Seasonal Dynamics of Non-Biting Midges (Diptera: Chironomidae) and Relevant Environmental Factors
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling
2.2. Species Identification
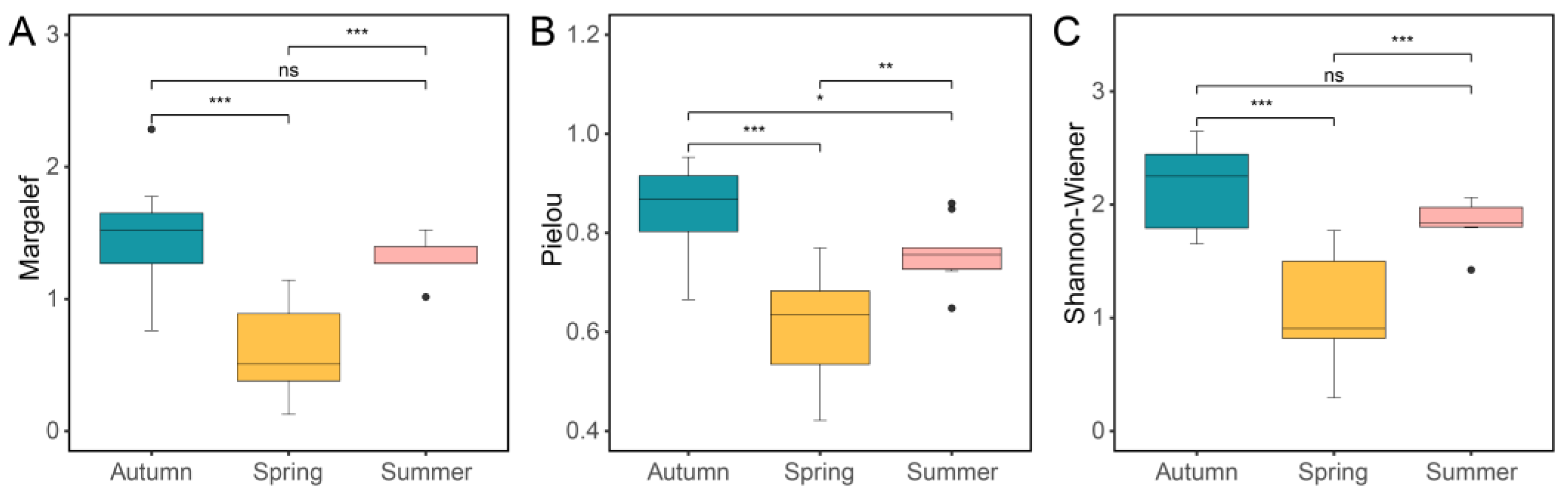
2.3. Alpha Diversity Analyses
2.4. Beta Diversity Analyses
3. Results
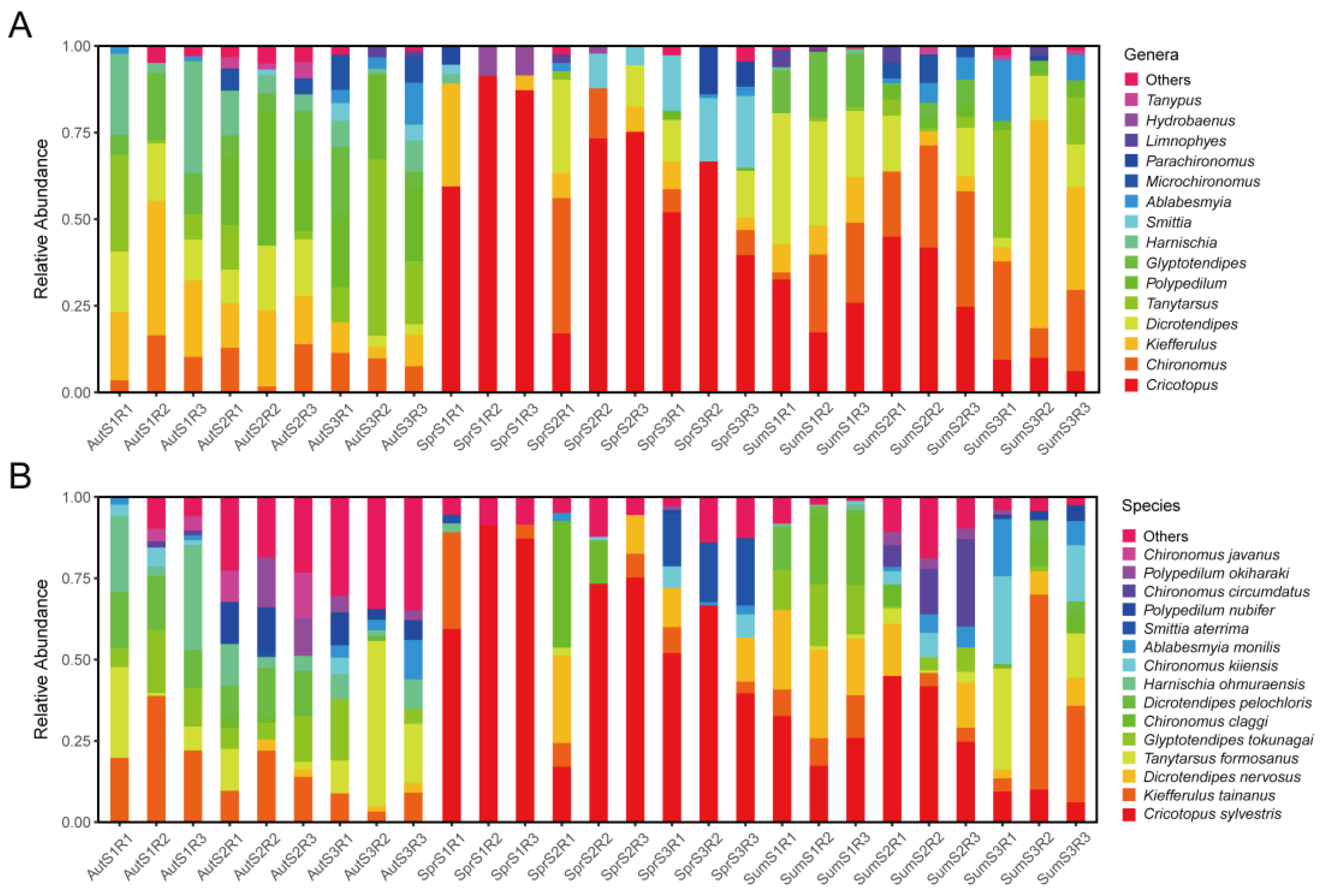
3.1. Composition of Non-Biting Midges
3.2. Alpha Diversity
3.3. Beta Diversity
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Karima, Z. Chironomidae: Biology, ecology and systematics. In The Wonders of Diptera—Characteristics, Diversity, and Significance for the World’s Ecosystems; IntechOpen: London, UK, 2021. [Google Scholar]
- Broza, M.; Gancz, H.; Halpern, M.; Kashi, Y. Adult non-biting midges: Possible windborne carriers of Vibrio cholerae non-O1 non-O139. Environ. Microbiol. 2005, 7, 576–585. [Google Scholar] [CrossRef] [PubMed]
- Hirabayashi, K.; Kubo, K.; Yamaguchi, S.; Fujimoto, K.; Murakami, G.; Nasu, Y. Studies of bronchial asthma induced by chironomid midges (Diptera) around a hypereutrophic lake in Japan. Allergy 1997, 52, 188–195. [Google Scholar] [CrossRef] [PubMed]
- McHugh, S.M.; Credland, P.F.; Tee, R.D.; Cranston, P.S. Evidence of allergic hypersensitivity to chironomid midges in an English village community. Clin. Allergy 1988, 18, 275–285. [Google Scholar] [CrossRef]
- Nandi, S.; Aditya, G.; Chowdhury, I.; Das, A.; Saha, G.K. Chironomid midges as allergens: Evidence from two species from West Bengal, Kolkata, India. Indian J. Med. Res. 2014, 139, 921–926. [Google Scholar] [PubMed]
- Contador Mejias, T.; Gañan, M.; Rendoll-Cárcamo, J.; Maturana, C.S.; Benítez, H.A.; Kennedy, J.; Rozzi, R.; Convey, P. A polar insect’s tale: Observations on the life cycle of Parochlus steinenii, the only winged midge native to Antarctica. Ecology 2023, 104, e3964. [Google Scholar] [CrossRef]
- Přidalová, M.S.; Hamerlík, L.; Novikmec, M.; Slobodníková, V.; Veselská, M.; Bitušík, P.; Svitok, M. Diversity and distribution of chironomids in Central European ponds. Ecol. Evol. 2024, 14, e11354. [Google Scholar] [CrossRef] [PubMed]
- Popović, N.; Marinković, N.; Čerba, D.; Raković, M.; Đuknić, J.; Paunović, M. Diversity patterns and assemblage structure of non-biting midges (Diptera: Chironomidae) in urban waterbodies. Diversity 2022, 14, 187. [Google Scholar] [CrossRef]
- Belle, S.; Klaus, F.; González Sagrario, M.d.l.Á.; Vrede, T.; Goedkoop, W. Unravelling chironomid biodiversity response to climate change in subarctic lakes across temporal and spatial scales. Hydrobiologia 2022, 849, 2621–2633. [Google Scholar] [CrossRef]
