Simple Summary
The Indian meal moth, Plodia interpunctella (Hübner, 1813) (Lepidoptera: Pyralidae), exhibited significant differences in oviposition preference between normal-oleic peanuts (NOPs) and high-oleic peanuts (HOPs). In this study, we found key volatile organic compounds (VOCs) from NOP and HOP peanuts that were attractive or repellent to P. interpunctella females by gas chromatography/mass spectrometry (GC-MS), gas chromatography/electroantennographic detection (GC-EAD), and electroantennogram (EAG) analysis, as well as behavioral responses in Y-tube olfactometer and wind tunnel bioassays. The identification of key VOCs may provide a foundation for developing food-based attractants and repellents for pest control.
Abstract
The Indian meal moth, Plodia interpunctella (Hübner, 1813) (Lepidoptera: Pyralidae), a primary stored peanut insect pest, exhibited a significant difference in oviposition preference among normal-oleic peanuts (NOPs) and high-oleic peanuts (HOPs). Identifying key volatile organic compounds (VOCs) that are attractive or repellent to P. interpunctella is of great significance for the ecological management of pests. The profiles and contents of VOCs among NOP and HOP varieties were measured and compared, and key bioactive VOCs were further confirmed via an electroantennogram (EAG) analysis, as well as behavioral responses in Y-tube olfactometer and wind tunnel bioassays. Females prefer to lay eggs on NOP varieties more than on HOP ones. Acetophenone, nonanal, decanal, dodecane, 2,5-dimethylbenzaldehyde, and 4-ethyl-benzaldehy derived from tested peanuts elicited stronger antennal EAG responses. The results of the Y-tube olfactometer and wind tunnel bioassay showed that the relative high levels of nonanal, dodecane, and unique VOC acetophenone in NOP varieties have a significant attraction to P. interpunctella. Conversely, 4-ethyl-benzaldehyde and the unique VOC 2,5-dimethyl-benzaldehyde commonly found in HOP varieties exhibit notable repellent effects on P. interpunctella. These VOCs could contribute to the development of attractants or repellents derived from special peanut varieties for pest management.
1. Introduction
The peanut, Arachis hypogaea (Linnaeus, 1753) (Fabales: Fabaceae), is an important oil and cash crop [1] and is widely distributed around the world. Peanut varieties are categorized as normal-oleic peanuts (NOPs) and high-oleic peanuts (HOPs) varieties based on the percentage of oleic acid oil. The proportion of oleic acid oil (18:1) in the total fatty acids of NOP is approximately within the range of 41–67%, whereas in HOP, it can be up to 80% [2]. HOP varieties have been widely promoted and commercially cultivated in many countries [3]. A series of HOP varieties have been developed in China, including the prominent varieties Kainong 301, Jihua 11, Jihua 13, Huayu 32, and so on, recently [4]. Peanuts are susceptible to storage insect infestations, such as P. interpunctella, Ephestia cautella (Walker, 1863) (Lepidoptera: Pyralidae), Tribolium castaneum (Herbst, 1797) (Coleoptera: Tenebrionidae), Corcyra cephalonica (Stainton, 1850) (Lepidoptera: Pyralidae), Oryzaephilus surinamensis (Linnaeus, 1767) (Coleoptera: Silvanidae), Oryzaephilus mercator (Fauvel, 1860) (Coleoptera: Silvanidae), Rhyzopertha dominica (Fabricius, 1792) (Coleoptera: Bostrichidae), and Cryptolestes ferrugineus (Stephens, 1831) (Coleoptera: Laemophloeidae) [5,6,7]. Among them, the Indian meal moth can cause serious damage to stored peanuts [8].
The larvae of the Indian meal moth feed on food and stored products, while adults can infest products by oviposition [9]. The moth evenly distributes its eggs based on a great many kinds and quantities of stored products [10]. Severe economic losses of stored grains and peanuts had been caused by this insect [11,12]. As an environmentally friendly strategy for pest management, attractant-baited traps have been used to monitor insect populations and capture insects in grain storage and food-processing facilities. Pheromone kairomone-baited dome traps and pheromone-baited flight traps effectively captured male P. interpunctella [13]. Food-based attractants have gained more attention and application due to their easy accessibility and low cost [14]. One female of this moth can lay 100–300 eggs in its lifespan and possesses a stronger flight performance [8]. By interfering with egg laying, targeting female attraction or repulsion based on food volatility could be further used for population control, either by attracting females into traps or expelling them from infected areas.
Insects can rely on volatile organic compounds (VOCs) to locate food [15], appropriate hosts [16,17], mating, oviposition sites [10], or to stay away from danger [18], and so on. Understanding insect behavior and identifying VOCs from foods may help develop attractants or repellents [19,20]. Hexanal, heptanal, and octanal collected from three kinds of dried fruits and whole-wheat flour attracted more P. interpunctella females to lay eggs in the screened net cages [19]. A mixture of VOCs ((E)-2-octenal, (E)-2-nonenal, and (E)-2-heptenal) from whole-wheat flour were oviposition attractants for mated females of P. interpunctella, but not for males and unmated females of the insect in pitfall olfactometers bioassay [21]. Inversely, VOCs 3-methyl-1-butanol from fungus-infected wheat was repellent to mated P. interpunctella females in the Y-tube olfactometer bioassay [22]. There is currently a lack of reports on typical VOCs emitted from peanuts that either attract or repel P. interpunctella.
According to our preliminary work, P. interpunctella females laid more eggs on peanuts compared to wheat, maize, and rice [23], and there were significant differences in oviposition preferences among 32 peanut varieties (including 25 NOP and 7 HOP varieties). We assume that the oviposition preference of P. interpunctella in different varieties of peanuts is mediated by some key VOCs derived from peanuts. The research aims to confirm this hypothesis and validate the key VOCs mediating oviposition preference or repelling in the tested peanut varieties. The purpose of the measurement is to (1) measure the oviposition preference of P. interpunctella females in the tested peanut varieties; (2) identify bioactive VOCs that trigger female antennal responses; and (3) verify individual active VOCs in female antenna and behavioral responses through electroantennography (EAG), Y-tube olfactometer, and wind tunnel bioassay. The research results can provide reference for the development of new attractants or repellents for the ecological management of this moth.
2. Material and Methods
2.1. Peanuts
The tested varieties of NOP (Yuhanghua 1, Yuhua 9326, Pukehua 5, Puhua 28, and Tianfu 3) and HOP (Jihua 13, Jihua 11, and Kainong 301) were kept at 4 °C in a freezer for one week after harvesting and drying at 42 °C for 48 h, provided by the Puyang Academy of Agricultural Sciences, Puyang, P R China. The shelled kernels were kept in a refrigerator at −15 °C two weeks for insect elimination. Then, the samples were kept in a room at 27 ± 1 °C 7 days before measurement. The chemical characteristics of each tested peanut were determined by proximate analysis [24], including proportions of protein, fat, and water (Table 1). The chemical characteristics of oleic acid and linoleic acid in peanuts were determined by using near-infrared spectroscopy (Table 1).

Table 1.
The chemical characteristics of eight varieties of peanuts.
