Adaptability of the Soybean Aphid Aphis glycines (Hemiptera: Aphididae) to Temperature and Photoperiod in a Laboratory Experiment
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Aphid and Hosts
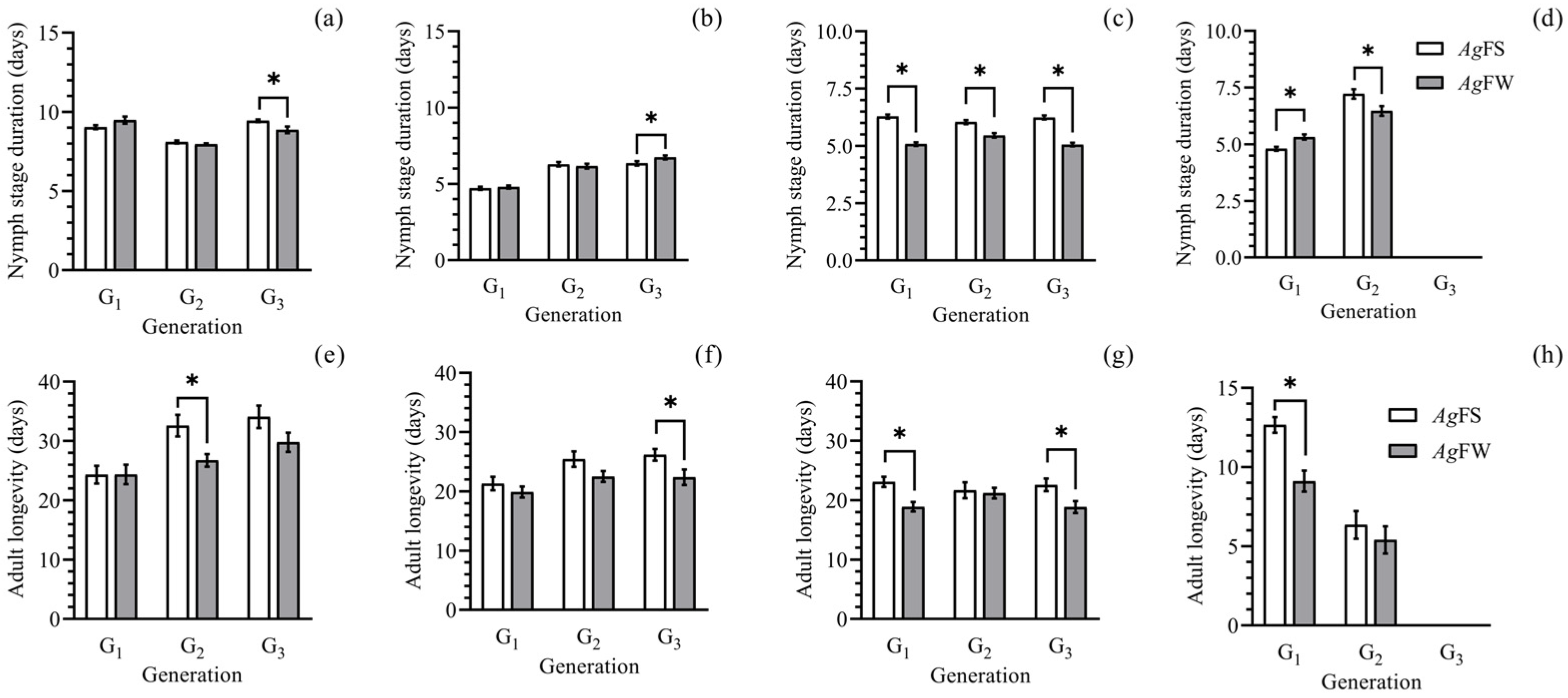
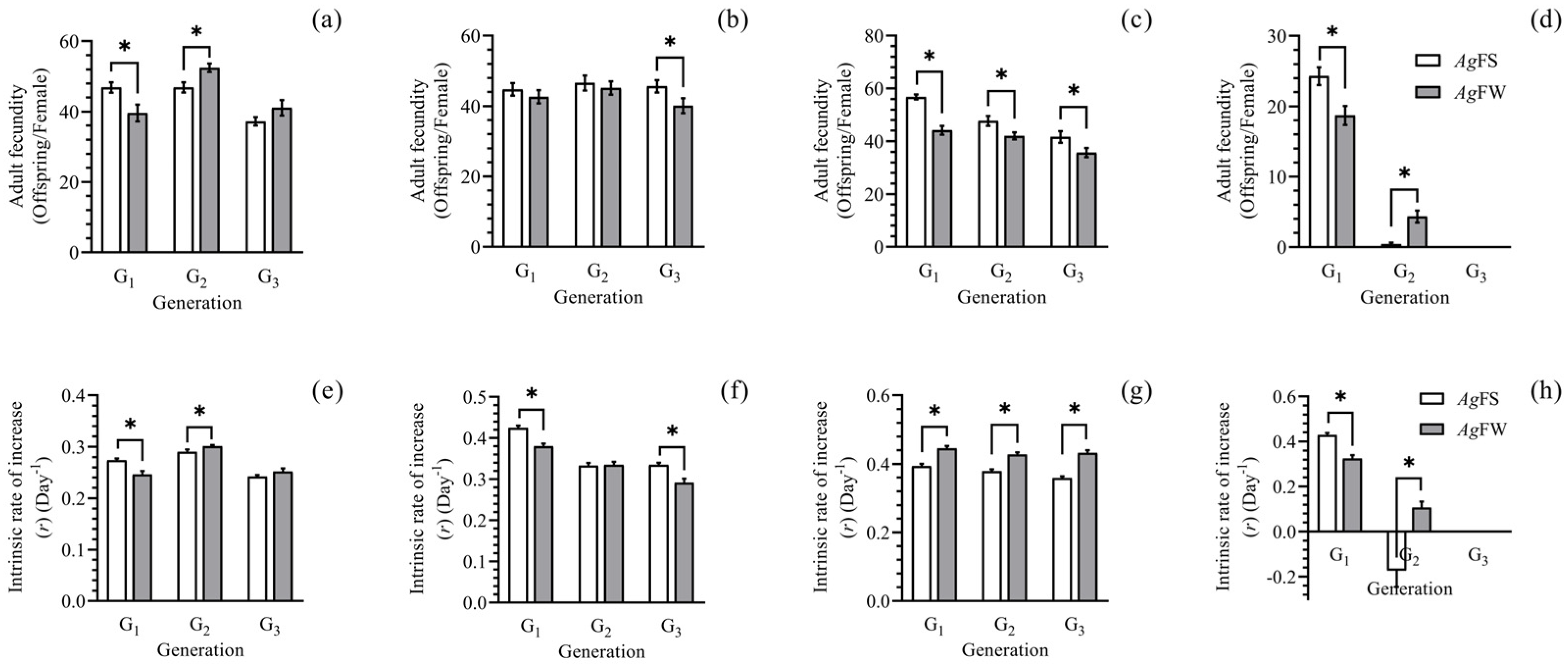
