Envenomation with Snake Venoms as a Cause of Death: A Forensic Investigation of the Decomposition Stages and the Impact on Differential Succession Pattern of Carcass-Attracted Coleopteran Beetles
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Site of Study and Meteorological Measurements
2.2. Animals and Venoms
2.3. In Vivo Determination of LD50 of Venoms
2.4. Envenomation of Rabbits
2.5. Collection and Identification of Beetles
2.6. Statistical Analyses
3. Results
3.1. Meteorological Data
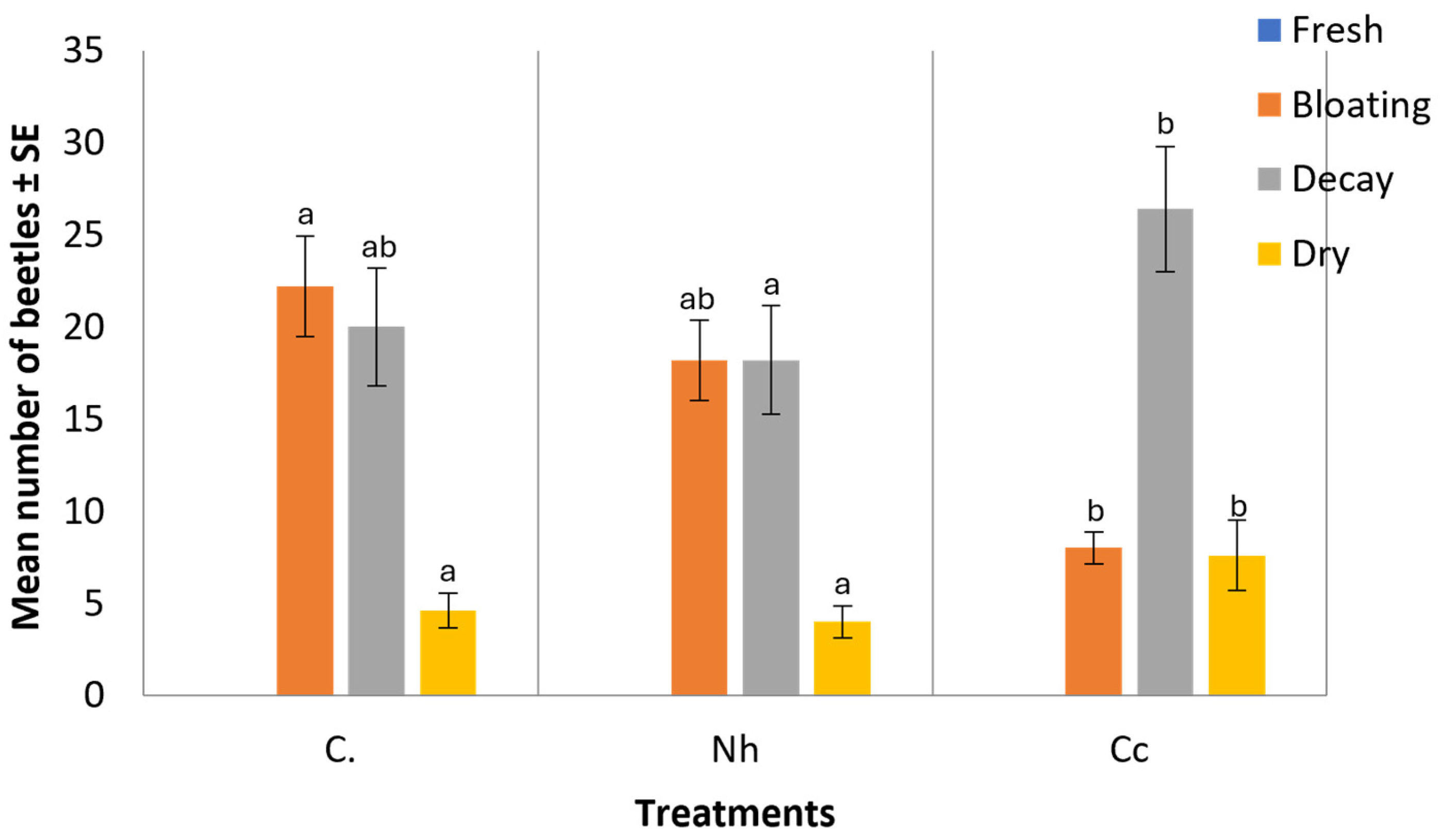
3.2. Decomposition Stages
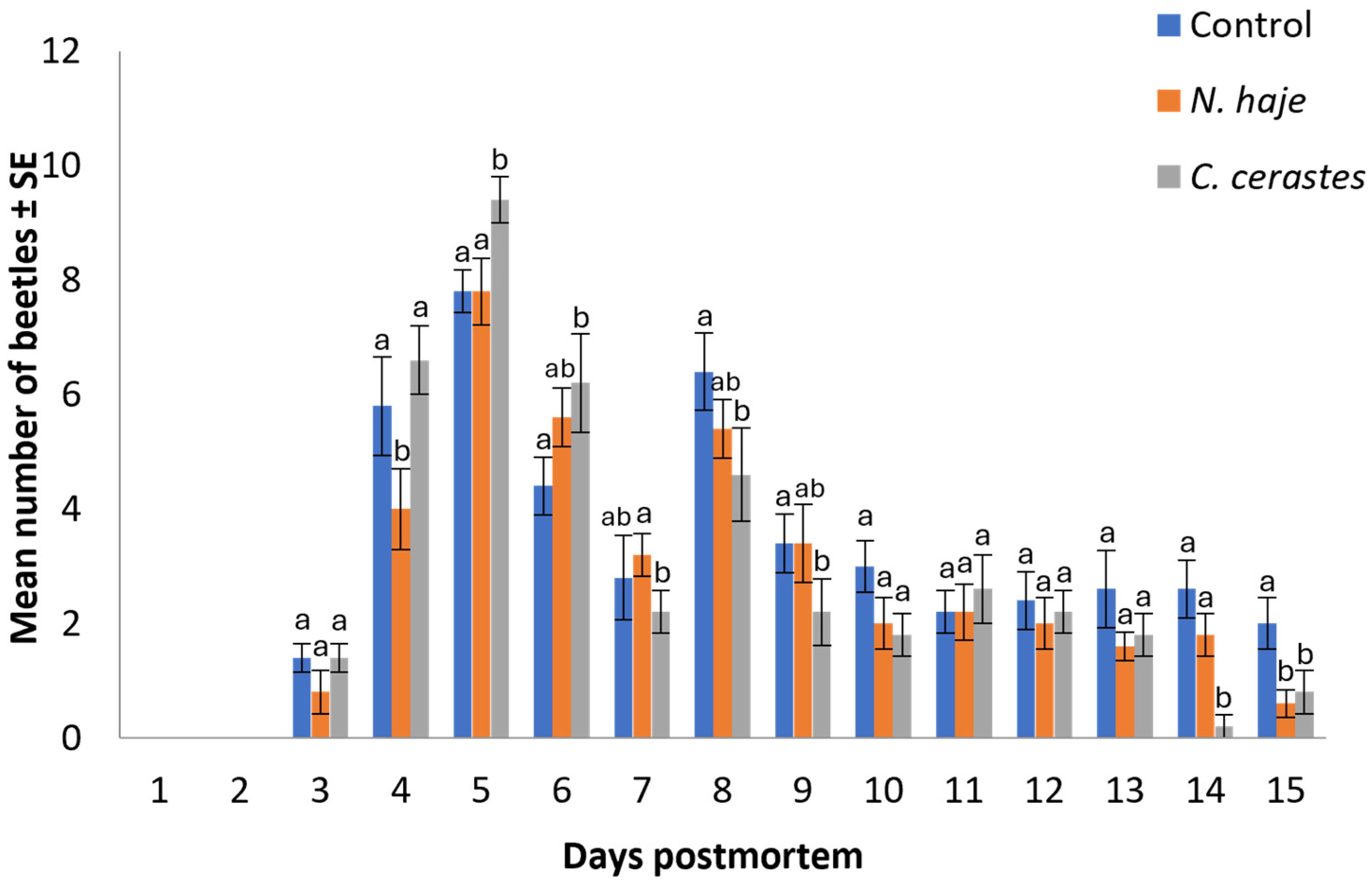
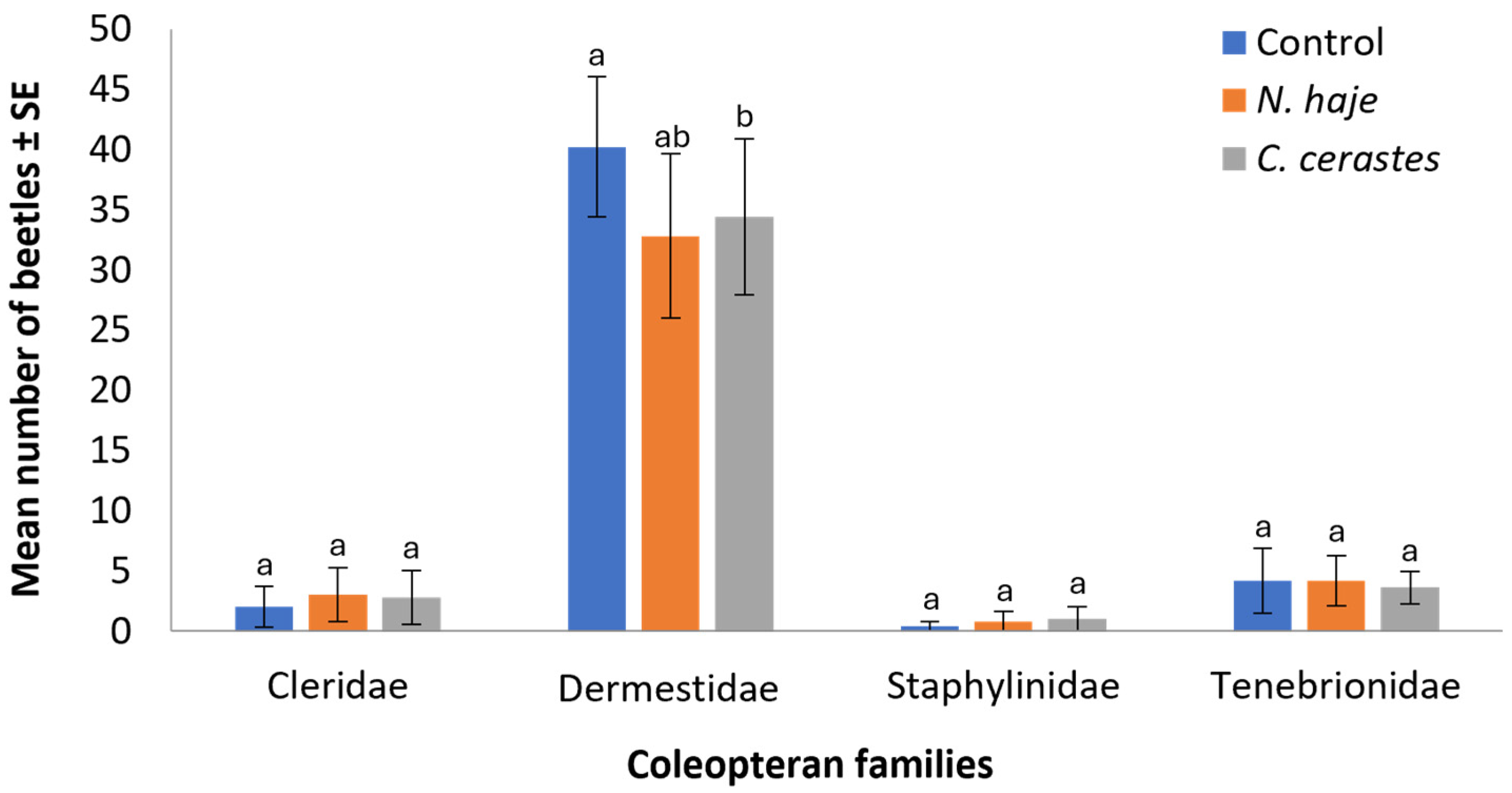
3.3. Differential Succession of Coleopterans
3.4. Abundance of Coleopterans Attracted to Carcasses
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Byrd, J.H.; Tomberlin, J.K. Forensic Entomology: The Utility of Arthropods in Legal Investigations; CRC Press: London, UK, 2019. [Google Scholar]
- Watson, E.J.; Carlton, C.E. Spring succession of necrophilous insectson wildlife carcasses in Louisiana. J. Med. Entomol. 2003, 40, 338–347. [Google Scholar] [CrossRef]
- Matuszewski, S.; Bajerlein, D.; Konwerski, S.; Szpila, K. Insect succession and carrion decomposition in selected forests of CentralEurope. Part 2: Composition and residency patterns of carrion fauna. Forensic Sci. Int. 2010, 195, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Mann, R.W.; Bass, W.M.; Meadows, L. Time since death and decomposition of the human body: Variables and observations incase and experimental field studies. J. Forensic Sci. 1990, 35, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Anderson, G.S. Factors that influence insect succession on carrion. In Forensic Entomology: The Utility of Arthropods in Legal Investigations; Byrd, J.H., Castner, J.L., Eds.; CRC Press: Boca Raton, FL, USA, 2010; pp. 201–250. [Google Scholar]
- Byrd, J.H.; Castner, J.L. Forensic Entomology: The Utility of Arthropods in Legal Investigations, 2nd ed.; CRC Press: BocaRaton, FL, USA, 2010. [Google Scholar]
- Schoenly, K.G.; Haskell, N.H.; Hall, R.D.; Gbur, J.R. Comparative performance and complementarity of four sampling methods and arthropod preference tests from human and porcine remains at the Forensic Anthropology Center in Knoxville, Tennessee. J. Med. Entomol. 2007, 44, 881–894. [Google Scholar] [CrossRef] [PubMed]
- Kulshrestha, P.; Satpathy, D.K. Use of beetles in forensic entomology. Forensic Sci. Int. 2001, 120, 15–17. [Google Scholar] [CrossRef] [PubMed]
