Reaction of Wood Ants to a Large-Scale European Spruce Bark Beetle Outbreak in Temperate Forests
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Field Work and Sampling
2.3. Calculations and Statistical Analyses
3. Results
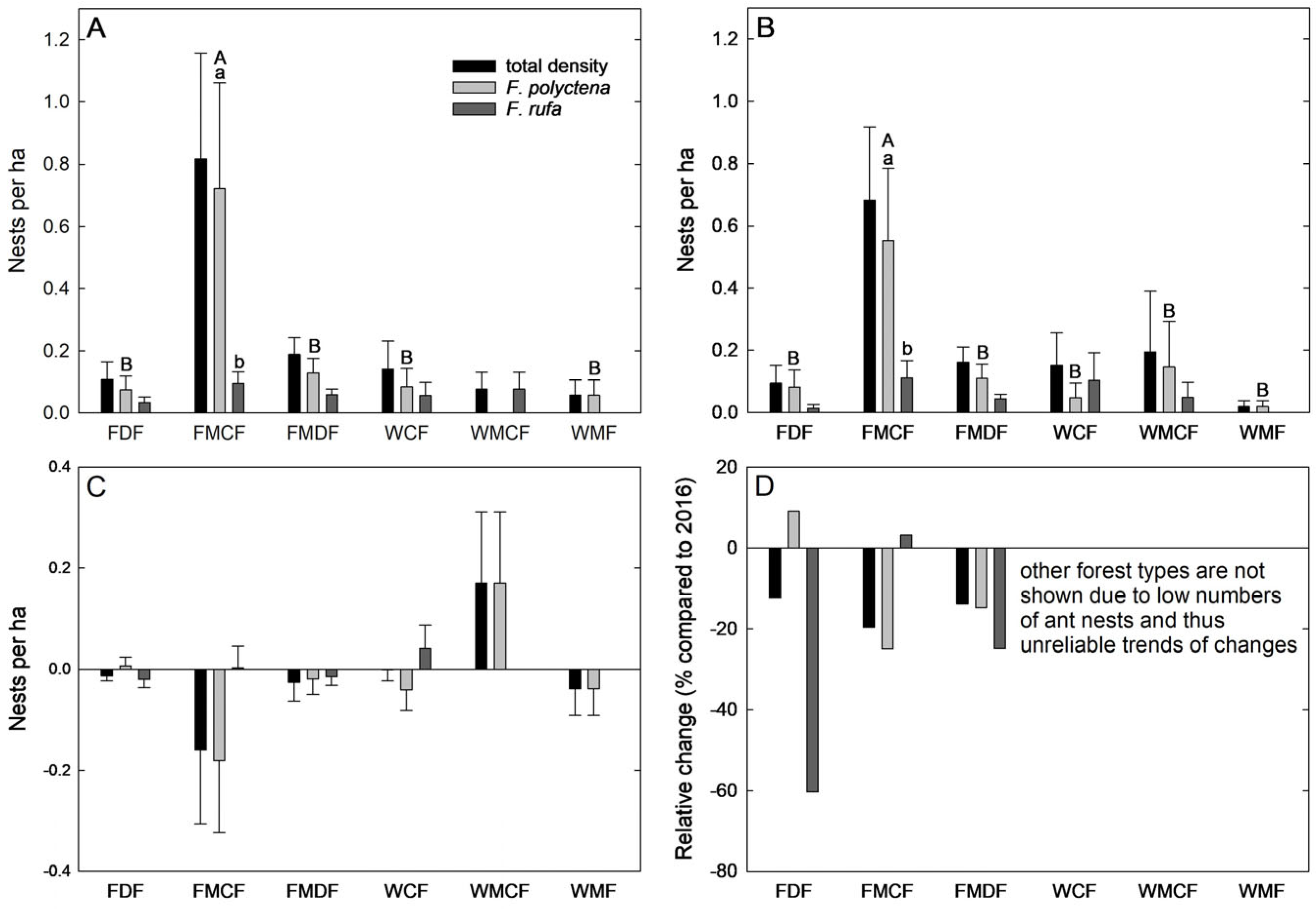
3.1. Wood Ant Nest Density and Abundance in 2022 after Bark Beetle Outbreak
3.2. Physical Parameters of Wood Ant Nests in 2022
3.3. Comparison of Wood Ant Nest Densities and Properties between 2016 and 2022
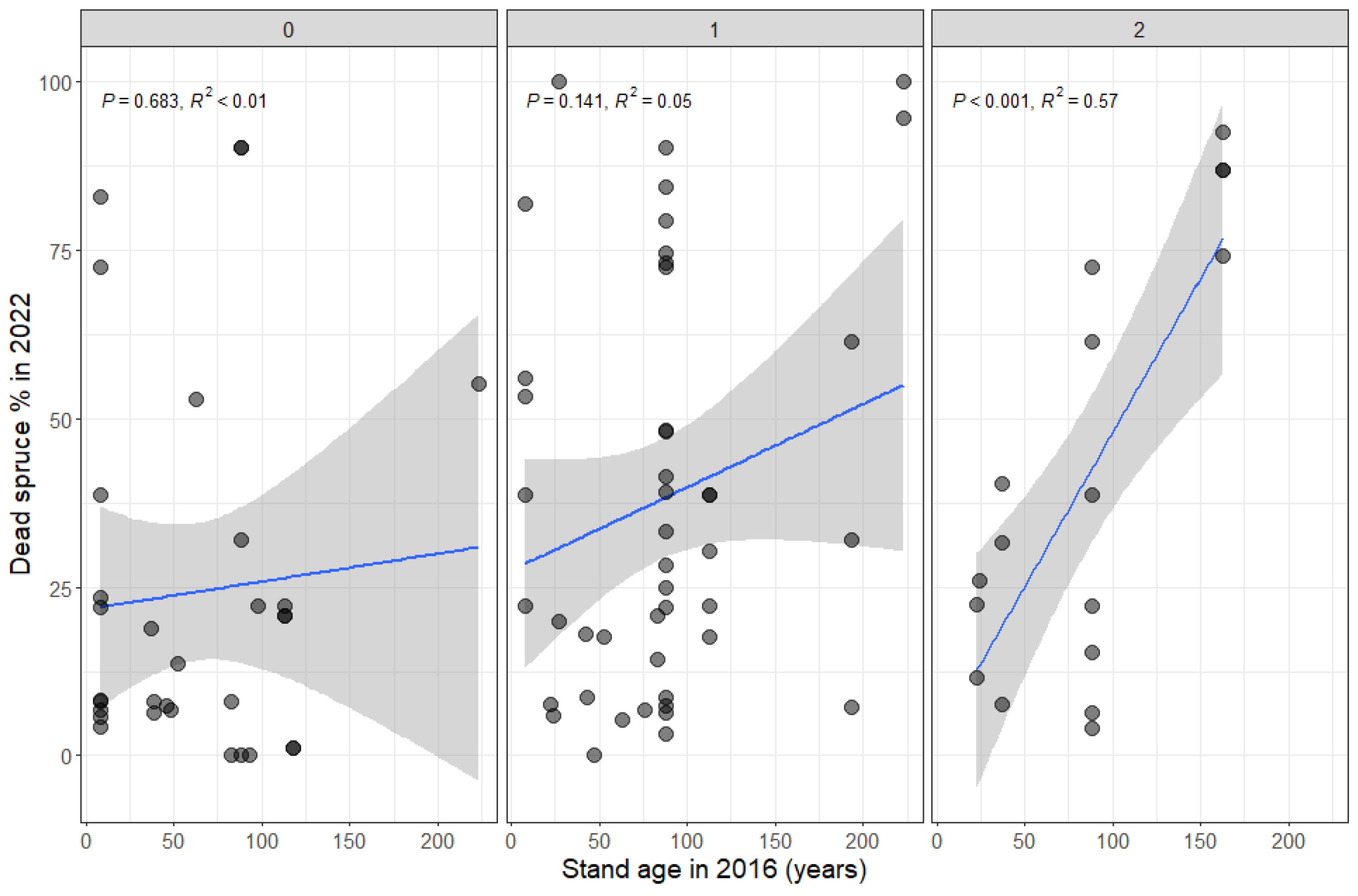
3.4. Factors Affecting the Dead Spruce Proportion
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Seidl, R.; Rammer, W.; Spies, T.A. Disturbance legacies increase the resilience of forest ecosystem structure, composition, and functioning. Ecol. Appl. 2014, 24, 2063–2077. [Google Scholar] [CrossRef]
- Thom, D.; Seidl, R. Natural disturbance impacts on ecosystem services and biodiversity in temperate and boreal forests. Biol. Rev. 2016, 91, 760–781. [Google Scholar] [CrossRef] [PubMed]
- Jaworski, T.; Hilszczański, J. The effect of temperature and humidity changes on insects development their impact on forest ecosystems in the context of expected climate change. For. Res. Pap. 2013, 74, 345–355. [Google Scholar] [CrossRef]
- Hagge, J.; Leibl, F.; Müller, J.; Plechinger, M.; Soutinho, J.G.; Thorn, S. Reconciling per control, nature conservation, and recreation in coniferous forests. Conserv. Lett. 2019, 12, e12615. [Google Scholar] [CrossRef]
- Hlásny, T.; Zimová, S.; Bentz, B. Scientific response to intensifying bark beetle outbreaks in Europe and North America. For. Ecol. Manag. 2021, 499, 119599. [Google Scholar] [CrossRef]
- Komonen, A.; Schroeder, M.L.; Weslien, J. Ips typographus population development after a severe storm in a nature reserve in southern Sweden. J. Appl. Entomol. 2011, 135, 132–141. [Google Scholar] [CrossRef]
- Marini, L.; Økland, B.; Jönsson, A.M.; Bentz, B.; Carroll, A.; Forster, B.; Grégoire, J.-C.; Hurling, R.; Nageleisen, L.M.; Netherer, S.; et al. Climate drivers of bark beetle outbreak dynamics in Norway spruce forests. Ecography 2017, 40, 1426–1435. [Google Scholar] [CrossRef]
- Wermelinger, B. Ecology and management of the spruce bark beetle Ips typographus—A review of recent research. For. Ecol. Manag. 2004, 202, 67–82. [Google Scholar] [CrossRef]
- Tanase, M.A.; Aponte, C.; Mermoz, S.; Bouvet, A.; Le Toan, T.; Heurich, M. Detection of windthrows and insect outbreaks by L-band SAR: A case study in the Bavarian Forest National Park. Remote Sens. Environ. 2018, 209, 700–711. [Google Scholar] [CrossRef]