2.2. Insect Rearing
The population of P. interpunctella was collected from a grain depot located in Zhengzhou, P R China, and reared with diets (oats, broken corn, and yeast in a specific proportion of 19:19:2) for several generations at 27 ± 1 °C, 70 ± 5% r.h., and 16:8 (L:D) photoperiod. The selected larvae of females and males were kept in containers (5 cm in diameter and 3 cm in height) separately with diets until adult emergence in preparation. Male and female larvae were distinguished during the larval stage by a dark patch along the median plane of the mid-dorsal abdomen in males, formed by the testis, while females exhibited no such patch [25]. New emerged virgin male and female moths were coupled for oviposition preference assay. Two-day-old mated females were chosen for electrophysiological, Y-tube olfactometer, and wind tunnel bioassays [21].
2.3. Oviposition Preference Measurement
The oviposition preference measurement was conducted in a multiple-choice oviposition assay apparatus, which consisted of a cubical steel sheeting chamber, a peanut sample board containing eight peanut cups to hold peanuts, and a suspended petri dish (for mating moth preparation). The cup contained 20 ± 0.01 g of the kernel for one variety of peanut, which was set and distributed with other varieties in a circle along the margin of the board (Figure 1). A couple of mating moths were released into a petri dish hung under the lid of the apparatus for oviposition performance measurement. Seventy-two hours later, the cups with eggs and kernels were removed, and the egg number was checked under a stereomicroscope. Nine parallel experiments were carried out for each batch with eight varieties of peanut.
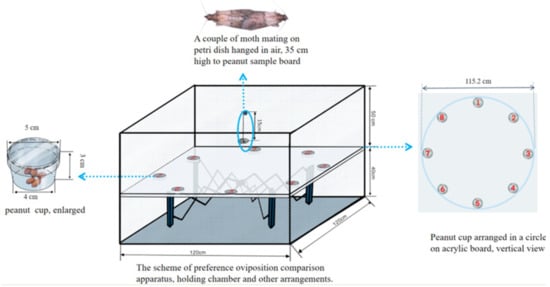
Figure 1.
The scheme of the multiple-choice oviposition apparatus and material arrangement.
2.4. Volatile Organic Compounds Collection and Chemical Analysis
The dynamic headspace sampling of VOCs was employed from tested varieties of peanuts. All sampling parts were connected with Teflon tubing. The dynamic headspace collection system is shown in Figure 2. An air pump (QC-1B, Institute of Labor Protection, Beijing, China) sequentially pulled an air stream through a vial containing activated carbon, a vial containing silica gel, a flowmeter, a glass jar (12.6 cm in diameter and 12.6 cm in height) containing peanut kernels, a VOC trap (0.9 cm in diameter, and 7 cm in height) filled with 100 mg PorapakTMQ adsorbent (Waters Company, Milford, MA, USA), and another air pump in same type (for airflow stream dewing out). The glass jar was cleaned with soap, washed with distilled water and acetone, and then heated at 40 °C for 6 h before collection/operation. The VOC trap was activated at 200 °C for 30 min before use. Four hundred grams of peanuts were sealed in a glass jar. The airflow was regulated at 300 mL/min by a flow meter. All dynamic headspace sampling parts without peanuts were run for 90 min for cleaning. The VOCs were sampled four times for each variety of peanut. Each collection was continued in a 12 h period. The air VOCs with no peanuts were collected as control. The VOCs were eluted from the adsorbent with 1 mL n-hexane (analytical grade, Aladdin, Shanghai, China), concentrated to 200 μL by a nitrogen evaporator (DN-36A, Shanghai Bilang Instrument Manufacturing Co., Ltd., Shanghai, China), and immediately stored at −80 °C. All measurements were carried out at 27 ± 1 °C, 70 ± 5% r.h., and a 16 L:8 D photoperiod.
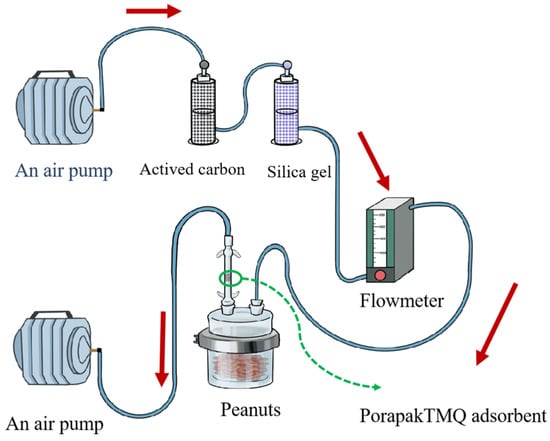
Figure 2.
A schematic diagram of dynamic headspace collection systems for VOCs from peanuts.
The VOCs were analyzed by a QP2010 gas chromatography (GC)/mass spectrometer (Shimadzu Co., Ltd., Kyoto, Japan) equipped with a DB-5MS quartz capillary column (30 m × 0.25 mm × 0.25 μm; Agilent Technologies, Santa Clara, CA, USA). The collected samples (1 μL) were injected in splitless mode at a 250 °C injector temperature. Helium was used as carrier gas at 1.0 mL/min. The analytical conditions were as follows: The column oven was set to 35 °C for 3 min and then ramped up to 125 °C at 4 °C/min and held for 3 min. Subsequently, the temperature was increased to 165 °C at 4 °C/min and maintained for 3 min, and it was ultimately heated to 250 °C at 10 °C/min and remained for 5 min. In electron ionization mass spectra (EI) mode, the ionization voltage was 70 eV, the ion source temperature was 230 °C, the detector temperature was 250 °C, and the scan range of the mass spectra was 45–500 m/z. The VOCs were preliminarily identified by their mass spectra by comparing them using the NIST14.0 and Wiley 275 libraries. Positive identifications were confirmed when the spectra displayed a library match factor exceeding 90%. Further, the VOCs’ identification was made by comparing their retention index to those from the NIST-08 mass spectra library [26]. The retention indices were calculated based on the retention time of a sequence of alkanes C8–C40 for each selected VOC.
2.5. Chemicals
The tested chemicals, simulated with VOCs from the peanuts, included dodecane (99%), tetradecane (95%), hexadecane (98%), heptadecane (95%), decanal (95%) acetophenone (98%), 4-ethyl-benzaldehyde (98%), and 3,4-dimethyl acetophenone (98%); they were purchased from Aladdin Biochemical Technology Co., Ltd. (Shanghai, China). Nonanal (95%), 2,5-dimethyl-benzaldehyde (95%), and 2,4-di-tert-butylphenol (99%) were purchased from Merck, Inc. (Shanghai, China).