- Hart, A.J.; Whitaker, A.P. Forensic entomology. Antenna 2006, 30, 159–164. [Google Scholar]
- Guo, Y.D.; Cai, J.F.; Xiong, F.; Wang, H.J.; Wen, J.F.; Li, J.B.; Chen, Y.Q. The utility of mitochondrial DNA fragments forgeneticidentificationofforensicallyimportantsarcophagidflies(Diptera:Sarcophagidae)inChina. Trop. Biomed. 2012, 29, 51–60. [Google Scholar]
- Biery, T.L. Venomous Arthropod Handbook: Envenomation Symptoms/Treatment, Identification, Biology and Control; Disease Surveillance Branch, Epidemiology Division: Austin, TX, USA, 1977. [Google Scholar]
- Edstrom, A. Venomous and Poisonous Animals; Krieger Publishing Company: Malabar, FL, USA, 1992. [Google Scholar]
- Forrester, J.A.; Weiser, T.G.; Forrester, J.D. An Update on Fatalities Due to Venomous and Non venomous Animals in the United States (2008–2015). Wilderness Environ. Med. 2018, 29, 36–44. [Google Scholar] [CrossRef]
- Forrester, J.D.; Forrester, J.A.; Tennakoon, L.; Staudenmayer, K. Mortality, hospital admission, and health care cost due to injury from venomous and non-venomous animal encounters in theUSA: 5-year analysis of the National Emergency Department Sample. Trauma. Surg. Acute Care Open 2018, 3, e000250. [Google Scholar] [CrossRef]
- Gutié rez, J.M.; Theakston, R.D.; Warrell, D.A. Confronting the neglected problem of snakebite envenoming: The need for a global partnership. PLoS Med. 2006, 3, e150. [Google Scholar]
- World Health Organization (WHO). Rabies and Envenomings: A Neglected Public Health Issue; WHO: Geneva, Switzerland, 2007. [Google Scholar]
- Chippaux, J.P.; Goyffon, M. Epidemiology of scorpionism: A global appraisal. Acta Trop. 2008, 107, 71–79. [Google Scholar] [CrossRef]
- Alirol, E.; Sharma, S.K.; Bawaskar, H.S.; Kuch, U.; Chappuis, F. Snake bite in SouthAsia: Areview. PLoS Negl. Trop. Dis. 2010, 4, e603. [Google Scholar] [CrossRef] [PubMed]
- Coelho, P.; Sousa, P.; Harris, D.J.; van der Meijden, A. Deep intraspecific divergences in the medically relevant fat-tailed scorpions (Androctonus, Scorpiones). Acta Trop. 2014, 134, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Cichutek, K.; Epstein, J.; Griffiths, E.; Hindawi, S.; Jivapaisarnpong, T.; Klein, H.; Minor, P.; Moftah, F.; Reddy, V.; Slamet, L. WHO Expert Committee on Biological Standardization sixty-seventh report. Tech. Rep. Ser. WHO 2017, 1004, 1–591. [Google Scholar]