- Hlásny, T.; Krokene, P.; Liebhold, A.; Montagné-Huck, C.; Müller, J.; Qin, H.; Raffa, K.; Schelhaas, M.-J.; Seidl, R.; Svoboda, M.; et al. Living with Bark Beetles: Impacts, Outlook and Management Options; From Science to Policy 8; European Forest Institute: Joensuu, Finland, 2019. [Google Scholar]
- Netherer, S.; Panassiti, B.; Pennerstorfer, J.; Bradley, M. Acute Drought Is an Important Driver of Bark Beetle Infestation in Austrian Norway Spruce Stands. Front. For. Glob. Change 2019, 2, 39. [Google Scholar] [CrossRef]
- Miścicki, S.; Grodzki, W. Can sanitation cutting contribute to reduced mortality of Norway spruce Picea abies (L.) H. Karst., due to infestation by Ips typographus (L.)? Sylwan 2022, 165, 749–762. [Google Scholar] [CrossRef]
- Hais, M.; Kučera, T. Surface temperature change of spruce forest as a result of bark beetle attack: Remote sensing and GIS approach. Eur. J. For. Res. 2008, 127, 327–336. [Google Scholar] [CrossRef]
- Olchev, A.; Radler, K.; Sogachev, A.; Panferov, O.; Gravenhorst, G. Application of a three-dimensional model for assessing effects of small clear-cuttings on radiation and soil temperature. Ecol. Model. 2009, 220, 3046–3056. [Google Scholar] [CrossRef]
- Beudert, B.; Bässler, C.; Thorn, S.; Noss, R.; Schröder, B.; Dieffenbach-Fries, H.; Foullois, N.; Müller, J. Bark Beetles Increase Biodiversity While Maintaining Drinking Water Quality. Conserv. Lett. 2015, 8, 272–281. [Google Scholar] [CrossRef]
- Stukalyuk, S.V. Structure of the ant assemblages (Hymenoptera, Formicidae) in the broad-leaved forests of Kiev. Entomol. Rev. 2015, 95, 370–387. [Google Scholar] [CrossRef]
- Sondej, I.; Domisch, T.; Finér, L.; Czechowski, W. Wood ants in the Białowieża Forest and factors affecting their distribution. Ann. Zool. Fenn. 2018, 55, 103–114. [Google Scholar] [CrossRef]
- Domisch, T.; Risch, A.C.; Robinson, E.J.H. Wood ant foraging and mutualism with aphids. In Wood Ant Ecology and Conservation; Stockan, A.J., Robinson, E.J.H., Eds.; Cambridge University Press: Cambridge, UK, 2016; pp. 145–176. [Google Scholar]
- Vandegehuchte, M.L.; Wermelinger, B.; Fraefel, M.; Baltensweiler, A.; Düggelin, C.; Brändli, U.-B.; Freitag, A.; Bernasconi, C.; Cherix, D.; Risch, A.C. Distribution and habitat requirements of red wood ants in Switzerland: Implications for conservation. Biol. Conserv. 2017, 212, 366–375. [Google Scholar] [CrossRef]
- Sondej, I.; Domisch, T.; Finér, L.; Czechowski, W. Wood ants prefer conifers to broadleaved trees in mixed temperate forests. Agric. For. Entomol. 2021, 23, 287–296. [Google Scholar] [CrossRef]