2.6. Electrophysiological Assays
The NOP variety Yuhanghua 1, which exhibited the highest oviposition preference, and the HOP variety Jihua 11, which exhibited the lowest oviposition behavior to P. interpunctella females, were chosen for further analysis. The active VOCs emitted from the varieties Yuhanghua 1 and Jihua 11, which elicited antennal responses in the two-day-old mated females, were identified on a gas chromatograph (8860, Agilent Technologies, Santa Clara, CA, USA) with a quartz capillary column (DB-5MS, 30 m × 0.25 mm × 0.25 μm; Agilent Technologies, Santa Clara, CA, USA) equipped with an electroantennographic detector (EAD) (Syntech, Hilversum, Germany). The collected sample (1 μL) was injected (splitless mode), with the detector set at 250 °C. The high-purity helium (99.99%) served as the carrier, with a 1 mL/min fixed flow rate, for flame ionization detection (FID) and EAD. The column effluent was split in a 1:1 ratio between the FID and EAD. The column heating procedure was consistent with gas chromatography/mass spectrometry (GC-MS) analysis. The diagram combining the GC-EAD analysis is shown in Figure 3a. The female moth head preparation (with antennae) for EAG recordings was indicated in Figure 3b. The moth head was quickly cut off, and then antennae with cut head and (with a 1 mm tip removed) were inserted between two glass capillaries (length of 100 mm, outer diameter of 1.2 mm, and inter diameter of 0.9 mm; Sutter, CA, USA) filled with 0.1 mol/L NaCl solution. The head was inserted into a reference electrode, and the antenna tip was inserted into a recording electrode (Figure 3c). The antenna was placed 1 cm away from the air outlet. The recorded signal was amplified and converted by an IDAC-2 data-acquisition controller (Syntech, Hilversum, Germany), and the data acquisition for GC with EAD was analyzed by GC-EAD version 2014 v 1.2.5 software (Syntech, Hilversum, Germany). The VOC was confirmed if the antennal EAD peak matched the FID peaks of VOCs eluting from GC. The verification was conducted with five or more individual insects separately.
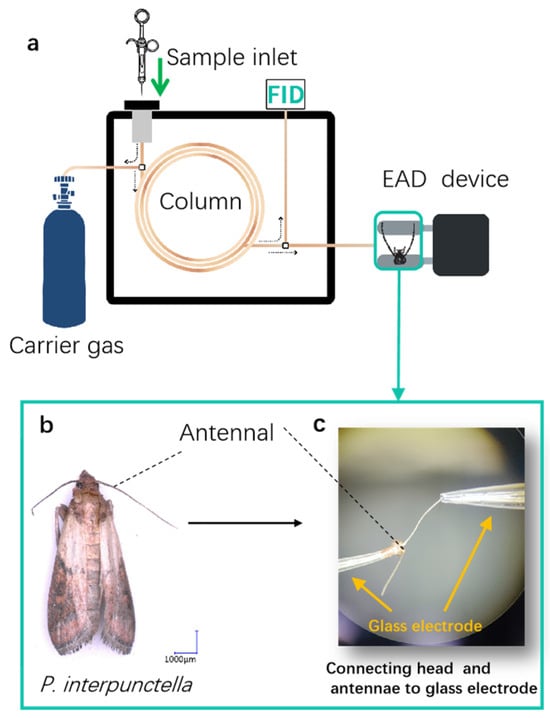
Figure 3.
Identification of bioactive VOCs eliciting antennal responses in Plodia interpunctella females through gas chromatography/electroantennographic detection (GC-EAD). (a) A schematic representation of GC-EAD analysis. Dashed arrows indicate the flow of the carrier gas. (b) Plodia interpunctella female, from which the head (including antenna) was harvested. (c) A close-up view reveals the detailed arrangement of the head, antennal, and glass electrodes.
Acetophenone, dodecane, 2,4-di-tert-butylphenol, heptadecane, 4-ethyl-benzaldehyde, 2,5-dimethyl-benzaldehyde, 3,4-dimethyl-phenylethanone, tetradecane, and hexadecane, along with nonanal and decanal, which were more abundant in NOP than in HOP varieties, were dissolved in paraffin oil with a 1, 10, 50, and 100 μg/μL concentration, respectively. The antennae were connected following the method described in GC-EAD operations. After evaporation for 30 s, the tested chemicals (10 μL) were dripped onto a 0.43 cm2 filtered paper and put in a glass Pasteur pipette. The tip of the pipette was placed into a small hole in the wall of a glass tube, in which a steady airflow (300 mL/min) was delivered onto the prepared antenna. The antennae were delivered as a 0.5 s pulse, with an interval of 60 s between two consecutive stimulations. A solvent control (10 μL of paraffin oil) was tested before and after chemical stimulations. Each VOC was tested at 1, 10, 50, and 100 µg/µL of concentration, respectively. EAG recordings were obtained from six females one by one for the VOCs. The EAG response values of the controls were averaged before and after stimulation for each VOC assay. The EAG response values were calculated as CT − (CK1 + CK2)/2, where CT was the EAG amplitude of VOCs, and CK1 and CK2 were EAG responses to the first and the second control stimuli.
The EAG response was assayed to be stronger by a value ≥ 0.5 mV, moderate by 0.15 mV–0.5 mV, and weak by a value < 0.15 mV.
2.7. Y-Tube Olfactometer Bioassay
The selecting behavior response of the females to 11 VOCs was evaluated by a Y-tube olfactometer with an inner diameter of 4 cm, a common tube 30 cm in length, and two arms of 20 cm in length. The angle of the two arms was 75°. Each VOC was prepared in paraffin oil at a concentration of 50 μg/μL, and paraffin oil served as the control. The airflow was 300 mL/min; it passed through an activated carbon air filter, a bottle of distilled water, a flowmeter, and two odor containers; and it entered the olfactory arms finally. Them 10 μL of test solution was dropped onto a filter paper strip (3 cm × 0.5 cm) and placed in the odor container. The moth was recorded as making a choice if it crossed over 10 cm into one of two olfactory arms in 5 min and stayed for 10 s or more. The olfactory arms were cleaned with anhydrous ethanol and distilled water and dried at 150 °C for each group of five moths. A total of 60 moths were tested separately for each VOC. The bioassay was conducted under dark conditions at 27 ± 1 °C and 70 ± 5% r.h. The results of Y-tube olfactometer bioassays were expressed in terms of attractive rate and repellent rate, according to the following formula:
Attractive rate (%) = the number of females in the treatment arm/the number of females tested × 100%
Repellent rate (%) = the number of females in the control arm/the number of females tested × 100%
2.8. Wind Tunnel Bioassay
Nonanal, decanal, dodecane, hexadecane, acetophenone, 4-ethylbenzaldehyde, and 2,5-dimethyl-benzaldehyde (50 μg/μL), which evoked olfactory reactions in the females in the Y-tube olfactometer assay, were measured further by the wind tunnel test bioassay, with paraffin oil as the control. A wind tunnel (200 cm length × 60 cm height × 60 cm width) was used at 25 ± 2 °C, 70 ± 5% r.h., under scotophase, from 17:00 to 00:00 [27]. The filtered air was blown by a centrifugal fan and exited by another same type of fan, with an airflow of 50 cm/s. Two lamps (40 W, fitted with a red filter) were placed 10 cm above the tunnel, with an intensity of 10 lux for light condition. The rubber soaked with 20 μL of tested solution was placed in a petri dish (10 cm in diameter) affixed to a metal stand (15 cm in height), which was positioned 15 cm from the upwind end of the wind tunnel. A two-day-old mated female moth was placed in a glass tube (2.5 cm in diameter and 12.5 cm in height) affixed to another metal stand, 15 cm from the floor and 195 cm downwind from the odor sources. A female was released downwind into the wind tunnel and tested for 5 min. After each test, clean air was introduced into the wind tunnel for 15 min to eliminate any potential air pollution. The behavior responses of females to VOCs were recorded as follows: taking off; upwind flight (reaching 100 cm from the VOCs or halfway through the wind tunnel); approaching the source of VOCs (reaching 20 cm from the VOCs); and landing on the source of VOCs. Each treatment was carried out with 30 females, and each female was used only once. Three replications were performed for each treatment. The wind tunnel was cleaned with 75% alcohol and distilled water before each treatment.