- Rossaro, B.; Marziali, L. Response of Chironomids (Diptera, Chironomidae) to environmental factors at different spatial scales. Insects 2024, 15, 272. [Google Scholar] [CrossRef]
- Głowacki, Ł.; Leszczyńska, J.; Grzybkowska, M.; Pyrzanowski, K.; Dukowska, M.; Przybylski, M. Determinants of chironomid species richness in mid-European temperate rivers—Environmental factors, regional influences, diversity, and seasons. Ecol. Indic. 2023, 147, 109838. [Google Scholar] [CrossRef]
- Delettre, Y.R.; Morvan, N. Dispersal of adult aquatic Chironomidae (Diptera) in agricultural landscapes. Freshw. Biol. 2000, 44, 399–411. [Google Scholar] [CrossRef]
- Furén, R.; Österlund, H.; Winston, R.J.; Tirpak, R.A.; Dorsey, J.D.; Smith, J.; Viklander, M.; Blecken, G.T. Concentration, distribution, and fractionation of metals in the filter material of 29 bioretention facilities: A field study. Environ. Sci. Water Res. Technol. 2023, 9, 3158–3173. [Google Scholar] [CrossRef]
- Mousavi, S.K.; Primicerio, R.; Amundsen, P. Diversity and structure of Chironomidae (Diptera) communities along a gradient of heavy metal contamination in a subarctic watercourse. Sci. Total Environ. 2003, 307, 93–110. [Google Scholar] [CrossRef]
- Balla, S.A.; Davis, J.A. Seasonal variation in the macroinvertebrate fauna of wetlands of differing water regime and nutrient status on the Swan Coastal Plain, Western Australia. Hydrobiologia 1995, 299, 147–161. [Google Scholar] [CrossRef]
- Sæther, O.A. The Chironomus group (Diptera: Chironomidae) in Lake Winnipeg, Canada. Zootaxa 2012, 3275, 1–19. [Google Scholar] [CrossRef]
- Makarchenko, E.A.; Makarchenko, M.A. On taxonomy of Hydrobaenus Fries, 1830 (Diptera: Chironomidae: Orthocladiinae) from the Russian Far East, with a key to species. Zootaxa 2014, 3760, 429–438. [Google Scholar] [CrossRef] [PubMed]
- Donato, M.; Siri, A.; Massaferro, J.; Brooks, S.J. Apedilum griseistriatum comb. nov., placement of Chironomus (Polypedilum) griseistriatum (Diptera, Chironomidae). Iheringia. Série Zool. 2015, 105, 5–11. [Google Scholar] [CrossRef]
- Liu, W.; Sun, B.; Yan, C.; Song, C.; Wang, X. Two new species and one new record of Parakiefferiella Thienemann, 1936 from China (Diptera, Chironomidae). ZooKeys 2015, 545, 139–148. [Google Scholar] [CrossRef]
- Zhang, R.; Song, C.; Wang, L.; Wang, X. Two new species of the acifer species group of Polypedilum subgenus Tripodura Townes from China (Diptera: Chironomidae). Zootaxa 2015, 3918, 571–578. [Google Scholar] [CrossRef][Green Version]