- Gibb, H.; Johansson, T. Forest succession and harvesting of hemipteran honeydew by boreal ants. Ann. Zool. Fenn. 2010, 47, 99–110. [Google Scholar] [CrossRef]
- Domisch, T.; Neuvonen, S.; Sundström, L.; Punttila, P.; Finér, L.; Kilpeläinen, J.; Niemelä, P.; Risch, A.C.; Ohashi, M.; Jurgensen, M.F. Sources of variation in the incidence of ant-aphid mutualism in boreal forests. Agric. For. Entomol. 2011, 13, 239–245. [Google Scholar] [CrossRef]
- Hijii, N. Density, biomass and guild structure of arboreal arthropods as related to their inhabited tree size in a Cryptomeria japonica plantation. Ecol. Res. 1986, 1, 97–118. [Google Scholar] [CrossRef]
- Véle, A.; Frouz, J. Bark Beetle Attacks Reduce Survival of Wood Ant Nests. Forests 2023, 14, 199. [Google Scholar] [CrossRef]
- Pisarski, B.; Vepsäläinen, K. Competition hierarchies in ant communities (Hymenoptera, Formicidae). Ann. Zool. Fenn. 1989, 4, 321–328. [Google Scholar]
- Pisarski, B.; Czechowski, W. Ways to reproductive success of wood ant queens. Memorab. Zool. 1994, 48, 181–186. [Google Scholar]
- Czechowski, W.; Radchenko, A.; Czechowska, W.; Vespäläinen, K. The Ants of Poland with Reference to the Myrmecofauna of Europe; Natura Optima Dux Foundation: Warsaw, Poland, 2012. [Google Scholar]
- Stockan, J.A.; Robinson, E.J.H. Wood Ant Ecology and Conservation; Cambridge University Press: Cambridge, UK, 2016. [Google Scholar]
- Seifert, B. The Ants of Central and North Europe; Lutra Verlags- und Vertriebsgesellschaf: Tauer, Germany, 2018. [Google Scholar]
- Rosengren, R.; Sundström, L. The interaction between redwood ants, Cinara aphids, and pines: Ghost of mutualism past. In Ant–Plant Interactions; Huxley, C.R., Culter, D.F., Eds.; Oxford Scientific Publications: Oxford, UK, 1991; pp. 80–91. [Google Scholar]
- Lenoir, L. Wood Ants (Formica spp.) as Ecosystem Engineers and Their Impact on the Soil Animal Community. Ph.D. Thesis, Swedish University of Agricultural Sciences, Uppsala, Sweden, 2001. [Google Scholar]
- Sipura, M. Contrasting effects of ants on the herbivory and growth of two willow species. Ecology 2002, 83, 2680–2690. [Google Scholar] [CrossRef]
- Gorb, E.; Gorb, S. Seed Dispersal by Ants in a Deciduous Forest Ecosystem: Mechanisms, Strategies, Adaptations; Kluwer Academic Publishers: Boston, MA, USA, 2003. [Google Scholar]
- Kilpeläinen, J.; Finér, L.; Niemelä, P.; Domisch, T.; Neuvonen, S.; Ohashi, M.; Risch, A.C.; Sundström, L. Carbon, nitrogen and phosphorus dynamics of ant mounds (Formica rufa group) in managed boreal forests of different successional stages. Appl. Soil Ecol. 2007, 36, 156–163. [Google Scholar] [CrossRef]