- Amr, Z.S.; AbuBaker, M.A.; Warrell, D.A. Terrestrial venomous snakes and snakebites in the Arab countries of the Middle East. Toxicon 2020, 177, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Kasturiratne, A.; Wickremasinghe, A.R.; de Silva, N.; Gunawardena, N.K.; Pathmeswaran, A.; Premaratna, R.; Savioli, L.; Lalloo, D.G.; de Silva, H.J. The global burden of snakebite: A literature analysis and modeling based on regional estimates of envenoming and deaths. PLoS Med. 2008, 5, e218. [Google Scholar] [CrossRef]
- Abdou, R.H.; Ibrahim, A.E. Effects of Egyptian cobra (Naja haje)venom on postmortem changes and some biochemical parameters in rats. Forensic Sci. 2015, 4, 186–190. [Google Scholar]
- Sant, S.M.; Purandare, N.M. Autopsy study of cases of snakebite with special reference to the renal lesions. J. Postgrad. Med. 1972, 18, 181–188. [Google Scholar]
- Pathmeswaran, A.; Kasturiratne, A.; Fonseka, M.; Nandasena, S.; Lalloo, D.G.; de Silva, H.J. Identifying the biting species in snakebite by clinical features: An epidemiological tool for community surveys. Trans. R. Soc. Trop. Med. Hyg. 2006, 100, 874–878. [Google Scholar] [CrossRef]
- Tan, N. The biochemistry of venoms of some venomous snakes of Malaysia: A review. Trop. Biomed. 1991, 8, 91–103. [Google Scholar]
- Omran, M.; Abdel-Nabi, I.; El-Naggar, M. Serum biochemical and hormonal parameters as biomarkers for the toxic effects of Egyptian cobra (Naja haje) envenomation. J. Nat. Toxins 1997, 6, 69–83. [Google Scholar]
- Schafer, A.I. Thrombocytosis. N. Engl. J. Med. 2004, 350, 1211–1219. [Google Scholar] [CrossRef] [PubMed]
- Cher, C.D.; Armugam, A.; Zhu, Y.Z.; Jeyaseelan, K. Molecular basis of cardiotoxicity upon cobra envenomation. Cell Mol. Life Sci. 2005, 62, 105–118. [Google Scholar] [CrossRef] [PubMed]
- Sejrsen, K.; Hvelplund, T.; Nielsen, M.O. Ruminant Physiology: Digestion, Metabolism and Impact of Nutrition on Gene Expression, Immunology and Stress; Wageningen Academic Publishers: Wageningen, The Netherlands, 2006. [Google Scholar]
- Fung, S.Y.; Tan, N.H.; Liew, S.H.; Sim, S.M.; Aguiyi, J.C. The protective effects of Mucuna pruriens seed extract against histopathological changes induced by Malayan cobra (Naja sputatrix) venom in rats. Trop. Biomed. 2009, 26, 80–84. [Google Scholar] [PubMed]
- Al-Sadoon, M.K.; Moneim, A.E.A.; Diab, M.; Bauomy, A. Hepatic and renal tissue damages induced by Cerastes cerastes gasperetti crude venom. Life Sci. J. 2013, 10, 191–197. [Google Scholar]