- Zakrzewska, M.; Krzemiński, W.; Giłka, W. Towards the diversity of non-biting midges of the tribe Tanytarsini from Eocene Baltic amber (Diptera: Chironomidae). Palaeontol. Electron. 2016, 19.2.18A. [Google Scholar] [CrossRef]
- Lin, X.L.; Stur, E.; Ekrem, T. DNA barcodes and morphology reveal unrecognized species in Chironomidae (Diptera). Insect Syst. Evol. 2017, 49, 329–398. [Google Scholar] [CrossRef]
- Dantas, G.P.S.; Amat, E.; Hernández-Rangel, S.M. A new Andean species of Ablabesmyia Johannsen from Colombia (Diptera: Chironomidae) with an updated taxonomic key for Neotropical species. Stud. Neotrop. Fauna Environ. 2019, 55, 96–102. [Google Scholar] [CrossRef]
- Makarchenko, E.A.; Makarchenko, M.A.; Semenchenko, A.A. Morphological description and DNA barcoding of Hydrobaenus laticaudus Sæther, 1976 (Diptera: Chironomidae: Orthocladiinae) from Amur River basin (Russian Far East). Zootaxa 2019, 4674, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, T.; Pal, G.; Hazra, N. On a new species of the genus Dicrotendipes Kieffer, 1913 from West Bengal, India with cladistic analysis and key to the Oriental species (Diptera: Chironomidae). Orient. Insects 2019, 54, 216–240. [Google Scholar] [CrossRef]
- Souto, J.; Reverter-Gil, O. Identity of bryozoan species described by Jullien & Calvet from the Bay of Biscay historically attributed to Smittia. Zootaxa 2019, 4545, 105–123. [Google Scholar] [CrossRef]
- Konar, S.; Majumdar, U. Two new species of the genus Glyptotendipes Kieffer (Diptera: Chironomidae: Chironominae) from West Bengal India. Orient. Insects 2020, 55, 386–399. [Google Scholar] [CrossRef]
- Stur, E.; Ekrem, T. The Chironomidae (Diptera) of Svalbard and Jan Mayen. Insects 2020, 11, 183. [Google Scholar] [CrossRef]
- Liu, W.; Zhao, C.; Kong, F.; Yan, C.; Wang, X. New species of Limnophyes Eaton (Diptera, Chironomidae) from China and synonymy of L. fuscipygmus Tokunaga, 1940. ZooKeys 2021, 1011, 51–61. [Google Scholar] [CrossRef]
- Aydin, G.B. Contributions to the faunistic knowledge of Chironomidae (Diptera) of Turkey based on the adult males collected around Hazar Lake (Elazığ). J. Entomol. Res. Soc. 2022, 24, 375–387. [Google Scholar] [CrossRef]
- Dantas, G.P.S.; Ramos-Pastrana, Y.; Hamada, N. A new species of Limnophyes Eaton, 1875 (Diptera: Chironomidae) from Llanos Orientales, Colombia. Zootaxa 2022, 5141, 295–300. [Google Scholar] [CrossRef]
- Giłka, W.; Zakrzewska, M.; Lukashevich, E.D.; Vorontsov, D.D.; Soszyńska-Maj, A.; Skibińska, K.; Cranston, P.S. Wanted, tracked down and identified: Mesozoic non-biting midges of the subfamily Chironominae (Chironomidae, Diptera). Zool. J. Linn. Soc. 2022, 194, 874–892. [Google Scholar] [CrossRef]