- Domisch, T.; Ohashi, M.; Finér, L.; Risch, A.C.; Sundström, L.; Kilpeläinen, J.; Niemelä, P. Decomposition of organic matter and nutrient mineralisation in wood ant (Formica rufa group) mounds in boreal coniferous forests of different age. Biol. Fertil. Soils 2008, 44, 539–545. [Google Scholar] [CrossRef]
- Frouz, J.; Rybniček, M.; Cudlin, P.; Chmeliková, E. Influence of the wood ant, Formica polyctena, on soil nutrient and the spruce tree growth. J. Appl. Entomol. 2008, 132, 281–284. [Google Scholar] [CrossRef]
- Jurgensen, M.F.; Finér, L.; Domisch, T.; Kilpeläinen, J.; Punttila, P.; Ohashi, M.; Niemelä, P.; Sundström, L.; Neuvonen, S.; Risch, A.C. Organic mound building ants: Their impact on soil properties in temperate and boreal forests. J. Appl. Entomol. 2008, 132, 266–275. [Google Scholar] [CrossRef]
- Jilková, V.; Matějiček, L.; Frouz, J. Changes in the pH and other soil chemical parameters in soil surrounding wood ant (Formica polyctena) nests. Eur. J. Soil Biol. 2011, 47, 72–76. [Google Scholar] [CrossRef]
- Sondej, I.; Domisch, T. Abandoned wood ant nests as sites for seedling germination. Forests 2022, 13, 764. [Google Scholar] [CrossRef]
- Otto, D. Die Roten Waldameisen; Die Neue Brehm-Bücherei: Westarp Wissenschaften: Hohenwarsleben, Germany, 2005. [Google Scholar]
- Parmentier, T.; Dekoninck, W.; Wenseleers, T. A highly diverse microcosm in hostile world: A review on the associates of red wood ants (Formica rufa group). Insect. Soc. 2014, 61, 229–237. [Google Scholar] [CrossRef]
- Frizzi, F.; Masoni, A.; Migliorini, M.; Fanciulli, P.P.; Cianferoni, F.; Balzani, P.; Giannotti, S.; Davini, G.; Wendt, C.F.; Santini, G. A comparative study of the fauna associated with nest mounds of native and introduced populations of the red wood ant Formica paralugubris. Eur. J. Soil Sci. 2020, 101, 103241. [Google Scholar] [CrossRef]
- Rosengren, R.; Vepsalainen, K.; Wuorenrinne, H. Distribution, nest densities, and ecological significance of wood ants (the Formica rufa group) in Finland. Bull. Srop 1979, 3, 181–213. [Google Scholar]
- Haemig, P.D. Effects of ants on the foraging of birds in spruce trees. Oecologia 1994, 97, 35–40. [Google Scholar] [CrossRef]
- Halaj, J.; Ross, D.W.; Moldenke, A.R. Negative effects of ant foraging on spiders in Douglas-fir canopies. Oecologia 1997, 109, 313–322. [Google Scholar] [CrossRef]
- Wegensteiner, R.; Wermelinger, B.; Herrmann, M. Natural Enemies of Bark Beetles: Predators, Parasitoids, Pathogens, and Nematodes. In Bark Beetles: Biology and Ecology of Native and Invasive Species; Vega, F., Hofstetter, R., Eds.; Academic Press: Amterdam, The Netherlands, 2015; pp. 247–304. [Google Scholar]
