Multigenerational Rearing on Non-Prey Foods Does Not Affect Prey (Aphid) Recognition Behavior of Coleomegilla maculata (Coleoptera: Coccinellidae)
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Coccinellid and Aphid Cultures
2.2. Experimental Design and Treatments
2.3. Statistical Analysis
3. Results
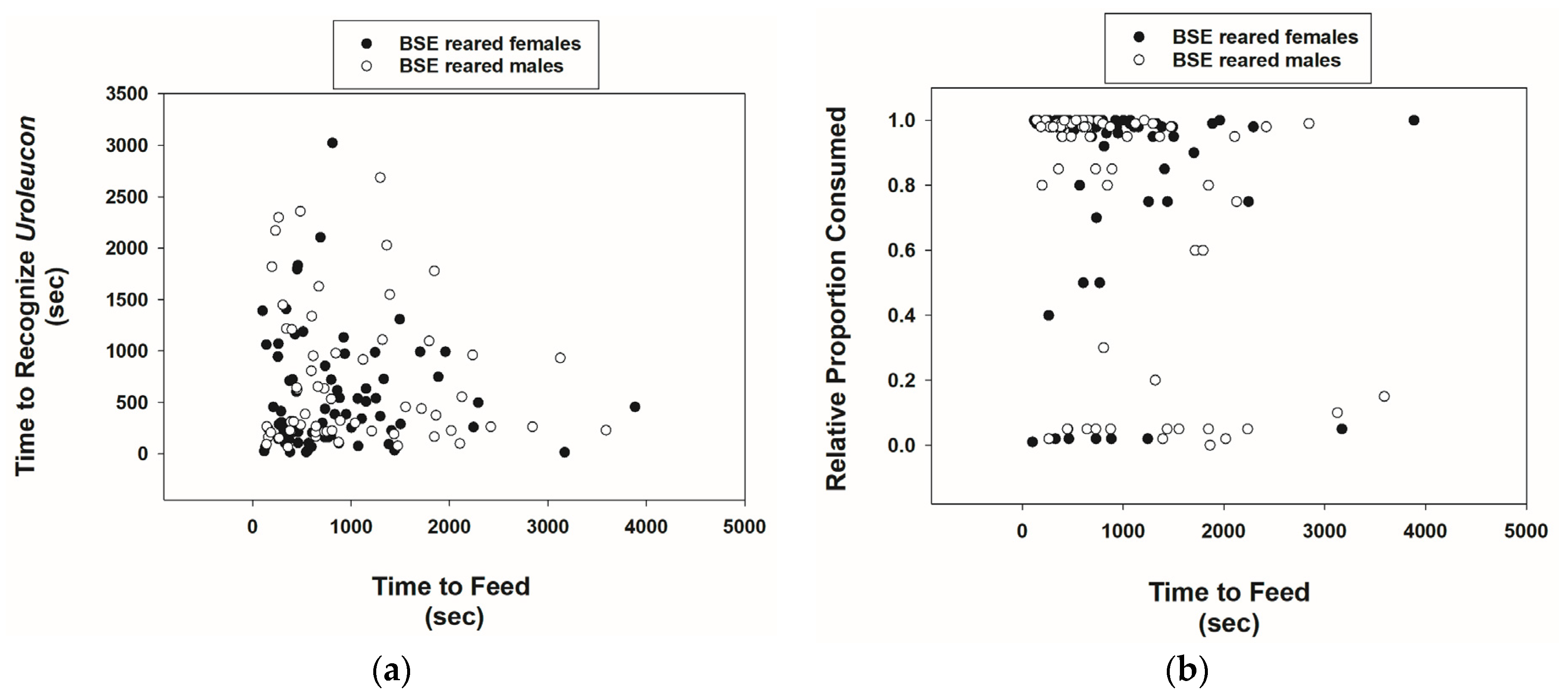
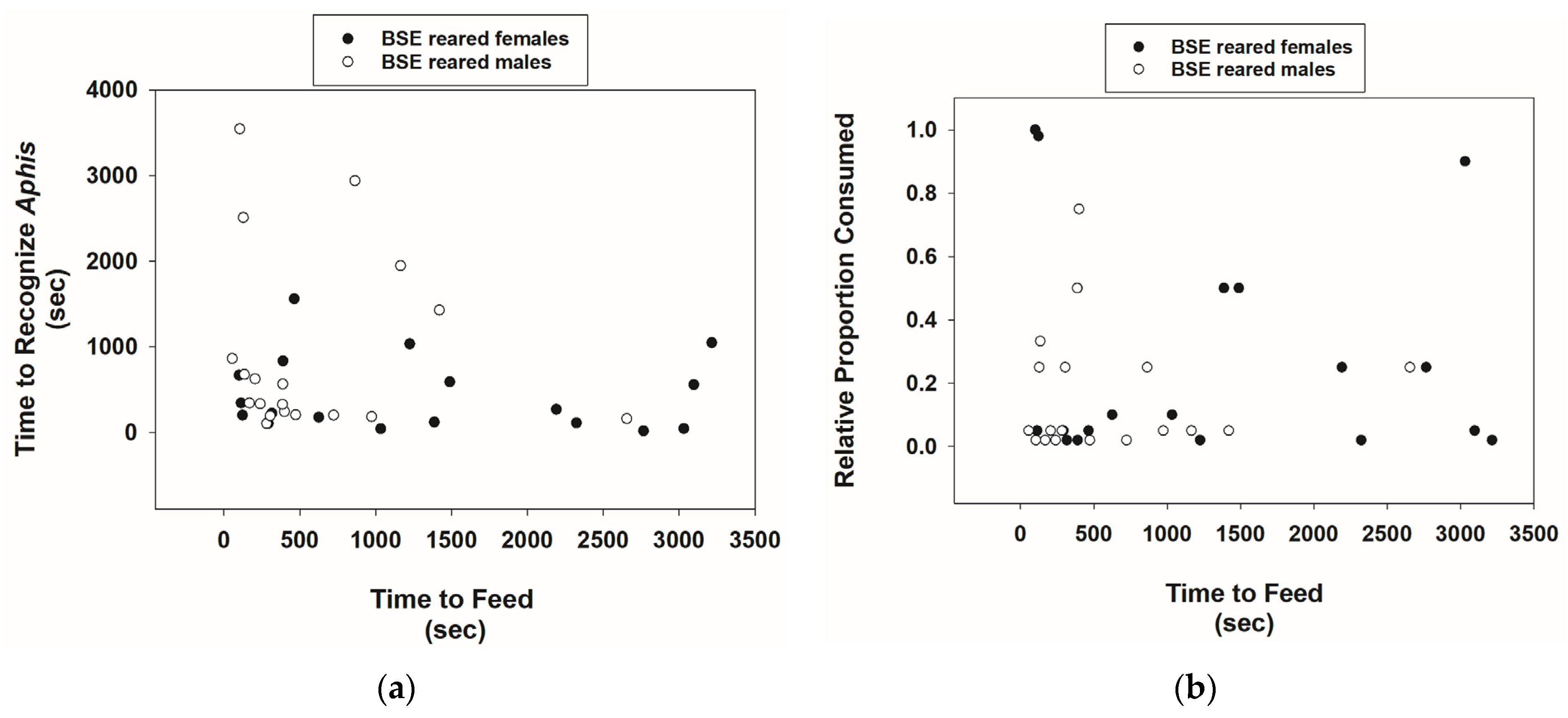
3.1. Prey Recognition and Consumption Behavior of Cmac Adults Reared on BSE Diet
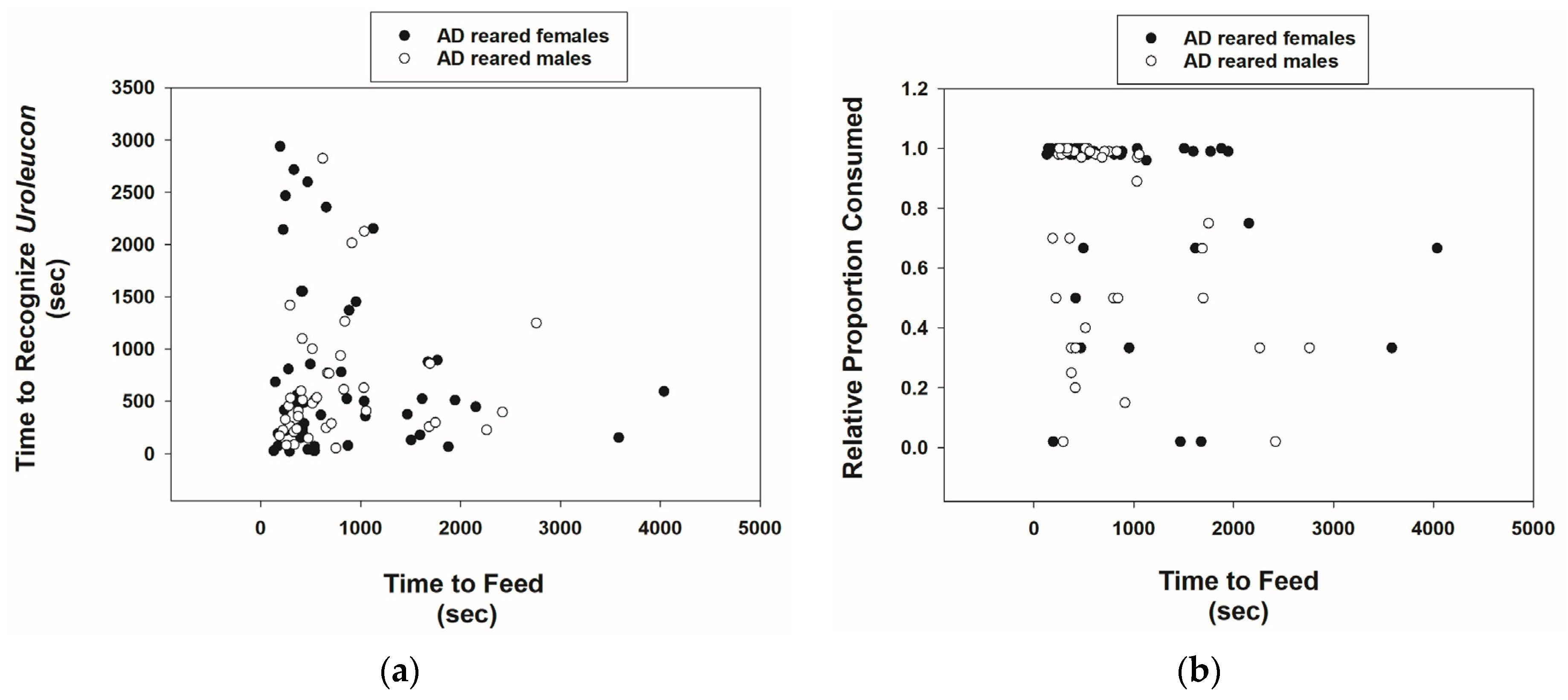
3.2. Prey Recognition and Consumption Behavior of Cmac Adults Reared on AD Diet
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gordon, R.D. The Coccinellidae (Coleoptera) of America north of Mexico. J. N. Y. Entomol. Soc. 1985, 93, 1–912. [Google Scholar]
- Hesler, L.; Brust, M. Expanded geographical distribution of Coleomegilla maculata lengi (Coleoptera: Coccinellidae) in North America. Insects 2024, 15, 305. [Google Scholar] [CrossRef] [PubMed]
- McAlpine, D.F.; Migneault, R.; Webster, R.P. Coleomegilla maculata lengi Timberlake, 1943 (Coleoptera: Coccinellidae), a native North American lady beetle new to Maritime Canada. J. Acadian Entomol. Soc. 2018, 14, 8–10. [Google Scholar]
- Michaud, J.P. Coccinellids in biological control. In Ecology and Behaviour of the Ladybird Beetles (Coccinellidae); Hodek, I., van Emden, H.F., Honek, A., Eds.; Wiley-Blackwell: Chichester, UK, 2012; pp. 488–519. [Google Scholar]
- Cottrell, T.E.; Yeargan, K.V. Effect of pollen on Coleomegilla maculata (Coleoptera: Coccinellidae) population density, predation, and cannibalism in sweet corn. Environ. Entomol. 1998, 27, 1402–1410. [Google Scholar] [CrossRef]