- Tohamy, A.A.; Mohamed, A.F.; Moneim, A.E.A.; Diab, M.S. Biological effects of Naja haje crude venom on the hepatic and renal tissues of mice. J. King Saud. Univ. Sci. 2014, 26, 205–212. [Google Scholar] [CrossRef]
- Khalil, A.; Zidan, M.M.M.; Alajmi, R.; Ahmed, A.M. Impact of Envenomation with Snake venoms on Rabbit Carcass Decomposition and Differential Adult Dipteran Succession Patterns. J. Med. Entomol. 2023, 60, 40–50. [Google Scholar] [CrossRef]
- Mapara, M.; Thomas, B.S.; Bhat, K.M. Rabbit as an animal model for experimental research. Dent. Res. J. 2012, 9, 111–118. [Google Scholar]
- Mashaly, A.; Al-Khalifa, M.; Al-Qahtni, A. Chrysomya albiceps Wiedemann (Diptera: Calliphoridae)colonizing poisoned rabbit carcasses. Entomol. Res. 2020, 50, 552–560. [Google Scholar] [CrossRef]
- Broad, A.; Sutherland, S.; Coulter, A.R. The lethality in mice of dangerous Australian and other snake venom. Toxicon 1979, 17, 661–664. [Google Scholar] [CrossRef]
- Conlee, K.M.; Stephens, M.L.; Rowan, A.N.; King, L.A. Carbondioxide for euthanasia: Concerns regarding pain anddistress, with special reference to mice and rats. Lab. Anim. 2005, 39, 137–161. [Google Scholar] [CrossRef] [PubMed]
- Gerstmeier, R. Checkered Beetles. Illustrated Key to the Cleridae and Thanerocleridae of the Western Palaearctic; ConchBooks: Harxheim, Germany, 1988. [Google Scholar]
- Háva, J. World keys to the genera and sub genera of Dermestidae (Coleoptera), with descriptions, nomenclature and distributional records. Acta Musei Nat. Pragae Ser. B Nat. Hist. 2004, 60, 149–164. [Google Scholar]
- Mazur, S. Family Histeridae. In Catalogue of Palaearctic Coleoptera: Hydrophiloidea—Staphylinoidea; Löbl, I., Löbl, D., Eds.; Brill: Boston, MA, USA, 2004; pp. 68–102. [Google Scholar]
- Shapiro, S.S.; Wilk, M.B. An analysis of variance test for normality (complete samples). Biometrika 1965, 52, 591–611. [Google Scholar] [CrossRef]
- Voss, S.C.; Cook, D.F.; Dadour, I.R. Decomposition and insect succession of clothed and unclothed carcasses in Western Australia. Forensic Sci. Int. 2011, 211, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Hyde, E.R.; Haarmann, D.P.; Petrosino, J.F.; Lynne, A.M.; Bucheli, S.R. Initial insights into bacterial succession during human decomposition. Int. J. Legal Med. 2015, 129, 661–671. [Google Scholar] [CrossRef]
- Roberts, L.G.; Spencer, J.R.; Dabbs, G.R. The Effect of Body Mass on Outdoor Adult Human Decomposition. J. Forensic Sci. 2017, 62, 1145–1150. [Google Scholar] [CrossRef]