- Khanday, A.L.; Buhroo, A.A.; Singh, S.; Ranjith, A.P.; Mazur, S. Survey of predators associated with bark beetles (Coleoptera: Curculionidae: Scolytinae) with redescription of Platysoma rimarium Erichson, 1834 from Kashmir, India. J. Asia-Pac. Biodivers 2018, 11, 353–360. [Google Scholar] [CrossRef]
- Trigos-Peral, G.; Juhász, O.; Kiss, P.J.; Módra, G.; Tenyér, A.; Maák, I. Wood ants as biological control of the forest pest beetles Ips spp. Sci. Rep. 2021, 11, 17931. [Google Scholar] [CrossRef]
- Grodzki, W. Mass outbreaks of the spruce bark beetle Ips typographus in the context of the controversies around the Białowieża Primeval Forest. For. Res. Pap. 2016, 77, 324–331. [Google Scholar] [CrossRef]
- Kamińska, A.; Lisiewicz, M.; Kraszewski, B.; Stereńczak, K. Mass outbreaks and factors related to the spatial dynamics of spruce bark beetle (Ips typographus) dieback considering diverse management regimes in the Białowieża forest. For. Ecol. Manag. 2021, 498, 119530. [Google Scholar] [CrossRef]
- Sorvari, J.; Hakkarainen, H. Wood ants are wood ants: Deforestation causes population declines in the polydomous wood ant Formica aquilonia. Ecol. Entomol. 2007, 32, 707–711. [Google Scholar] [CrossRef]
- Żmihorski, M. Distribution of red wood ants (Hymenoptera: Formicidae) in the clear-cut areas of a managed forest in Western Poland. J. For. Res 2010, 15, 145–148. [Google Scholar] [CrossRef]
- Rosengren, R.; Pamilo, P. Effect of winter timber felling on behaviour of foraging wood ants (Formica rufa group) in early spring. Memorab. Zool. 1978, 29, 143–155. [Google Scholar]
- Miścicki, S.; Kuberski, Ł.; Paluch, R.; Pilch, K.; Stereńczak, K. Wood resources of Białowieża Forest in 2015–2019—status and dynamics. In The Current State of Białowieża Forest Based on the Results of the LIFE+ ForBioSensing Project; Stereńczak, K., Ed.; Forest Research Institute: Sękocin Stary, Poland, 2022; pp. 36–79. [Google Scholar]
- Kamińska, A.; Lisiewicz, M.; Kraszewski, B.; Stereńczak, K. Comprehensive analysis of spruce dieback between 2015 and 2019 in the Białowieża Forest. In The Current State of Białowieża Forest Based on the Results of the LIFE+ ForBioSensing Project; Stereńczak, K., Ed.; Forest Research Institute: Sękocin Stary, Poland, 2022; pp. 283–296. [Google Scholar]
- Czechowski, W.; Vepsäläinen, K. Territory size of wood ants (Hymenoptera: Formicidae): A search for limits of existence of Formica polyctena Först. an inherently polygynic and polycalic species. Ann. Zool. Fenn 2009, 59, 179–187. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: https://www.R-project.org/ (accessed on 10 February 2022).