- Lundgren, J.G.; Wiedenmann, R.N. Nutritional suitability of corn pollen for the predator Coleomegilla maculata (Coleoptera: Coccinellidae). J. Insect Physiol. 2004, 50, 567–575. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.-X.; Hao, Y.-N.; Riddick, E.W.; Liu, T.-X. Factitious prey and artificial diets for predatory lady beetles: Current situation, obstacles, and approaches for improvement: A review. Biocontrol Sci. Technol. 2017, 27, 601–619. [Google Scholar] [CrossRef]
- Riddick, E.W. Production of coleopteran predators. In Mass Production of Beneficial Organisms: Invertebrates and Entomopathogens, 2nd ed.; Morales-Ramos, J.A., Rojas, M.G., Shapiro-Ilan, D.I., Eds.; Academic Press: London, UK, 2023; Chapter 2; pp. 13–36. [Google Scholar]
- Huynh, M.P.; Shelby, K.S.; Coudron, T.A. Recent advances in insect rearing methodology to promote scientific research and mass production. Insects 2021, 12, 961. [Google Scholar] [CrossRef] [PubMed]
- van Lenteren, J.C. Need for quality control of mass-produced biological control agents. In Quality Control and Production of Biological Control Agents: Theory and Testing Procedures; van Lenteren, J.C., Ed.; CABI Publishing: Oxon, UK, 2003; Chapter 1; pp. 1–18. [Google Scholar]
- Grenier, S.; DeClercq, P. Comparison of artificially vs. naturally reared natural enemies and their potential for use in biological control. In Quality Control and Production of Biological Control Agents: Theory and Testing Procedures; van Lenteren, J.C., Ed.; CABI Publishing: Oxon, UK, 2003; Chapter 9; pp. 115–131. [Google Scholar]
- van Lenteren, J.C.; Tommasini, M.G. Mass production, storage, shipment, and release of natural enemies. In Quality Control and Production of Biological Control Agents: Theory and Testing Procedures; van Lenteren, J.C., Ed.; CABI Publishing: Oxon, UK, 2003; Chapter 12; pp. 181–189. [Google Scholar]