- Liu, W.B.; Wang, Y.; Zhao, K.Z.; Wang, C.Y.; Zhang, J.Y.; Yan, C.C.; Lin, X.L. New species, a new combination, and DNA barcodes of Parachironomus Lenz, 1921 (Diptera, Chironomidae). ZooKeys 2023, 1153, 121–140. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, B.; Hazra, N. Taxonomic studies on Harnischia complex from India (Diptera: Chironomidae). Zootaxa 2023, 5278, 239–263. [Google Scholar] [CrossRef] [PubMed]
- Giłka, W.; Dantas, G.P.S.; Andersen, T.; Armitage, B.J. Tanytarsus deimos group (Chironomidae, Diptera) for two distinctive species from the Neotropics. Zootaxa 2024, 5428, 589–596. [Google Scholar] [CrossRef]
- Folmer, O.; Black, M.; Hoeh, W.; Lutz, R.; Vrijenhoek, R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotechnol. 1994, 3, 294–299. [Google Scholar]
- Pons, J.; Barraclough, T.G.; Gomez-Zurita, J.; Cardoso, A.; Duran, D.P.; Hazell, S.; Kamoun, S.; Sumlin, W.D.; Vogler, A.P.; Hedin, M. Sequence-based species delimitation for the DNA taxonomy of undescribed insects. Syst. Biol. 2006, 55, 595–609. [Google Scholar] [CrossRef]
- Monaghan, M.T.; Wild, R.; Elliot, M.; Fujisawa, T.; Balke, M.; Inward, D.J.G.; Lees, D.C.; Ranaivosolo, R.; Eggleton, P.; Barraclough, T.G.; et al. Accelerated species inventory on Madagascar using coalescent-based models of species delineation. Syst. Biol. 2009, 58, 298–311. [Google Scholar] [CrossRef]
- Margalef, R. Information theory in ecology. Gen. Syst. 1958, 3, 36–71. [Google Scholar]
- Pielou, E.C. An Introduction to Mathematical Ecology; Wiley-Interscience: New York, NY, USA, 1969. [Google Scholar]
- Shannon, C.E.; Weaver, W. The Mathematical Theory of Communication; University of Illinois Press: Urbana, IL, USA, 1949. [Google Scholar]
- Rosa, B.J.F.V.; Rodrigues, L.F.T.; de Oliveira, G.S.; da Gama Alves, R. Chironomidae and Oligochaeta for water quality evaluation in an urban river in southeastern Brazil. Environ. Monit. Assess. 2014, 186, 7771–7779. [Google Scholar] [CrossRef]
- Gadawski, P.; Riss, H.W.; Płóciennik, M.; Meyer, E.I. City channel chironomids—benthic diversity in urban conditions. River Res. Appl. 2016, 32, 1978–1988. [Google Scholar] [CrossRef]
- Copeland, R.S.; Nkubaye, E.; Nzigidahera, B.; Epler, J.H.; Cuda, J.P.; Overholt, W.A. The diversity of Chironomidae (Diptera) associated with Hydrilla verticillata (Alismatales: Hydrocharitaceae) and other aquatic macrophytes in Lake Tanganyika, Burundi. Ann. Entomol. Soc. Am. 2012, 105, 206–224. [Google Scholar] [CrossRef]