- Jones, A.W.; Holmgren, A. Concentration distributions of the drugs most frequently identified in post-mortem femoral blood representing all causes of death. Med. Sci. Law. 2009, 49, 257–273. [Google Scholar] [CrossRef]
- Zhou, C.; Byard, R.W. Factors and processes causing accelerated decomposition in human cadavers—An over view. J. Forensic Leg. Med. 2011, 18, 6–9. [Google Scholar] [CrossRef]
- Byrd, J.H.; Peace, M.R. Entomotoxicology: Drugs, toxins, and insects. In Forensic Chemistry Handbook; Kobilinsky, L., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2012; pp. 483–499. [Google Scholar]
- Jones, A.W.; Holmgren, A.; Ahlner, J. Concentrations of free-morphine in peripheral blood after recent use of heroin in overdose deaths and in apprehended drivers. Forensic Sci. Int. 2012, 215, 18–24. [Google Scholar] [CrossRef]
- Abd El-Aziz, F.E.; Eldeeb, S.M.; Abdellah, N.Z.; Shaltout, E.S.; Ebrahem, N.E. Influence of Scorpion Venom on Decomposition and Arthropod Succession. Egypt. Acad. J. Biol. Sci. B Zool. 2022, 14, 209–219. [Google Scholar] [CrossRef]
- Goff, M.L. Estimation of Postmortem Interval Using Arthropod Development and Successional Patterns. Forensic Sci. Rev. 1993, 5, 81–94. [Google Scholar] [PubMed]
- Carvalho, L.d.; Thyssen, P.; Goff, M.; Linhares, A. Observations on the succession patterns of necrophagous insects on a pigcarcass in an urban area of South eastern Brazil. J. Forensic Med. Toxicol. 2004, 5, 33–39. [Google Scholar]
- Martinez, E.; Duque, P.; Wolff, M. Succession pattern of carrion-feeding insects in Paramo, Colombia. Forensic Sci. Int. 2007, 166, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Gomes, L.; Gomes, G.; Desuó, I.C. A preliminary study of insect fauna on pig carcasses located in sugar cane in winter in south eastern Brazil. Med. Vet. Entomol. 2009, 23, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Tembe, D.; Mukaratirwa, S. Insect Succession and Decomposition Pattern on Pig Carrion During Warm and Cold Seasons in Kwazulu-Natal Province of South Africa. J. Med. Entomol. 2021, 58, 2047–2057. [Google Scholar] [CrossRef] [PubMed]
- Thümmel, L.; Lutz, L.; Geissenberger, J.; Pittner, S.; Heimer, J.; Amendt, J. Decomposition and insect succession of pig cadavers in tents versus outdoors—A preliminary study. Forensic Sci. Int. 2023, 346, 111640. [Google Scholar] [CrossRef]
- Voss, S.C.; Spafford, H.; Dadour, I.R. Annual and seasonal patterns of insect succession on decomposing remains at two locations in Western Australia. Forensic Sci. Int. 2009, 193, 26–36. [Google Scholar] [CrossRef]