- van Lenteren, J.C.; Hale, A.; Klapwijk, J.N.; van Schelt, J.; Steinberg, S. Guidelines for quality control of commercially produced natural enemies. In Quality Control and Production of Biological Control Agents: Theory and Testing Procedures; van Lenteren, J.C., Ed.; CABI Publishing: Oxon, UK, 2003; Chapter 19; pp. 265–303. [Google Scholar]
- Leppla, N.C. Concepts and methods of quality assurance for mass-reared parasitoids and predators. In Mass Production of Beneficial Organisms: Invertebrates and Entomopathogens, 2nd ed.; Morales-Ramos, J.A., Rojas, M.G., Shapiro-Ilan, D.I., Eds.; Academic Press: London, UK, 2023; Chapter 9; pp. 261–290. [Google Scholar]
- Morales-Ramos, J.A.; Rojas, M.G.; Coudron, T.A.; Huynh, M.P.; Zou, D.; Shelby, K.S. Artificial diet development for entomophagous arthropods. In Mass Production of Beneficial Organisms: Invertebrates and Entomopathogens, 2nd ed.; Morales-Ramos, J.A., Rojas, M.G., Shapiro-Ilan, D.I., Eds.; Academic Press: London, UK, 2023; Chapter 8; pp. 233–260. [Google Scholar]
- Riddick, E.W.; Walker, R.C.; Rojas, M.G.; Morales-Ramos, J.A. Evaluation of black soldier fly Hermetia illucens as food for pink-spotted lady beetle Coleomegilla maculata. Insects 2023, 14, 902. [Google Scholar] [CrossRef] [PubMed]
- Rojas, M.G.; Morales-Ramos, J.A.; Riddick, E.W. Use of Tenebrio molitor (Coleoptera: Tenebrionidae) powder to enhance artificial diet formulation for Coleomegilla maculata (Coleoptera: Coccinellidae). Biol. Control 2016, 100, 70–78. [Google Scholar] [CrossRef]
- Hesler, L.S. Risk to native Uroleucon aphids (Hemiptera: Aphididae) from non-native lady beetles (Coleoptera: Coccinellidae). Entomol. Amer. 2013, 119, 14–22. [Google Scholar] [CrossRef]
- Jensen, A.S.; Miller, G.L.; Carmichael, A. Host range and biology of Uroleucon (Lambersius) erigeronense (Thomas 1878), and its synonymy with Uroleucon (Lambersius) escalantii (Knowlton) 1928 (Hemiptera: Aphidae). Proc. Entomol. Soc. Wash. 2010, 112, 239–245. [Google Scholar] [CrossRef]
