 × ALD + kairomone/Fem 7 × ALD | 1.75 | 0.78 | 1.26 | 0.23 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Crook, D.; Wickham, J.; Ren, L.; Xu, Z.; Jones, T.H.; Warden, M.; Cossé, A. Further Evidence That Female Anoplophora glabripennis (Coleoptera: Cerambycidae) Utilizes Photo-Degradation to Produce Volatiles That Are Attractive to Adult Males. Insects 2024, 15, 923. https://doi.org/10.3390/insects15120923
Crook D, Wickham J, Ren L, Xu Z, Jones TH, Warden M, Cossé A. Further Evidence That Female Anoplophora glabripennis (Coleoptera: Cerambycidae) Utilizes Photo-Degradation to Produce Volatiles That Are Attractive to Adult Males. Insects. 2024; 15(12):923. https://doi.org/10.3390/insects15120923
Chicago/Turabian StyleCrook, Damon, Jacob Wickham, Lili Ren, Zhichun Xu, Tappey H. Jones, Melissa Warden, and Allard Cossé. 2024. "Further Evidence That Female Anoplophora glabripennis (Coleoptera: Cerambycidae) Utilizes Photo-Degradation to Produce Volatiles That Are Attractive to Adult Males" Insects 15, no. 12: 923. https://doi.org/10.3390/insects15120923
APA StyleCrook, D., Wickham, J., Ren, L., Xu, Z., Jones, T. H., Warden, M., & Cossé, A. (2024). Further Evidence That Female Anoplophora glabripennis (Coleoptera: Cerambycidae) Utilizes Photo-Degradation to Produce Volatiles That Are Attractive to Adult Males. Insects, 15(12), 923. https://doi.org/10.3390/insects15120923