2.9. Data Analysis
The data on egg number on tested peanuts, EAG response value among tested concentrations of each VOC, and the behavior response of female in the wind tunnel bioassay were analyzed by one-way analysis of Variance (ANOVA), followed by Duncan’s multiple range test (p < 0.05). The behavioral response of female moths to VOCs was analyzed via the χ2 test to examine the null hypothesis that the moths showed equal preference for the VOCs and the control. All statistical analyses were performed using IBM SPSS Statistics 26.0 (IBM Corp., Armonk, NY, USA).
3. Results
3.1. The Oviposition Preference of Plodia interpunctella Females
There was a significant difference in the number of eggs among the eight tested peanut varieties (F = 9.163; df = 7, 63; p < 0.001) (Figure 4), especially between NOP varieties (20–28 eggs) and HOP ones (9–12 eggs) (p < 0.05) (Figure 4). The order of egg production of peanut varieties from high to low is Yuhanghua 1 > Yuhua 9326 > Puhua 28 > Pukehua 5 > Tianfu 3 > Jihua 13 > Kainong 301 > Jihua 11. The NOP variety Yuhanghua 1 exhibited the highest preference for oviposition, with an egg production 3.1 times that of HOP Jihua 11.
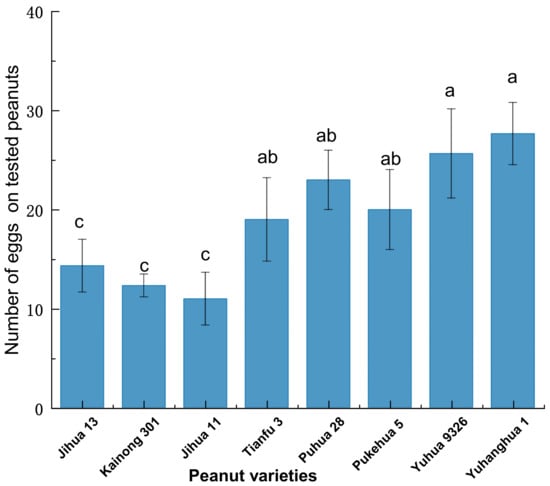
Figure 4.
The oviposition preference comparison of Plodia interpunctella females among eight peanut varieties. Bars represent the number of eggs (mean ± SE) deposited by an individual female in 72 h. Distinct lowercase letters are employed to indicate statistically significant differences according to Duncan’s multiple range test (p < 0.05).
3.2. Chemical Analysis
Forty-two VOCs from all of the tested varieties were analyzed via the GC-MS method, including 23 VOCs from Yuhanghua 1, 17 VOCs from Yuhua 9326, 10 VOCs from Puhua 28, 14 VOCs from Pukehua 5, 11 VOCs from Tianfu 3, 15 VOCs from Jihua 13, 16 VOCs from Kainong 301, and 20 VOCs from Jihua 11 (Table 2). These VOCs mainly included alkanes, aldehydes, and ketones, along with fewer types of alcohols, acids, and esters.

Table 2.
Volatile organic compounds detected from eight varieties of peanuts by GC-MS method.
Dodecane was common to all NOP and HOP varieties. The relative content of dodecane in NOP varieties was higher than that in HOP varieties. Hexadecane was identified in all tested varieties, except for Pukehua 5, while 3,7-dimethyldecane was detected in all except for Jihua 13. Heptadecane and tetradecane were detected in most varieties, but heptane was not found in Pukehua 5 and Tianfu 3. Similarly, tetradecane was not found in the varieties Pukehua 5, Tianfu 3, and Jihua 13. Tridecane was identified in both Yuhua 9326 and Pukehua 5. The VOCs 5-methyl-5-propylnonane and 2-methyl hexacosane were measured in Yuhanghua 1, while decane was only detected in Pukehua 5.
Nonanal was the common VOC in all NOP and HOP varieties. In addition, decanal was absent from the NOP varieties Puhua 28 and Jihua 13. The relative contents of nonanal and decanal in NOP varieties were higher compared to those in HOP varieties. Benzaldehyde was uniquely identified in Pukehua 5, whereas 2,5-dimethyl-benzaldehyde was found in the HOP variety Jihua 11. The relative content of 4-ethyl-benzaldehyde in three HOP varieties was higher compared to that in the NOP variety Jihua 11 (p < 0.05).
The VOC 3,4-dimethyl-phenylethanone was identified in two NOP varieties, Yuhanghua 1 and Yuhua 9326, along with three HOP varieties. The relative content of 3,4-dimethyl-phenylethanone in three HOP varieties was higher compared to two NOP ones (p < 0.05). Acetophenone was detected in two NOP varieties, Yuhanghua 1 and Yuhua 9326. The VOC 6-methyl-5-hepten-2-one was identified in Pukehua 5.
The VOC 2,4-di-tert-butylphenol was identified in two NOP varieties, Yuhanghua 1 and Yuhua 9326, along with three HOP varieties. Additionally, 3-butyn-1-ol was identified in Tianfu 3 and Pukehua 5; 1-hexanol was detected in Puhua28 and Tianfu 3. The VOC 1-octen-3-ol was identified in Tianfu 3.
Hexanoic acid was identified in Pukehua 5 and Tianfu 3, while benzoic acid was uniquely detected in Tianfu 3. Additionally, butanedioic acid and diethyl ester were analyzed in the HOP variety Jihua 13.
3.3. Antennal Responses of the Females to Volatile Organic Compounds
Acetophenone, 3,7-dimethyl-decane, dodecane, 3,4-dimethyl phenylmethanone, hexadecane, heptadecane, tetradecane, 4-ethyl-benzaldehyde, 2,5-dimethyl-benzaldehyde, and 2,4-di-tert-butylphenol elicited the EAG responses in females (Figure 5).
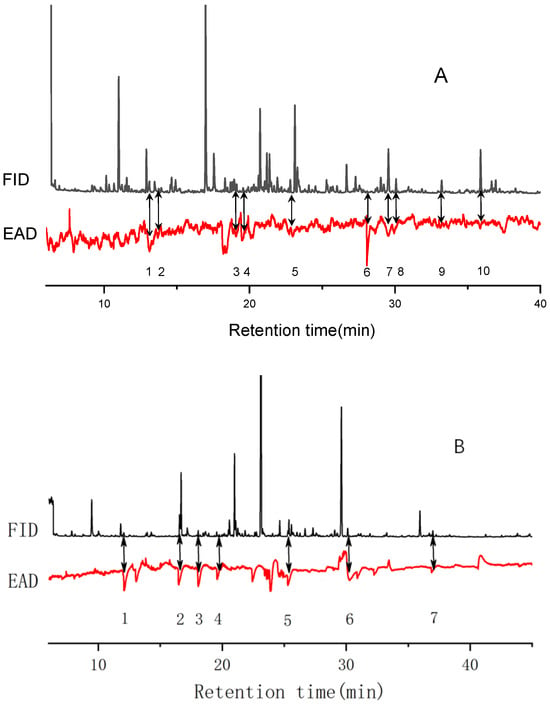
Figure 5.