- Zhang, L.; Shao, S.; Liu, C.; Xu, T.; Fan, C. Forms of nutrients in rivers flowing into Lake Chaohu: A comparison between urban and rural rivers. Water 2015, 7, 4523–4536. [Google Scholar] [CrossRef]
- Lin, K.N.; Lim, Y.C.; Chen, C.W.; Chen, C.F.; Kao, C.M.; Dong, C.D. Spatiotemporal variation and ecological risk assessment of heavy metals in industrialized urban river sediments: Fengshan River in Southern Taiwan as a case study. Appl. Sci. 2022, 12, 1013. [Google Scholar] [CrossRef]
- Molineri, C.; Tejerina, E.G.; Torrejón, S.E.; Pero, E.J.I.; Hankel, G.E. Indicative value of different taxonomic levels of Chironomidae for assessing the water quality. Ecol. Indic. 2020, 108, 105703. [Google Scholar] [CrossRef]
- Kaiser, T.S.; Poehn, B.; Szkiba, D.; Preussner, M.; Sedlazeck, F.J.; Zrim, A.; Neumann, T.; Nguyen, L.T.; Betancourt, A.J.; Hummel, T.; et al. The genomic basis of circadian and circalunar timing adaptations in a midge. Nature 2016, 540, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Miao, J.; Guo, P.; Li, H.; Wei, C.; Liu, Q.; Gong, Z.; Duan, Y.; Li, T.; Jiang, Y.; Fen, H.; et al. Low barometric pressure enhances tethered-flight performance and reproductive of the oriental armyworm, Mythimna separate (Lepidoptera: Noctuidae). J. Econ. Entomol. 2021, 114, 620–626. [Google Scholar] [CrossRef]
- Welti, E.A.R.; Zajicek, P.; Frenzel, M.; Ayasse, M.; Bornholdt, T.; Buse, J.; Classen, A.; Dziock, F.; Engelmann, R.A.; Englmeier, J.; et al. Temperature drives variation in flying insect biomass across a German malaise trap network. Insect Conserv. Divers. 2022, 15, 168–180. [Google Scholar] [CrossRef]
- Martini, X.; Rivera, M.; Hoyte, A.; Sétamou, M.; Stelinski, L. Effects of wind, temperature, and barometric pressure on Asian citrus psyllid (Hemiptera: Liviidae) flight behavior. J. Econ. Entomol. 2018, 11, 2570–2577. [Google Scholar] [CrossRef]
- Gaylord, M.L.; Williams, K.K.; Hofstetter, R.W.; Mcmillin, J.D.; Degomez, T.E.; Wagner, M.R. Influence of temperature on spring flight initiation for southwestern ponderosa pine bark beetles (Coleoptera: Curculionidae, Scolytinae). Environ. Entomol. 2008, 37, 57–69. [Google Scholar] [CrossRef]
- Holmes, L.A.; Vanlaerhoven, S.L.; Tomberlin, J.K. Relative humidity effects on the life history of Hermetia illucens (Diptera: Stratiomyidae). Environ. Entomol. 2012, 41, 971–978. [Google Scholar] [CrossRef]
- Chen, Y.; Seybold, S.J. Crepuscular flight activity of an invasive insect governed by interaction abiotic factors. PLoS ONE 2014, 9, e105945. [Google Scholar] [CrossRef]