- Risch, A.C.; Ellis, S.; Wiswell, H. Where and why? Wood ant population ecology. In Wood Ant Ecology and Conservation; Stockan, A.J., Robinson, E.J.H., Eds.; Cambridge University Press: Cambridge, UK, 2016; pp. 81–105. [Google Scholar]
- Dyachenko, N.G. Composition of ant species from Formica L. genus of Białowieża Forest and peculiarities of their ecology. Park. Nar. I Rezerwaty Przyr. 1999, 18, 81–90. (In Russian) [Google Scholar]
- Fitzpatrick, B.R.; Baltensweiler, A.; Düggelin, C.; Fraefel, M.; Freitag, A.; Vandegehuchte, M.L.; Wermelinger, B.; Risch, A.C. The distribution of a group of keystone species is not associated with anthropogenic habitat disturbance. Divers. Distrib. 2021, 27, 72–584. [Google Scholar] [CrossRef]
- Balzani, P.; Dekoninck, W.; Feldhaar, H.; Freitag, A.; Frizzi, F.; Frouz, J.; Masoni, A.; Robinson, E.; Sorvari, J.; Santini, G. Challenges and a call to action for protecting European red wood ants. Conserv. Biol. 2022, 36, e13959. [Google Scholar] [CrossRef]
- Bruchwald, A.; Dmyterko, E.; Brzeziecki, B. Dynamika i główne kierunki zmian w drzewostanach zagospodarowanej części Puszczy Białowieskiej. Sylwan 2018, 162, 897–906. [Google Scholar]
- Mabelis, A.A. Flying as a survival strategy for wood ants in a fragmented landscape (Hymenoptera, Formicidae). Memorab. Zool. 1994, 48, 147–170. [Google Scholar]
- Hölldobler, B.; Wilson, E.O. The Ants; The Belknap Press of Harvard University Press: Cambridge, MA, USA, 1990. [Google Scholar]
- Tinya, F.; Ódor, P. Congruence of the spatial pattern of light and understory vegetation in an old-growth, temperate mixed forest. For. Ecol. Manag. 2016, 381, 84–92. [Google Scholar] [CrossRef]
- Müller, M.; Olsson, P.O.; Eklundh, L.; Jamali, S.; Ardö, J. Features predisposing forest to bark beetle outbreaks and their dynamics during drought. For. Ecol. Manag. 2022, 523, 120480. [Google Scholar] [CrossRef]
- Kautz, M.; Peter, F.J.; Harms, L.; Kammen, S.; Delb, H. Patterns, drivers and detectability of infestation symptoms following attacks by the European spruce bark beetle. J. Pest. Sci. 2023, 96, 403–414. [Google Scholar] [CrossRef]
- Hýsek, Š.; Radim, L.; Turčáni, M. What Happens to Wood after a Tree Is Attacked by a Bark Beetle? Forests 2021, 12, 1163. [Google Scholar] [CrossRef]
- Rozwałka, Z. Zasady Hodowli Lasu. DGLP. Warszawa. PGL Lasy Państwowe 2012: Instrukcja Urządzania Lasu. Cz. II. Instrukcja Wyróżniania i Kartowania w Lasach Państwowych Typów Siedliskowych Lasu Oraz Zbiorowisk Roślinnych; Ośrodek Rozwojowo-Wdrożeniowy Lasów Państwowych: Bedoń, Poland, 2002. (In Polish) [Google Scholar]
- Kliczkowska, A.; Zielony, R. (Eds.) Siedliskowe Podstawy Hodowli Lasu. Załącznik Do Zasad Hodowli Lasu; Ośrodek Rozwojowo-Wdrożeniowy Lasów Państwowych: Bedoń, Poland, 2004. (In Polish) [Google Scholar]