- Colvin, S.M.; Yeargan, K.V. Predator fauna associated with oleander aphids on four milkweed species and the effect of those host plants on the development and fecundity of Cycloneda munda and Harmonia axyridis. J. Kans. Entomol. Soc. 2014, 87, 280–298. [Google Scholar] [CrossRef]
- Colvin, S.M.; Snyder, J.C.; Thacker, R.; Yeargan, K.V. Thinking outside the Asclepias box: Oleander aphids and honeyvine milkweed. Ann. Entomol. Soc. Am. 2013, 106, 214–221. [Google Scholar] [CrossRef]
- Rothschild, M.; Euw, J.v.; Reichstein, T. Cardiac glycosides in the oleander aphid, Aphis nerii. J. Insect Physiol. 1970, 16, 1141–1145. [Google Scholar] [CrossRef] [PubMed]
- Malcolm, S.B. Chemical defence in chewing and sucking insect herbivores: Plant-derived cardenolides in the monarch butterfly and oleander aphid. Chemoecology 1990, 1, 12–21. [Google Scholar] [CrossRef]
- Omkar; Srivastava, S. Influence of six aphid species on development and reproduction of a ladybird beetle, Coccinella septempunctata. BioControl 2003, 48, 379–393. [Google Scholar] [CrossRef]
- Osawa, N. The effect of prey availability on ovarian development and oosorption in the ladybird beetle Harmonia axyridis (Coleoptera: Coccinellidae). Eur. J. Entomol. 2005, 102, 503–511. [Google Scholar] [CrossRef]


| Cmac Diet | Cmac Sex | Prey | Time (s) to Recognize Prey | Time (s) of Feeding | Proportion of Prey Consumed |
|---|---|---|---|---|---|
| BSE | Male | U. erigeronense | 698.33 ± 78.91 a | 1017.10 ± 101.05 a | 0.713 ± 0.050 a |
| Female | U. erigeronense | 542.17 ± 62.33 a | 833.21 ± 78.35 a | 0.843 ± 0.035 b | |
| t = 1.67 | t = 1.44 | t = 2.16 | |||
| df = 148 | df = 134 | df = 134 | |||
| p = 0.097 | p = 0.15 | p = 0.032 | |||
| BSE | Male | A. nerii | 900.35 ± 229.55 a | 582.37 ± 145.32 a | 0.183 ± 0.048 a |
| Female | A. nerii | 439.00 ± 103.18 a | 1343.33 ± 269.17 b | 0.271 ± 0.083 a | |