- Benbow, M.E.; Pechal, J.L.; Lang, J.M.; Erb, R.; Wallace, J.R. The Potential of High-throughput Metagenomic Sequencing of Aquatic Bacterial Communities to Estimate the Postmortem Submersion Interval. J. Forensic Sci. 2015, 60, 1500–1510. [Google Scholar] [CrossRef]
- Iqbal, M.A.; Ueland, M.; Forbes, S.L. Recent advances in the estimation of post-mortem interval in forensic taphonomy. Austr. J. Forensic Sci. 2020, 52, 107–123. [Google Scholar] [CrossRef]
- Al-Qahtni, A.; Mashaly, A.; Haddadi, R.; Al-Khalifa, M. Seasonal Impact of Heroin on Rabbit Carcass Decomposition and Insect Succession. J. Med. Entomol. 2021, 58, 567–575. [Google Scholar] [CrossRef]
- Al-Khalifa, M.; Mashaly, A.; Al-Qahtni, A. Impacts of antemortem ingestion of alcoholic beverages on insect successional patterns. Saudi J. Biol. Sci. 2021, 28, 685–692. [Google Scholar] [CrossRef] [PubMed]
- Binh, D.; Thanh, T.; Chi, P. Proteomic characterization of the thermostable toxins from Naja naja venom. J. Venom. Anim. Toxins Incl. Trop. Dis. 2010, 16, 631–638. [Google Scholar] [CrossRef]
- Pepys, M.B.; Hirschfield, G.M. C-reactive protein: A critical update. J. Clin. Investig. 2003, 111, 1805–1812. [Google Scholar] [CrossRef] [PubMed]
- Fahim, A. Biochemical effects of crude venoms of two elapidae from Saudi fauna on male albino rats. J. Egypt. Ger. Soc. Zool. 2001, 36, 191–204. [Google Scholar]
- El-Refaei, M.F.; Sarkar, N.H. Snake venom inhibits the growth of mouse mammary tumor cells in vitro and in vivo. Toxicon 2009, 54, 33–41. [Google Scholar] [CrossRef]
- Evangelista, I.L.; Martins, A.M.; Nascimento, N.R.; Havt, A.; Evangelista, J.S.; de Norões, T.B.; Toyama, M.H.; Diz-Filho, E.B.; Toyama Dde, O.; Fonteles, M.C.; et al. Renal and cardiovascular effects of Bothrops marajoensis venom and phospholipaseA2. Toxicon 2010, 55, 1061–1070. [Google Scholar] [CrossRef]
- Schneemann, M.; Cathomas, R.; Laidlaw, S.T.; ElNahas, A.M.; Theakston, R.D.; Warrell, D.A. Life-threatening envenoming by the Saharan horned viper (Cerastes cerastes) causing micro-angiopathic haemolysis, coagulopathy and acute renal failure: Clinical cases and review. Qjm 2004, 97, 717–727. [Google Scholar] [CrossRef]
- Bazaa, A.; Marrakchi, N.; El Ayeb, M.; Sanz, L.; Calvete, J.J. Snake venomics: Comparative analysis of the venom proteomes of the Tunisian snakes Cerastes cerastes, Cerastes vipera and Macrovipera lebetina. Proteomics 2005, 5, 4223–4235. [Google Scholar] [CrossRef]