- Vebrová, L.; van Nieuwenhuijzen, A.; Kolář, V.; Boukal, D.S. Seasonality and weather conditions jointly drive flight activity patterns of aquatic and terrestrial chironomids. BMC Ecol. 2018, 18, 19. [Google Scholar] [CrossRef] [PubMed]

| Code | Season | Sampling Site | Barometric Pressure (hPa) | Temperature (°C) | Relative Humidity (%) | Wind Speed (m/s) |
|---|---|---|---|---|---|---|
| AutS1R1 | Autumn | Site 1 | 1010.72 | 18.28 | 70.26 | 4.29 |
| AutS1R2 | Autumn | Site 1 | 1014.36 | 20.94 | 71.34 | 1.29 |
| AutS1R3 | Autumn | Site 1 | 1013.29 | 22.71 | 72.17 | 3.40 |
| AutS2R1 | Autumn | Site 2 | 1014.12 | 22.10 | 70.92 | 3.72 |
| AutS2R2 | Autumn | Site 2 | 1011.04 | 19.10 | 72.08 | 2.11 |
| AutS2R3 | Autumn | Site 2 | 1012.27 | 18.60 | 70.36 | 2.67 |
| AutS3R1 | Autumn | Site 3 | 1013.54 | 18.36 | 70.56 | 1.45 |
| AutS3R2 | Autumn | Site 3 | 1014.17 | 21.95 | 71.73 | 2.28 |
| AutS3R3 | Autumn | Site 3 | 1013.11 | 20.57 | 70.40 | 2.04 |
| SprS1R1 | Spring | Site 1 | 1012.79 | 18.40 | 78.43 | 2.79 |
| SprS1R2 | Spring | Site 1 | 1013.19 | 17.64 | 77.66 | 1.41 |
| SprS1R3 | Spring | Site 1 | 1009.73 | 15.88 | 75.72 | 2.81 |
| SprS2R1 | Spring | Site 2 | 1011.52 | 16.44 | 78.21 | 2.71 |
| SprS2R2 | Spring | Site 2 | 1012.96 | 16.85 | 77.29 | 1.71 |
| SprS2R3 | Spring | Site 2 | 1014.01 | 16.65 | 78.58 | 2.30 |
| SprS3R1 | Spring | Site 3 | 1010.87 | 16.51 | 77.98 | 2.44 |
| SprS3R2 | Spring | Site 3 | 1010.86 | 17.42 | 77.56 | 2.69 |
| SprS3R3 | Spring | Site 3 | 1011.03 | 17.70 | 74.73 | 1.70 |
| SumS1R1 | Summer | Site 1 | 1004.28 | 26.15 | 89.64 | 2.80 |
| SumS1R2 | Summer | Site 1 | 1003.30 | 29.99 | 79.06 | 2.97 |
| SumS1R3 | Summer | Site 1 | 999.21 | 32.13 | 74.06 | 4.35 |
| SumS2R1 | Summer | Site 2 | 1003.78 | 30.83 | 92.62 | 3.09 |
| SumS2R2 | Summer | Site 2 | 998.05 | 28.28 | 85.63 | 4.15 |
| SumS2R3 | Summer | Site 2 | 1003.91 | 27.96 | 75.53 | 3.67 |
| SumS3R1 | Summer | Site 3 | 1000.72 | 30.72 | 83.46 | 4.76 |
| SumS3R2 | Summer | Site 3 | 1002.28 | 29.91 | 81.54 | 3.10 |
| SumS3R3 | Summer | Site 3 | 1003.01 | 29.48 | 88.45 | 3.59 |
| Subfamily | Tribe | Species | Search Database | Top Similarity (%) |
|---|---|---|---|---|
| Chironominae | Chironomini | Benthalia carbonaria | BOLD | 99.23 |
| Chironomus agilis | NCBI | 99.37 | ||
| Chironomus circumdatus | BOLD | 99.65–100 | ||
| Chironomus claggi | NCBI | 99.56 | ||
| Chironomus flaviplumus | BOLD | 99.48–100 | ||
| Chironomus fujitertius | BOLD | 98.62–98.79 | ||
| Chironomus javanus | BOLD | 99.82–100 | ||
| Chironomus kiiensis | BOLD | 99.65–100 | ||