| Forest Type | Area (ha) | Area (% of Total) | Number of Expected Nests | Number of Observed Nests | % of Observed Nests | Density of Nests (Nests ha−1) |
|---|---|---|---|---|---|---|
| FMDF | 589.8 | 44.1 | 63 | 67 | 46.9 | 0.11 |
| FDF | 191.3 | 14.3 | 20 | 13 | 9.1 | 0.07 |
| FMCF | 138.3 | 10.3 | 15 | 50 | 35 | 0.36 |
| WDF | 135.3 | 10.1 | 14 | 0 | 0 | 0 |
| WMF | 87.3 | 6.5 | 9 | 1 | 0.7 | 0.01 |
| AW | 63.6 | 4.8 | 7 | 0 | 0 | 0 |
| AAW | 47.8 | 3.6 | 5 | 0 | 0 | 0 |
| MMF | 40.4 | 3 | 4 | 0 | 0 | 0 |
| WCF | 23.1 | 1.7 | 2 | 8 | 5.6 | 0.35 |
| WMCF | 20.7 | 1.5 | 2 | 4 | 2.8 | 0.19 |
| MCF | 1 | 0.1 | 0 | 0 | 0 | 0 |
| TOTAL | 1338.5 | 100 | 143 | 143 | 100 | 0.11 |
| Age Class | Area (ha) | Area (% of Total) | Number of Expected Nests | Number of Observed Nests | % of Observed Nests | Density of Nests (Nests ha−1) |
|---|---|---|---|---|---|---|
| 0–20 | 43.3 | 3.2 | 5 | 19 | 13.3 | 0.44 |
| 21–40 | 112.6 | 8.4 | 12 | 14 | 9.8 | 0.12 |
| 41–60 | 240.3 | 18 | 26 | 24 | 16.8 | 0.1 |
| 61–80 | 139.5 | 10.4 | 15 | 4 | 2.8 | 0.03 |
| 81–100 | 512.1 | 38.3 | 55 | 49 | 34.3 | 0.1 |
| 101–120 | 92.3 | 6.9 | 10 | 3 | 2.1 | 0.03 |
| 121–140 | 41.8 | 3.1 | 4 | 17 | 11.9 | 0.41 |
| 141–160 | 49.4 | 3.7 | 5 | 1 | 0.7 | 0.02 |
| 161–180 | 48.1 | 3.6 | 5 | 7 | 4.9 | 0.15 |
| 181–200 | 33.8 | 2.5 | 4 | 0 | 0 | 0 |
| 201–220 | 24.3 | 1.8 | 3 | 2 | 1.4 | 0.08 |
| 221–240 | 1.1 | 0.1 | 0 | 3 | 2.1 | 2.83 |
| TOTAL | 1338.5 | 100 | 143 | 143 | 100 | 0.11 |
| 2016 | 2022 | |||
|---|---|---|---|---|
| F. polyctena (mean ± SE) | F.rufa (mean ± SE) | F. polyctena (mean ± SE) | F. rufa (mean ± SE) | |
| Diameter (cm) | 125.63 ± 5.14 a | 110.73 ± 9.27 b | 146.74 ± 6.65 a | 113.03 ± 7.60 b |
| Height (cm) | 33.28 ± 1.39 a | 30.50 ± 2.17 b | 38.32 ± 1.70 a | 29.14 ± 2.22 b |
| Volume (m3) | 0.40 ± 0.04 a | 0.29 ± 0.07 b | 0.65 ± 0.07 a | 0.26 ± 0.04 b |
| df | F | p | df | F | p | df | F | p | |
|---|---|---|---|---|---|---|---|---|---|
| Year | Ant species | Year: Ant species | |||||||
| Ant species and year | |||||||||
| Diameter | 1 | 5.049 | 0.025 | 1 | 9.253 | 0.003 | 1 | 1.475 | 0.226 |
| Height | 1 | 3.111 | 0.079 | 1 | 7.937 | 0.005 | 1 | 2.491 | 0.116 |
| Volume | 1 | 7.726 | 0.006 | 1 | 11.239 | <0.001 | 1 | 3.933 | 0.050 |
| Longest slope direction | 1 | 0.798 | 0.372 | 1 | 0.536 | 0.464 | 1 | 0.146 | 0.702 |
| Distance to tree | 1 | 0.468 | 0.494 | 1 | 0.702 | 0.403 | 1 | 0.412 | 0.522 |
| Year | Light | Year: light | |||||||
| Light condition and year | |||||||||
| Diameter | 1 | 5.402 | 0.020 | 2 | 16.980 | <0.001 | 2 | 0.319 | 0.727 |
| Height | 1 | 3.163 | 0.076 | 2 | 8.727 | <0.001 | 2 | 0.091 | 0.913 |
| Volume | 1 | 7.925 | 0.005 | 2 | 11.690 | <0.001 | 2 | 0.977 | 0.378 |