Responses of P. interpunctella females to Yuhanghua 1 (A) and Jihua11 (B) VOC detected by GC−EAD. In the panel A, the numbers of 1-10 represent the compounds of unidentified, acetophenone, dodecane, unidentified, tetradecane, unidentified, unidentified, 2,4-di-tert-butylphenol, hexadecane, unidentified, respectively; In the panel B, the numbers of 1-7 represent the compounds of 3,7-dimethyl-decane, 4-ethyl-benzaldehyde, 2,5-dimethyl-benzaldehyde, 3,4-dimethyl-phenylethanone, tetradecane, 2,4-di-tert-butylphenol, heptadecane, respectively.
As the VOC dose increased from 1 to 100 μg/μL, the EAG response of females to 2,4-di-tert-butylphenol, 2,5-dimethylbenzaldehyde, and tetradecane increased, while the responses to heptadecane decreased (Figure 6). The EAG responses of females to decanal, hexadecane, nonanal, 4-ethyl-benzaldehyde, 3,4-dimethyl-phenylethanone, acetophenone, and dodecane increased with concentrations and saturated at 50 μg/μL before decreasing (Figure 6). The highest EAG response was elicited by 50 μg/μL of 4-ethyl-benzaldehyde (EAG value of 1.39 mV, as noted below), 100 μg/μL of 2,5-dimethylbenzaldehyde (0.78 mV), 50 μg/μL of decanal (0.56 mV), and 50 μg/μL of acetophenone (0.45 mV), followed by nonanal, 3,4-dimethyl-phenylethanone, and hexadecane at 50 μg/μL, as well as heptadecane at 1 μg/μL (between 0.15 and 0.3 mV). The responses elicited by dodecane, tetradecane, and 2,4-di-tert-butylphenol are very weak.
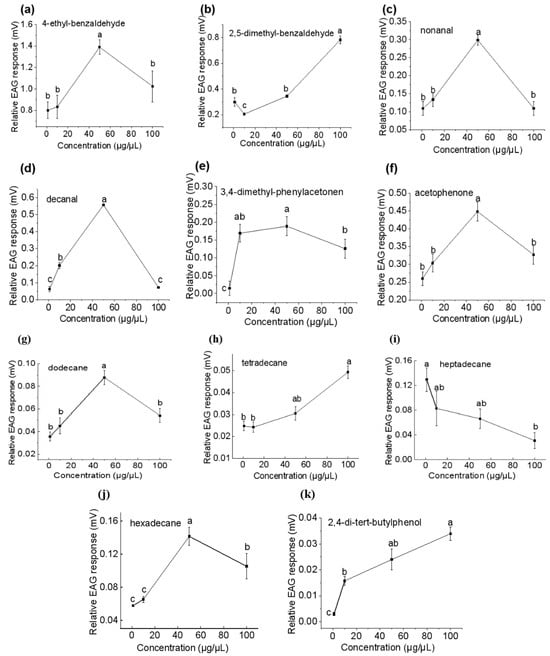
Figure 6.
Comparative electroantennography responses (mean ± SE) of Plodia interpunctella females to volatile organic compounds at different concentrations (1, 10, 50, and 100 µg/µL): (a) 4-ethylbenzaldehyde; (b) 2,5-dimethyl benzaldehyde; (c) nonanal; (d) decanal; (e) 3,4-dimethyl phenylethanone; (f) acetophenone; (g) dodecane; (h) tetradecane; (i) heptadecane; (j) hexadecane; and (k) 2,4-di-tert-butylphenol. Different letters indicate significant differences (one-way ANOVA, Duncan’s multiple range test, p < 0.05).
3.4. Behavioral Responses
In the bioassay of Y-tube olfactometer, females were significantly more attracted to dodecane (χ2 = 15.338; p < 0.001), acetophenone (χ2 = 7.200; p = 0.007), hexadecane (χ2 = 13.474; p < 0.001), and nonanal (χ2 = 14.450; p < 0.001) than that to the paraffin oil control. The females had weaker attraction to decanal (χ2 = 4.457; p = 0.035), significant repulsion to 2,5-dimethyl-benzaldehyde (χ2 = 12.188; p < 0.001), and weak repulsion to 4-ethyl-benzaldehyde (χ2 = 5.232; p = 0.022). However, the females were neither attracted nor repelled by tetradecane, 2,4-di-tert-butylphenol (χ2 = 0.156; p = 0.814), heptadecane (χ2 = 0.690; p = 0.406), and 3,4-dimethyl phenylmethanone (χ2 = 0.462; p = 0.497) (Figure 7).
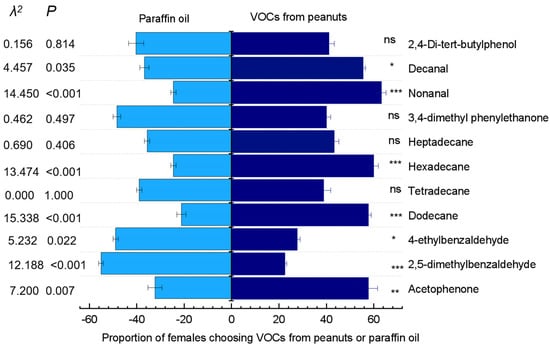
Figure 7.
Behavioral responses of Plodia interpunctella females in the Y-tube olfactometer to test compounds at 50 µg/µL and the paraffin oil control. Thirty insects were tested per treatment. The dark blue bars on the right side and the light blue bars on the left side represent the responses to the test volatiles and paraffin oil, respectively. * p < 0.05; ** p < 0.01; *** p < 0.001; “ns” indicates no significant difference (χ2-test).
In the wind tunnel bioassay (Figure 8), compared with the control group, acetophenone, nonanal, decanal, and dodecane significantly increased the proportion of female responding in all behavior responses, from taking off to landing on source of VOCs, while 2,5-dimethylbenzaldehyde and 4−ethylbenzaldehyde significantly reduced the proportion of female responding in all behavior responses (p < 0.05), and no significant difference was revealed in female responses to hexadecane (p > 0.05). Among reactive females, acetophenone elicited the highest proportion of all behavior responses, followed by nonanal, decanal, and then dodecane (p < 0.05). Acetophenone and nonanal attracted 29.16% and 22.67% of females to land on the VOC source, respectively, while neither 2,5−dimethylbenzaldehyde nor 4-ethylbenzaldehyde attracted any females to land on the VOC source.
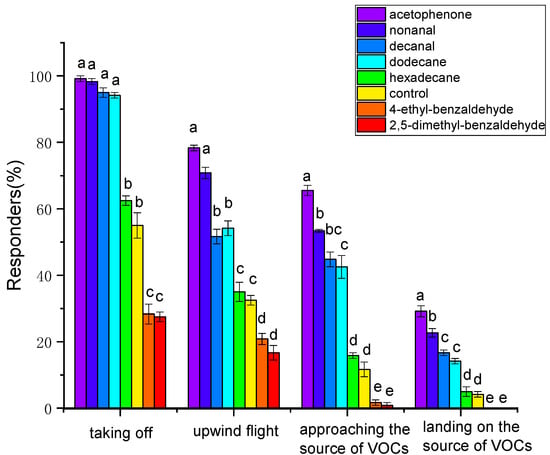
Figure 8.
Behavioral response of Plodia interpunctella females towards 50 µg/µL of test volatile organic compounds in the wind tunnel. Different letters indicate significant differences among different volatile organic compounds (one-way ANOVA, Duncan’s multiple range test, p < 0.05).