| t = 1.84 | t = 2.42 | t = 0.93 | |||
| df = 36 | df = 35 | df = 35 | |||
| p = 0.074 | p = 0.021 | p = 0.36 | |||
| AD | Male | U. erigeronense | 813.71 ± 128.618 a | 770.77 ± 99.79 a | 0.720 ± 0.052 a |
| Female | U. erigeronense | 727.83 ± 112.15 a | 833.96 ± 114.25 a | 0.862 ± 0.039 b | |
| t = 0.890 | t = 0.154 | t = 2.23 | |||
| df = 102 | df = 89 | df = 89 | |||
| p = 0.376 | p = 0.878 | p = 0.028 | |||
| AD | Male | A. nerii | 468.78 ± 83.19 a | 1304.71 ± 239.44 a | 0.131 ± 0.037 a |
| Female | A. nerii | 366.87 ± 56.15 a | 1388.75 ± 296.73 a | 0.111 ± 0.060 a | |
| t = 0.814 | t = 0.047 | t = 0.29 | |||
| df = 32 | df = 31 | df = 31 | |||
| p = 0.421 | p = 0.963 | p = 0.776 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Riddick, E.W.; Rojas, M.G.; Morales-Ramos, J.A. Multigenerational Rearing on Non-Prey Foods Does Not Affect Prey (Aphid) Recognition Behavior of Coleomegilla maculata (Coleoptera: Coccinellidae). Insects 2024, 15, 852. https://doi.org/10.3390/insects15110852
Riddick EW, Rojas MG, Morales-Ramos JA. Multigenerational Rearing on Non-Prey Foods Does Not Affect Prey (Aphid) Recognition Behavior of Coleomegilla maculata (Coleoptera: Coccinellidae). Insects. 2024; 15(11):852. https://doi.org/10.3390/insects15110852
Chicago/Turabian StyleRiddick, Eric W., Maria Guadalupe Rojas, and Juan A. Morales-Ramos. 2024. "Multigenerational Rearing on Non-Prey Foods Does Not Affect Prey (Aphid) Recognition Behavior of Coleomegilla maculata (Coleoptera: Coccinellidae)" Insects 15, no. 11: 852. https://doi.org/10.3390/insects15110852
APA StyleRiddick, E. W., Rojas, M. G., & Morales-Ramos, J. A. (2024). Multigenerational Rearing on Non-Prey Foods Does Not Affect Prey (Aphid) Recognition Behavior of Coleomegilla maculata (Coleoptera: Coccinellidae). Insects, 15(11), 852. https://doi.org/10.3390/insects15110852