| Longest slope direction | 1 | 0.811 | 0.368 | 2 | 1.134 | 0.323 | 2 | 2.797 | 0.060 |
| Distance to tree | 1 | 0.487 | 0.486 | 2 | 6.556 | 0.002 | 2 | 1.080 | 0.341 |
| Year | Forest type | Year: Forest type | |||||||
| Forest type and year | |||||||||
| Diameter | 1 | 4.890 | 0.030 | 6 | 1.245 | 0.283 | 4 | 0.345 | 0.848 |
| Height | 1 | 3.175 | 0.086 | 6 | 3.802 | 0.001 | 4 | 0.520 | 0.721 |
| Volume | 1 | 7.434 | 0.007 | 6 | 1.522 | 0.171 | 4 | 0.515 | 0.725 |
| Longest slope direction | 1 | 0.808 | 0.369 | 6 | 1.007 | 0.421 | 4 | 1.622 | 0.169 |
| Distance to tree | 1 | 0.465 | 0.496 | 6 | 1.085 | 0.371 | 4 | 0.126 | 0.973 |
| Year | Age class | Year: Age class | |||||||
| Age class and year | |||||||||
| Diameter | 1 | 4.900 | 0.028 | 11 | 0.783 | 0.687 | 7 | 1.313 | 0.244 |
| Height | 1 | 3.078 | 0.080 | 11 | 0.832 | 0.608 | 7 | 1.990 | 0.060 |
| Volume | 1 | 7.314 | 0.007 | 11 | 0.519 | 0.890 | 7 | 1.220 | 0.291 |
| Longest slope direction | 1 | 0.817 | 0.367 | 11 | 1.143 | 0.328 | 7 | 1.610 | 0.132 |
| Distance to tree | 1 | 0.482 | 0.488 | 11 | 2.096 | 0.210 | 7 | 0.412 | 0.895 |
| Well-Lit Condition | Moderate Shade | Full Shade | |
|---|---|---|---|
| Diameter (cm) | 104.08 ± 5.36 a | 130.52 ± 4.90 | 156.34 ± 7.17 b |
| Height (cm) | 29.64 ± 1.33 | 33.59 ± 1.46 | 39.67 ± 2.34 |
| Volume (m3) | 0.28 ± 0.04 a | 0.41 ± 0.04 | 0.70 ± 0.08 b |
| Forest Type | Mean ± SE |
|---|---|
| FMCF | 32.9 ± 3.6 a |
| WMCF | 6.7 ± 0.2 b |
| WCF | 5.8 ± 1.2 b |
| FMDF | 36.2 ± 3.2 a |
| WMF | 18.0 a,b |
| FDF | 38.9 ± 8.0 a |
| Ant species | |
| F. polyctena | 38.7 ± 2.8 a |
| F. rufa | 16.4 ± 2.8 b |
| NA (abandoned nests) | 38.5 ± 6.5 a |
| Light condition | |
| Well lit (0) | 27.0 ± 4.1 a |
| Moderate shade (1) | 23.0 ± 3.1 a |
| Full shade (2) | 42.9 ± 3.5 b |
| Type of nest | |
| “Old nests” | 32.8 ± 3.4 |
| Abandoned nests | 38.7 ± 6.5 |
| “New nests” | 32.0 ± 3.2 |
| Total | 33.3 ± 2.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sondej, I.; Domisch, T. Reaction of Wood Ants to a Large-Scale European Spruce Bark Beetle Outbreak in Temperate Forests. Insects 2024, 15, 840. https://doi.org/10.3390/insects15110840
Sondej I, Domisch T. Reaction of Wood Ants to a Large-Scale European Spruce Bark Beetle Outbreak in Temperate Forests. Insects. 2024; 15(11):840. https://doi.org/10.3390/insects15110840
Chicago/Turabian StyleSondej, Izabela, and Timo Domisch. 2024. "Reaction of Wood Ants to a Large-Scale European Spruce Bark Beetle Outbreak in Temperate Forests" Insects 15, no. 11: 840. https://doi.org/10.3390/insects15110840
APA StyleSondej, I., & Domisch, T. (2024). Reaction of Wood Ants to a Large-Scale European Spruce Bark Beetle Outbreak in Temperate Forests. Insects, 15(11), 840. https://doi.org/10.3390/insects15110840