4. Discussion
Grains and some foods can be either attractive or repellent to stored-product pests [28,29]. There is little attention paid to the oviposition preference of insects for storing peanuts in their preferences for certain storage products. Our valuable measurements demonstrate that compared to wheat, rice, and corn, some peanuts are significantly more attractive to P. interpunctella females [23]. We also found that P. interpunctella females had higher egg production in normal oleic peanut varieties than in high oleic ones. The larva and adults of R. dominica showed the strongest olfactory preference for wheat, followed by rice, maize, sorghum, and soybean [30]. Males and female maize weevil Sitophilus zeamais (Motschulsky, 1855) (Coleoptera: Curculionidae) were significantly attracted by maize and wheat seeds but were significantly repelled by alligator pepper, ginger, and black pepper [31]. The Stegobium paniceum (Linnaeus, 1758) (Coleoptera: Ptinidae) showed significant preferences for Panax notoginseng (Burkill, 1903) (Apiales: Araliaceae), followed by Angelica sinensis (Oliv., 1872) (Apiales: Apiaceae), Gastrodia elata (Blume, 1826) (Asparagales: Orchidaceae), and Peucedanum praeruptorum (Blume, 1826) (Asparagales: Orchidaceae) [28].
Insects can find and locate suitable food sources, mating, and ovipositing sites by VOCs [30,32]. The behavior responses of several stored grain pests to volatile organic compounds in grains was studied [33,34,35]. P. interpunctella females were attracted by 1-hexanol, phenylacetaldehyde, and 3-methyl-1-butanol, and (E)-2-nonenal from grains [21,22] and repelled by 4-oxoisophorone from the fungi-uninfected grain [22]. This study reports for the first time the behavioral response of P. interpunctella female to volatile organic compounds in different peanut varieties, which may provide valuable insights for the control of P. interpunctella in peanuts.
Active VOCs play an important role in the behavior response of insects and can be usually screened via the GC-EAD method [36,37]. Eleven special VOCs were selected through GC-EAD and GC-MS analyses in our treatments. There were significant differences in the number of compound kinds and relative content of VOCs among NOP and HOP varieties. Acetophenone was checked in NOP Yuhanghua 1 and Yuhua 9326 and 2,5-dimethyl-benzaldehyde only measured in HOP variety Jihua 11. The VOC 4-ethyl-benzaldehyde was more abundant in three HOP varieties, Kainong 301, Jihua 11, and Jihua 11, than in NOP variety Yuhua 9326. The relative content of dodecane and nonanal in three NOP varieties was higher than that in three HOP ones. The common VOCs, nonanal, dodecane, hexadecane, and decanal, from tested peanuts were also identified from wheat and rice [23]. The acetophenone was unique to NOP varieties and is considered to be a sweet odor; it has been reported in runner-type peanuts (Arachis hypogaea L., variety Georgia Green) [38] and may attract P. interpunctella.
Female moths exhibited strong EAG responses to 4-ethyl-benzaldehyde, 2,5-dimethyl-benzaldehyde, decanal, acetophenone, and nonanal. These results were similar to behaviors of Oryzaephilus surinamensis [39] and Sitophilus oryzae [40]. Y-tube and wind-tunnel assays revealed that dodecane, nonanal, decanal, and acetophenone were attractive to this moth, whereas 4-ethyl-benzaldehyde and 2,5-dimethyl-benzaldehyde had a repellent effect on females.
Nonanal and decanal from NOP and HOP varieties have been taken as attractants to Callosobruchus maculatus (F.) (Fabricius, 1775) (Coleoptera: Chrysomelidae) [41], O. surinamensis [39], T. castaneum [42], and Sitotroga cerealella (Olivier, 1795) (Lepidoptera: Gelechiidae) [43] in Y-tube olfactometer bioassays. Nonanal from whole-wheat flour attracted female moths of P. interpunctella for oviposition at low doses but had no attractive effect at high doses in Y-tube olfactometer bioassay [21]. Low concentrations of 1-hexanol and nonanal were attractive to mated female moths of P. interpunctella in the same bioassay [22]. Our results are consistent with the fact that the relative content of nonanal in NOP is higher than that in HOP ones (p < 0.05), and this fact may explain the significantly higher egg number of P. interpunctella on NOP varieties compared to HOP ones. Decanal was also a major component of the aggregation pheromone for the greater wax moth, Galleria mellonella (Linnaeus, 1758) (Lepidoptera: Pyralidae) [44]. Our result demonstrated decanal had a significant attraction to P. interpunctella females. Similarly, the relative content of decanal in NOP varieties is significantly higher than that in HOP ones (p < 0.05), which contributed to the oviposition preference of P. interpunctella females for NOP varieties.
Acetophenone was primarily detected in preferred oviposition stored products of P. interpunctella, such as wheat and dried fruits [45,46], and also measured in NOP varieties in this research here. In the tested NOP varieties, the unique VOC acetophenone appeared to have a synergistic effect with other VOCs, enhancing the oviposition preference of P. interpunctella. The VOC 4-ethyl-benzaldehyde, which repelled females, was detected to be significantly high level than that in three HOP varieties, Kainong 301, Jihua11, and Jihua 13, compared with NOP variety Yuhua 9326 (p < 0.05). The low oviposition preference in HOP varieties likely resulted from the high content of these deterrent VOCs. The unique 2,5-dimethyl-benzaldehyde, found in HOP Jihua 11 only, may synergistically interact with 4-ethyl-benzaldehyde to enhance its repellent effect on females. This combination of these VOCs likely played a significant role in the low oviposition number observed in Jihua 11. The VOC 3,4-diethylacetophenone, identified as having a high relative content in HOP and NOP varieties, despite eliciting strong electroantennographic (EAG) responses to the females, may not necessarily exert significant attractive effects.
5. Conclusions
Our results demonstrated that P. interpunctella females lay more eggs on NOP than on HOP varieties. The higher relative content of dodecane and nonanal, along with the unique presence of acetophenone in NOP varieties, showed effective attractiveness to females in Y-tube olfactometer and wind tunnel bioassays. In contrast, the higher relative levels of 4-ethyl-benzaldehyde and the exclusive presence of 2,5-dimethyl-benzaldehyde in HOP varieties acted as repellents for the females. The VOCs identified from NOP varieties could be utilized in the development of volatile-based attractants, while some VOCs originating from HOP varieties may serve as ingredients for repellents. This approach could play a crucial role in the integrated management of P. interpunctella for stored product protection. Certainly, future studies should further evaluate the field effectiveness of key VOCs in grain depots or food-processing facilities. Additionally, it is necessary to broaden the range of peanut varieties to provide insights into the diversity of VOC profiles and their impact on P. interpunctella and other stored product-insect behaviors based on these results.
Author Contributions
Conceptualization, D.W. and L.C.; methodology, C.W., X.Z. (Xi Zhu) and X.Z. (Xinxin Zhao); investigation, C.W., F.Z. and X.Z. (Xi Zhu); data analysis, C.W., Y.L. and J.Y.; writing—original draft preparation, C.W., J.Y. and Y.L.; writing—review and editing, D.W., X.Z. (Xi Zhu) and L.C.; supervision, F.Z., X.Z. (Xinxin Zhao) and L.C.; project administration, F.Z. and L.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by China Agriculture Research System of MOF and MARA (CARS-13).