- Vyas, V.K.; Brahmbhatt, K.; Bhatt, H.; Parmar, U. Therapeutic potential of snake venom in cancer therapy: Current perspectives. Asian Pac. J. Trop. Biomed. 2013, 3, 156–162. [Google Scholar] [CrossRef]
- Ozverel, C.S.; Damm, M.; Hempel, B.F.; Göçmen, B.; Sroka, R.; Süssmuth, R.D.; Nalbantsoy, A. Investigating the cytotoxic effects of the venom proteome of two species of the Viperidae family(Cerastes cerastes and Cryptelytrops purpureomaculatus) from various habitats. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2019, 220, 20–30. [Google Scholar] [CrossRef]
- Mashaly, A.M. Carrion beetles succession in three different habitats in Riyadh, Saudi Arabia. Saudi J. Biol. Sci. 2017, 24, 430–435. [Google Scholar] [CrossRef] [PubMed]
- Sonker, R.; Rawat, S.; Singh, K. Succession and life cycle of beetles on the exposed carcass. Int. J. Sci. Innov. Res. 2015, 1, 46–50. [Google Scholar]
- Al-Dakhil, A.A.; Alharbi, S.A. A preliminary investigation of the entomofauna composition of forensically important necrophagous insects in Al-Madinah Al-Munawwarah region, Kingdom of Saudi Arabia. J. Taibah Univ. Sci. 2020, 14, 1127–1133. [Google Scholar] [CrossRef]
- Early, M.; Goff, M.L. Arthropod succession patterns in exposed carrion on the island of O’ahu, Hawaiian Islands, USA. J. Med. Entomol. 1986, 23, 520–531. [Google Scholar] [CrossRef] [PubMed]
- Mabika, N.; Masendu, R.; Mawera, G. An initial study of insect succession on decomposing rabbit carrions in Harare, Zimbabwe. Asian Pac. J. Trop. Biomed. 2014, 4, 561–565. [Google Scholar] [CrossRef]
- VanLaerhoven, S.L.; Anderson, G.S. Insect succession on buried carrion in two biogeoclimatic zones of BritishColumbia. J. Forensic Sci. 1999, 44, 32–43. [Google Scholar] [CrossRef]
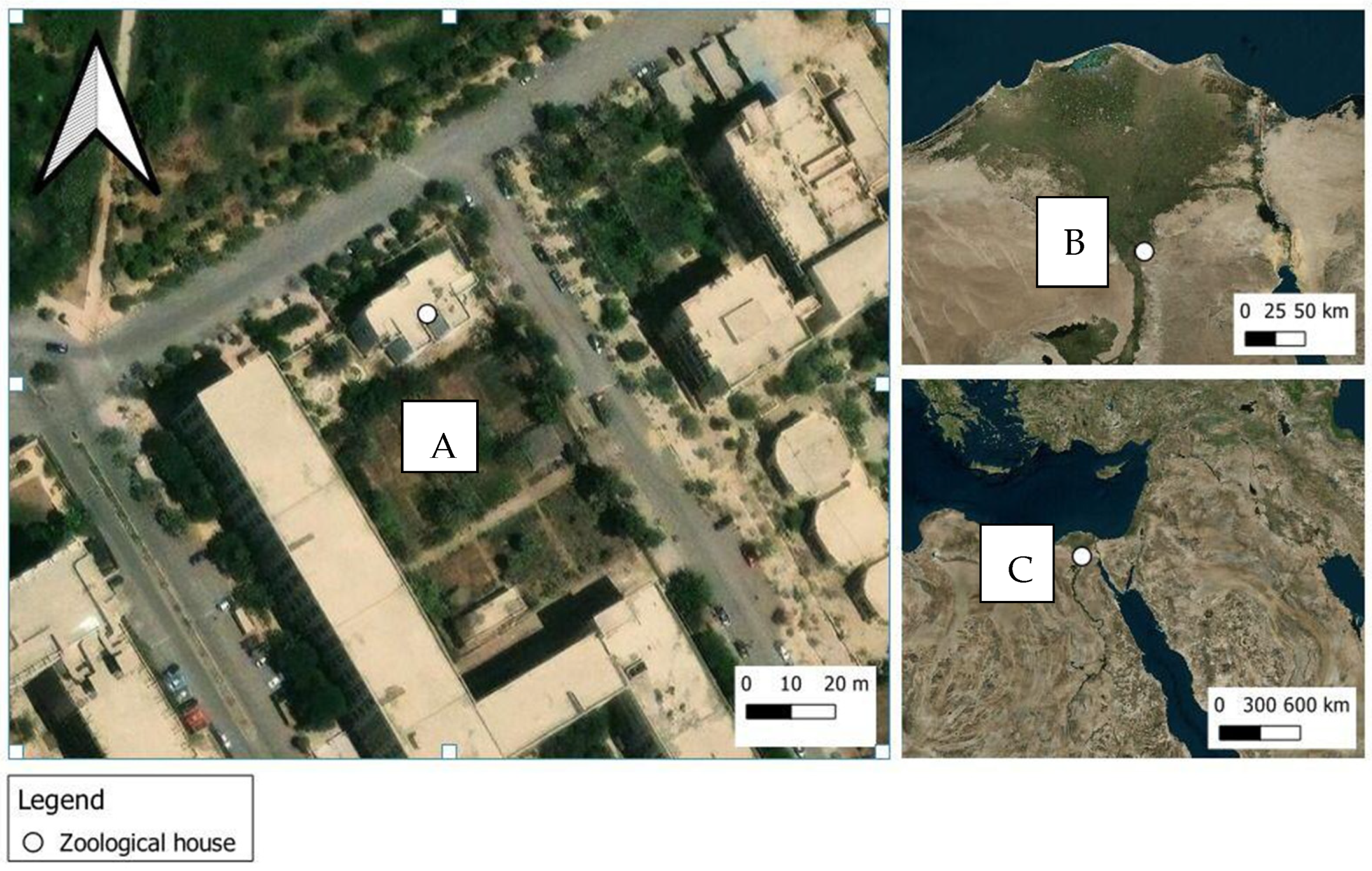
| Atmospheric Temperature (°C) | Atmospheric Relative Humidity (%) | Soil Temperature (°C) | Soil Humidity (%) | Wind Speed (km/h) | Carcasses Temperature (°C) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Maximum | Minimum | Maximum | Minimum | Control | N. haje | C. cerastes | |||
| 36.80 ± 0.39 | 24.93 ± 0.18 | 85.40 ± 0.42 | 30.06 ± 0.06 | 42.93 ± 0.34 | 59.13 ± 0.43 | 15.26 ±0.33 | 15.87 ± 1.59 | 15.63 ± 1.71 | 14.93 ± 1.92 |
| Treatments | Days Postmortem ± SE | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | |
| Control | 2 ± 0.14a * | 5 ± 0.16a | 6 ± 0.08a | 2 ± 0.17a | |||||||||||
| N. haje | 1 ± 0.16b | 5 ± 0.07a | 6 ± 0.21a | 3 ± 0.16ab | |||||||||||