| Chironomus nippodorsalis | BOLD | 99.85 | ||
| Chironomus striatipennis | BOLD | 99.65–100 | ||
| Dicrotendipes nervosus | BOLD | 99.65–100 | ||
| Dicrotendipes pelochloris | BOLD | 99.14–100 | ||
| Endochironomus pekanus | BOLD | 100 | ||
| Glyptotendipes tokunagai | BOLD | 100 | ||
| Harnischia longispuria | BOLD | 100 | ||
| Harnischia ohmuraensis | BOLD | 99.14–99.66 | ||
| Kiefferulus glauciventris | BOLD | 99.48 | ||
| Kiefferulus tainanus | BOLD | 99.83–100 | ||
| Microchironomus tabarui | BOLD | 99.83–100 | ||
| Microchironomus tener | BOLD | 98.08 | ||
| Parachironomus gracilior | BOLD | 99.31 | ||
| Polypedilum okiharaki | BOLD | 98.62–99.66 | ||
| Polypedilum harteni | BOLD | 99.31 | ||
| Polypedilum johannseni | BOLD | 99.83 | ||
| Polypedilum masudai | BOLD | 99.31 | ||
| Polypedilum nubifer | BOLD | 99.83–100 | ||
| Polypedilum sp. | BOLD | 100 | ||
| Polypedilum tigrinum | BOLD | 99.48–99.83 | ||
| Tanytarsini | Tanytarsus formosanus | BOLD | 100 | |
| Orthocladiinae | / | Cricotopus sylvestris | BOLD | 99.83–100 |
| / | Hydrobaenus kondoi | BOLD | 100 | |
| / | Limnophyes minimus | BOLD | 100 | |
| / | Limnophyes verpus | BOLD | 99.83 | |
| / | Parakiefferiella bathophila | BOLD | 99.83 | |
| / | Propsilocerus akamusi | BOLD | 99.14 | |
| / | Smittia aterrima | BOLD | 99.83–100 | |
| / | Smittia leucopogon | BOLD | 100 | |
| / | Smittia sp. | BOLD | 100 | |
| Tanypodinae | Pentaneurini | Ablabesmyia monilis | BOLD | 99.66–99.83 |
| Procladini | Procladius choreus | BOLD | 100 | |
| Procladius sp. | BOLD | 99.48 | ||
| Tanypodini | Tanypus chinensis | BOLD | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lei, T.; Gu, J.; Zhao, M.; Chen, Y.; Song, C.; Qi, X. Seasonal Dynamics of Non-Biting Midges (Diptera: Chironomidae) and Relevant Environmental Factors. Insects 2024, 15, 921. https://doi.org/10.3390/insects15120921
Lei T, Gu J, Zhao M, Chen Y, Song C, Qi X. Seasonal Dynamics of Non-Biting Midges (Diptera: Chironomidae) and Relevant Environmental Factors. Insects. 2024; 15(12):921. https://doi.org/10.3390/insects15120921
Chicago/Turabian StyleLei, Teng, Jingjing Gu, Mengyao Zhao, Yuqiu Chen, Chao Song, and Xin Qi. 2024. "Seasonal Dynamics of Non-Biting Midges (Diptera: Chironomidae) and Relevant Environmental Factors" Insects 15, no. 12: 921. https://doi.org/10.3390/insects15120921
APA StyleLei, T., Gu, J., Zhao, M., Chen, Y., Song, C., & Qi, X. (2024). Seasonal Dynamics of Non-Biting Midges (Diptera: Chironomidae) and Relevant Environmental Factors. Insects, 15(12), 921. https://doi.org/10.3390/insects15120921