Data Availability Statement
The data presented in this study are available upon request from the corresponding authors.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Wadood, S.A.; Nie, J.; Li, C.L.; Rogers, K.M.; Zhang, Y.Z.; Yuan, Y.W. Geographical origin classification of peanuts and processed fractions using stable isotopes. Food Chem.-X 2022, 16, 104456–104463. [Google Scholar] [CrossRef] [PubMed]
- Gong, A.N.; Shi, A.M.; Liu, H.Z.; Yu, H.W.; Li, L.; Lin, W.J.; Qiang, W. Relationship of chemical properties of different peanut varieties to peanut butter storage stability. J. Integr. Agric. 2018, 17, 1003–1010. [Google Scholar] [CrossRef]
- Jastrombek, J.M.; Faguerazzi, M.M.; de Cássio Pierezan, H.; Rufato, L.; Sato, A.J.; da Silva Ricce, W.; Marques, V.V.; Leles, N.R.; Roberto, S.R. Hop: An emerging crop in subtropical areas in Brazil. Horticulturae 2022, 8, 393. [Google Scholar] [CrossRef]
- Qiang, W.C.; Zhang, J.T.; Yan, Y.S.; Wang, S. Current Situation and Future Directions of High Oleic Peanut Breeding in China. Shandong Agric. Sci. 2018, 50, 171–176. [Google Scholar]
- Ranga Rao, G.; Rameshwar Rao, V.; Nigam, S. Post-Harvest Insect Pests of Groundnut and Their Management; Information Bulletin No. 84; International Crops Research Institute for the Semi-Arid Tropics: Patancheru, India, 2010; 20p, ISBN 978-92-9066-528-1. [Google Scholar]
- Arthur, F.H. Effects of cleaning peanuts on insect damage, insect population growth and insecticide efficacy. Peanut Sci. 2010, 16, 100–105. [Google Scholar] [CrossRef]
- Nesci, A.; Montemarani, A.; Etcheverry, M. Assessment of mycoflora and infestation of insects, vector of Aspergillus section Flavi, in stored peanut from Argentina. Mycotoxin Res. 2011, 27, 5–12. [Google Scholar] [CrossRef]
- Mohandass, S.; Arthur, F.H.; Zhu, K.Y.; Throne, J.E. Biology and management of Plodia interpunctella (Lepidoptera: Pyralidae) in stored products. J. Stored Prod. Res. 2007, 43, 302–311. [Google Scholar] [CrossRef]
- Yaman, M.; Saǧlam, T.; Ertürk, Ö. First Record, Distribution and Occurrence of A Protistan Entomopathogen, Adelina mesnili Perez (Coccidia: Adeleidae) in the Indian Meal Moth, Plodia interpunctella (Hübner) (Lepidoptera: Pyralidae) Populations in Türkiye. Türkiye Parazitolojii Derg. 2023, 47, 151–155. [Google Scholar] [CrossRef]
- Mankin, R.W.; Hagstrum, D.W.; Nansen, C.; Meikle, W.G. Almond moth oviposition patterns in continuous layers of peanuts. J. Stored Prod. Res. 2014, 59, 48–54. [Google Scholar] [CrossRef]
- Warsi, S.; Mbata, G.N. Impact of Peanut Depth and Container Size on the Parasitism of Diapausing and Nondiapausing Larvae of Indian Meal Moth (Lepidoptera: Pyralidae) by Habrobracon hebetor (Hymenoptera: Braconidae). Environ. Entomol. 2018, 47, 1226–1232. [Google Scholar] [CrossRef]
- Pearson, T.; Brabec, D.L. Detection of wheat kernels with hidden insect infestations with an electrically conductive roller mill. Appl. Eng. Agric. 2007, 23, 639–645. [Google Scholar] [CrossRef]
- Perez, J.; Moore, R.; Abney, M.; Toews, M. Species Composition, Temporal Abundance and Distribution of Insect Captures Inside and Outside Commercial Peanut Shelling Facilities. Insects 2020, 11, 110. [Google Scholar] [CrossRef] [PubMed]
- ISO/TC34/SC4; Stored Cereal Grains and Pulses—Guidance on the Detection of Infestation by Live Invertebrates by Trapping. ISO: Geneva, Switzerland, 2004.
- Michereff, M.F.; Borges, M.; Aquino, M.F.; Laumann, R.A.; Mendes Gomes, A.C.; Blassioli-Moraes, M.C. The influence of volatile semiochemicals from stink bug eggs and oviposition-damaged plants on the foraging behaviour of the egg parasitoid Telenomus podisi. Bull. Entomol. Res. 2016, 106, 663–671. [Google Scholar] [CrossRef] [PubMed]
- Wu, A.; Li, X.; Yan, X.; Fan, W.; Hao, C. Electroantennogram responses of Plutella xylostella (L.) to sex pheromone components and host plant volatile semiochemicals. J. Appl. Entomol. 2020, 144, 396–406. [Google Scholar] [CrossRef]
- Zhang, Z.Z. Odorant-binding proteins and chemosensory proteins potentially involved in host plant recognition in the Asian citrus psyllid, Diaphorina citri. Pest Manag. Sci. 2020, 76, 2609–2618. [Google Scholar] [CrossRef]
- Magalhães, D.M.; Borges, M.; Laumann, R.A.; Blassioli Moraes, M.C. Influence of multiple- and single-species infestations on herbivore-induced cotton volatiles and Anthonomus grandis behaviour. J. Pest Sci. 2018, 91, 1019–1032. [Google Scholar] [CrossRef]
- Zhao, J.Y.; Zheng, R.R.; Li, X.F.; Lyu, Z.S.; Ma, L.; Song, C.F.; Qie, X.T.; Yan, X.Z.; Hao, C. Electrophysiological and Behavioral Responses of Plodia interpunctella (Hübner) Females to Aldehyde Volatiles from Dried Fruits. J. Agric. Food Chem. 2023, 71, 17253–17262. [Google Scholar] [CrossRef]
- Ndomo-Moualeu, A.F.; Ulrichs, C.; Adler, C. Behavioral responses of Callosobruchus maculatus to volatile organic compounds found in the headspace of dried green pea seeds. J. Pest Sci. 2016, 89, 107–116. [Google Scholar] [CrossRef]
- Uechi, K.; Matsuyama, S.; Suzuki, T. Oviposition attractants for Plodia interpunctella (Hübner) (Lepidoptera: Pyralidae) in the volatiles of whole wheat flour. J. Stored Prod. Res. 2007, 43, 193–201. [Google Scholar] [CrossRef]
- Būda, V.; Apšegaitė, V.; Blažytė-Čereškienė, L.; Butkienė, R.; Nedveckytė, I.; Pečiulytė, D. Response of moth Plodia interpunctella to volatiles of fungus-infected and uninfected wheat grain. J. Stored Prod. Res. 2016, 69, 152–158. [Google Scholar] [CrossRef]
- Jiang, B.R.; Wang, D.X.; Zhang, L.K.; Chen, L.; Jing, J.G.; Li, Z.H.; Tang, P.A. Comparison on Oviposition Preference of Plodia interpunctella (Hübner) on Grain and Peanut Kernels. J. Henan Univ. Technol. 2019, 40, 86–93. [Google Scholar]