| C. cerastes | 1 ± 0.10b | 3 ± 0.15b | 6 ± 013a | 5 ± 0.20b | |||||||||||
| Key: | Fresh | Bloated | Decayed | Dried | |||||||||||
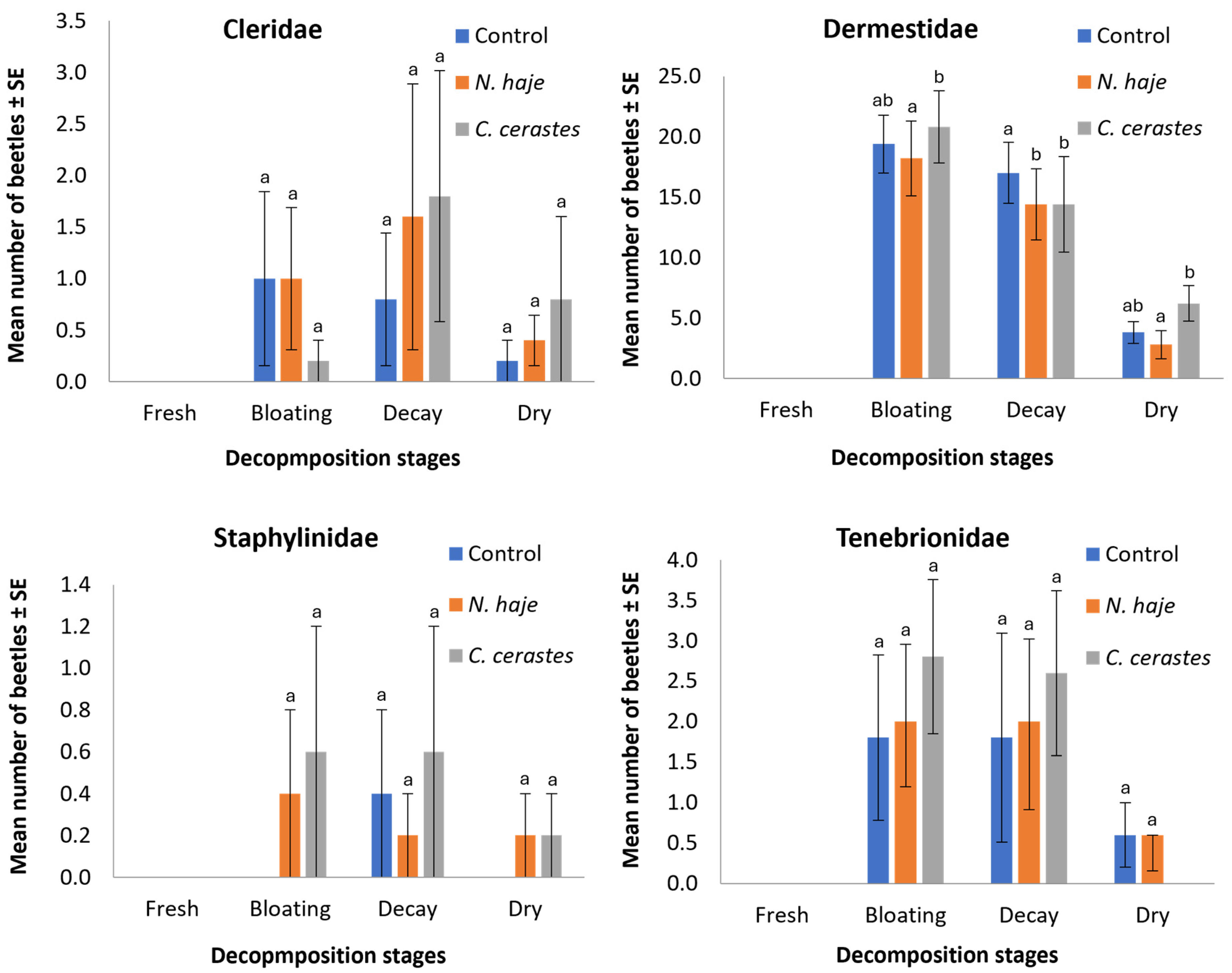
| Coleopteran Families (Number/Five Carcasses) | Beetle Species (Number/Five Carcasses) | Control | N. hajie | C. cerastes | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fr | Bl | De | Dr | Fr | Bl | De | Dr | Fr | Bl | De | Dr | ||
| Cleridae (39) | Necrobia rufipes (Fabricius, 1781) (39) | − | + | + | + | − | + | + | + | − | + | + | + |
| Dermestidae (532) | Attagenus sp. (67) | − | + | + | + | − | + | + | + | − | + | + | + |
| Dermestes Frischii Kugelann, 1792 (119) | − | + | + | + | − | + | + | + | − | + | + | + | |
| D. maculatus De Geer, 1774 (346) | − | + | + | + | − | + | + | + | − | + | + | + | |
| Staphylinidae (11) | Bledius sp. (11) | − | − | + | − | − | + | + | + | − | + | + | + |
| Tenebrionidae (65) | Apentanodes sp. (65) | − | + | + | + | − | + | + | + | − | + | + | − |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khalil, A.; Salem, A.M.; Shaurub, E.-S.H.; Ahmed, A.M.; Al-Khalaf, A.A.; Zidan, M.M. Envenomation with Snake Venoms as a Cause of Death: A Forensic Investigation of the Decomposition Stages and the Impact on Differential Succession Pattern of Carcass-Attracted Coleopteran Beetles. Insects 2024, 15, 902. https://doi.org/10.3390/insects15110902
Khalil A, Salem AM, Shaurub E-SH, Ahmed AM, Al-Khalaf AA, Zidan MM. Envenomation with Snake Venoms as a Cause of Death: A Forensic Investigation of the Decomposition Stages and the Impact on Differential Succession Pattern of Carcass-Attracted Coleopteran Beetles. Insects. 2024; 15(11):902. https://doi.org/10.3390/insects15110902
Chicago/Turabian StyleKhalil, Abdelwahab, Abeer M. Salem, El-Sayed H. Shaurub, Ashraf M. Ahmed, Areej A. Al-Khalaf, and Mahmoud M. Zidan. 2024. "Envenomation with Snake Venoms as a Cause of Death: A Forensic Investigation of the Decomposition Stages and the Impact on Differential Succession Pattern of Carcass-Attracted Coleopteran Beetles" Insects 15, no. 11: 902. https://doi.org/10.3390/insects15110902
APA StyleKhalil, A., Salem, A. M., Shaurub, E.-S. H., Ahmed, A. M., Al-Khalaf, A. A., & Zidan, M. M. (2024). Envenomation with Snake Venoms as a Cause of Death: A Forensic Investigation of the Decomposition Stages and the Impact on Differential Succession Pattern of Carcass-Attracted Coleopteran Beetles. Insects, 15(11), 902. https://doi.org/10.3390/insects15110902