- Astuti, L.P.; Rizali, A.; Tanzilia, S. Seed coat and variety of peanut inhibit host preference and development of Oryzaephilus mercator. J. Stored Prod. Res. 2018, 78, 98–104. [Google Scholar] [CrossRef]
- Huang, Y.L.; Wang, D.X.; Jian, F.J. Survival of Plodia interpunctella (Hübner) larvae treated with 98% N2 and the life history of their next generation. Bull. Entomol. Res. 2023, 113, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.S.; Zhao, N.N.; Liu, Q.Z.; Liu, Z.L.; Du, S.S.; Zhou, L.G.; Deng, Z.W. Repellent Constituents of Essential Oil of Cymbopogon distans Aerial Parts against Two Stored-Product Insects. J. Agric. Food Chem. 2011, 59, 9910–9915. [Google Scholar] [CrossRef] [PubMed]
- Dev, P.; Kennedy, J.; Srinivasan, T.; Arthanari, P.M. Dose Optimization in a Wind Tunnel to Determine the Effective Concentration preferred by Male Fall Armyworm Moths. Biol. Forum 2022, 14, 96–100. [Google Scholar]
- Cao, Y.; Li, S.; Benelli, G.; Germinara, G.S.; Yang, J.; Yang, W.J.; Li, C. Olfactory responses of stegobium paniceum to different chinese medicinal plant materials and component analysis of volatiles. J. Stored Prod. Res. 2018, 76, 122–128. [Google Scholar] [CrossRef]
- Trematerra, P.; Sciarreta, A.; Tamasi, E. Behavioural responses of Oryzaephilus surinamensis, Tribolium castaneum and Tribolium confusum to naturally and artificially damaged durum wheat kernels. Entomol. Exp. Appl. 2000, 94, 195–200. [Google Scholar] [CrossRef]
- Cao, Y.; Jian, L.; Athanassiou, C.G.; Yang, Y.; Hu, Q.; Zhang, X.Y.; Dai, F.L.; Maggi, F. Behavioral responses of Rhyzopertha dominica (F.) to volatiles of different stored grains. J. Stored Prod. Res. 2024, 105, 102235–102242. [Google Scholar] [CrossRef]
- Ukeh, D.A.; Birkett, M.A.; Bruce, T.J.; Allan, E.J.; Pickett, J.A.; Luntz, A.J. Behavioural responses of the maize weevil, Sitophilus zeamais, to host (stored-grain) and non-host plant volatiles. Pest Manag. Sci. 2010, 66, 44–50. [Google Scholar] [CrossRef]
- Tsuchida, T. Molecular basis and ecological relevance of aphid body colors. Curr. Opin. Insect Sci. 2016, 17, 74–80. [Google Scholar] [CrossRef]
- Germinara, G.S.; De Cristofaro, A.; Rotundo, G. Behavioral responses of adult Sitophilus granarius to individual cereal volatiles. J. Chem. Ecol. 2008, 34, 523–529. [Google Scholar] [CrossRef] [PubMed]
- Olsson, P.O.; Anderbrant, O.; Lofstedt, C.; Borg-Karlson, A.K.; Liblikas, I. Electrophysiological and behavioral responses to chocolate volatiles in both sexes of the pyralid moths Ephestia cautella and Plodia interpunctella. J. Chem. Ecol. 2005, 31, 2947–2961. [Google Scholar] [CrossRef] [PubMed]
- Nansen, C.; Phillips, T.W. Ovipositional Responses of the Indianmeal Moth, Plodia interpunctella (Hübner) (Lepidoptera: Pyralidae) to Oils. Ann. Entomol. Soc. Am. 2003, 96, 524–531. [Google Scholar] [CrossRef]
- Li, C.; Cao, J.; Wang, X.; Xu, P.; Wang, X.; Ren, G. Efficacy of an improved method to screen semiochemicals of insect. PeerJ 2021, 9, e11510. [Google Scholar] [CrossRef]
- Xiang, L.; Zhang, X.G.; Chun, X.; Gao, Y.L.; Dong, W.X. Behavioral responses of potato tuber moth (Phthorimaea operculella) to tobacco plant volatiles. J. Integr. Agric. 2020, 19, 325–332. [Google Scholar]
- Schirack, A.; Drake, M.; Sanders, T.; Sandeep, K. Characterization of aroma-active compounds in microwave blanched peanuts. J. Food Sci. 2006, 71, C513–C520. [Google Scholar] [CrossRef]
- Sabier, M.; Wang, J.R.; Zhang, T.; Jin, J.D.; Wang, Z.J.; Shen, B.; Deng, J.; Liu, X.; Zhou, G. The attractiveness of a food-based lure and its component volatiles to the stored-grain pest Oryzaephilus surinamensis (L.). J. Stored Prod. Res. 2022, 98, 102000–102011. [Google Scholar] [CrossRef]
- Cao, Y.; Hu, Q.; Huang, L.; Athanassiou, C.G.; Maggi, F.; D’Isita, I.; Liu, Y.Y.; Pistillo, O.M.; Miao, M.Z.; Germinara, G.S.; et al. Attraction of Sitophilus oryzae (L.) (Coleoptera: Curculionidae) to the semiochemical volatiles of stored rice materials. J. Stored Prod. Res. 2024, 97, 73–85. [Google Scholar] [CrossRef]
- Adhikary, P.; Mukherjee, A.; Barik, A. Attraction of Callosobruchus maculatus (F.) (Coleoptera: Bruchidae) to four varieties of Lathyrus sativus L. seed volatiles. Bull. Entomol. Res. 2015, 105, 187–201. [Google Scholar] [CrossRef]
- Balakrishnan, K.; Holighaus, G.; Weissbecker, B.; Schutz, S. Electroantennographic responses of red flour beetle Tribolium castaneum Herbst (Coleoptera: Tenebrionidae) to volatile organic compounds. J. Appl. Entomol. 2017, 141, 477–486. [Google Scholar] [CrossRef]
- Fouad, H.A.; Faroni, L.R.D.; Vilela, E.F.; de Lima, E.R. Flight responses of Sitotroga cerealella (Lepidoptera: Gelechiidae) to corn kernel volatiles in a wind tunnel. Arthropod-Plant Interact. 2013, 7, 651–658. [Google Scholar] [CrossRef]
- Kwadha, C.A.; Mutunga, J.M.; Irungu, J.; Ongamo, G.; Ndegwa, P.; Raina, S.; Fombong, A.T. Decanal as a major component of larval aggregation pheromone of the greater wax moth, Galleria mellonella. J. Appl. Entomol. 2019, 143, 417–429. [Google Scholar] [CrossRef]
- Chen, Q.; Zhu, X.; Kang, G.; Yu, Q.; Liu, Q.; Du, L.; Yang, Y.; He, X.; Zhao, Y.; Zhang, J.; et al. Identification and functional characterization of female antennae-biased odorant receptor 23 involved in acetophenone detection of the Indian meal moth Plodia interpunctella. Insect Sci. 2024, 31, 59–78. [Google Scholar] [CrossRef] [PubMed]
- Mattiolo, E.; Licciardello, F.; Lombardo, G.M.; Muratore, G.; Anastasi, U. Volatile profiling of durum wheat kernels by HS–SPME/GC–MS. Eur. Food Res. Technol. 2017, 243, 147–155. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).