2.2. Development and Reproduction of AgFS and AgFW in Three Generations
2.3. Development and Reproduction of AgFS on Wild Soybean and AgFW on Soybean
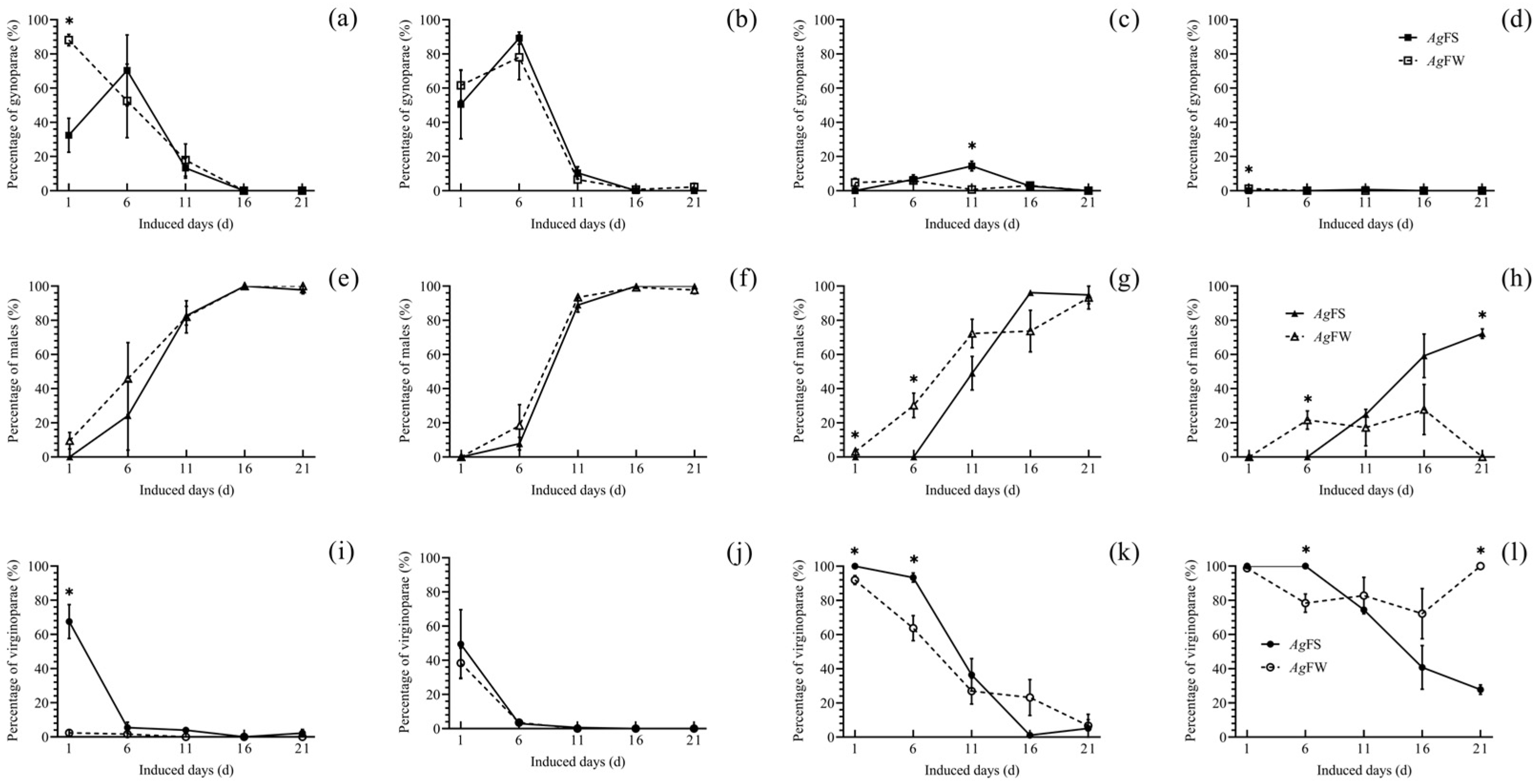
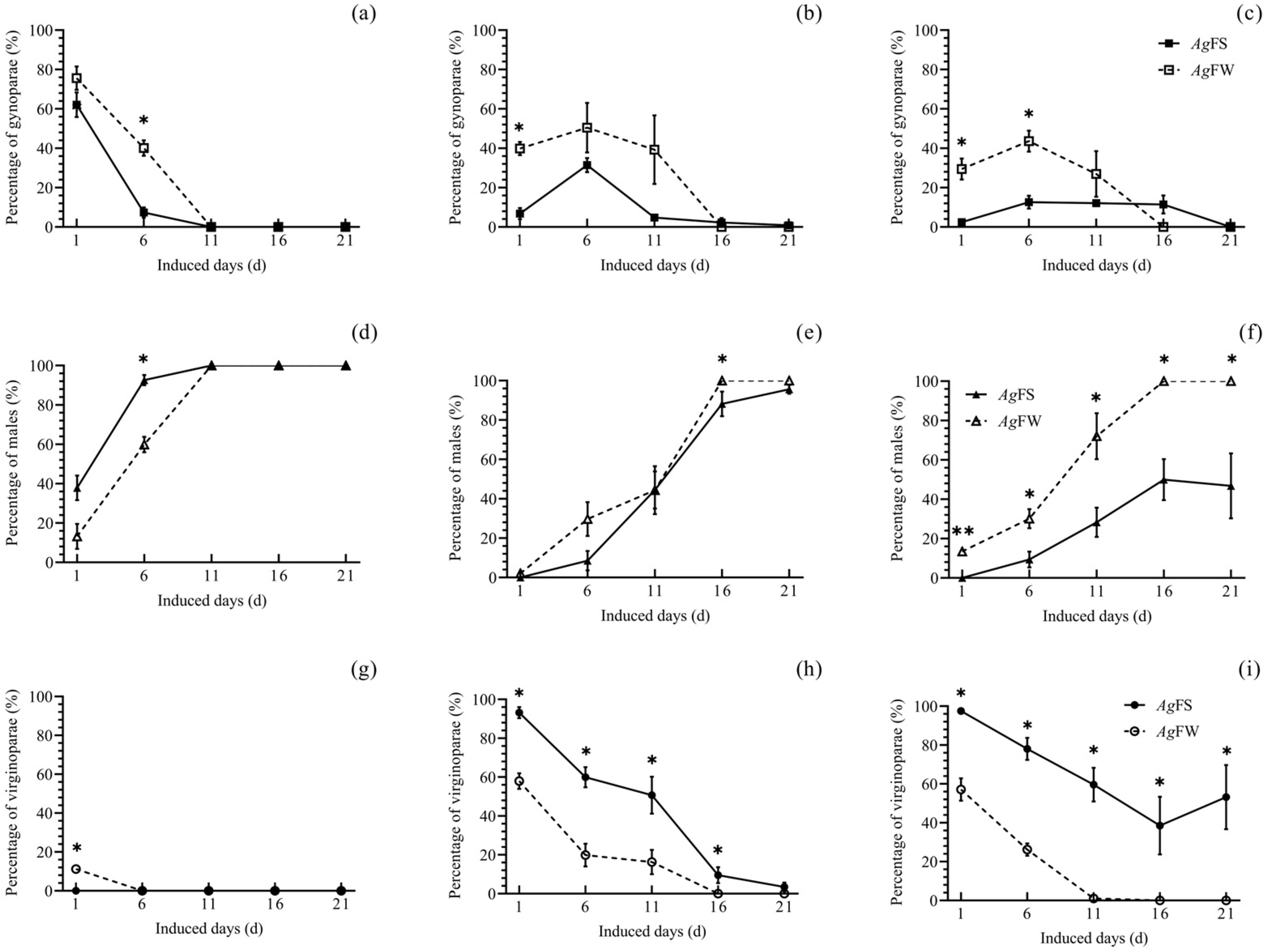
2.4. Morph Differentiation of AgFS and AgFW at Different Temperatures and Photoperiods
2.5. Data Analyses
3. Results
3.1. Development and Reproduction of AgFS and AgFW
3.2. Morph Differentiation of AgFS and AgFW at Different Temperatures and Photoperiods
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ragsdale, D.W.; Voegtlin, D.J.; O’Neil, R.J. Soybean aphid biology in North America. Ann. Entomol. Soc. Am. 2004, 97, 204–208. [Google Scholar] [CrossRef]
- Wu, Z.; Schenk-Hamlin, D.; Zhan, W.; Ragsdale, D.W.; Heimpel, G.E. The soybean aphid in China: A historical review. Ann. Entomol. Soc. Am. 2004, 97, 209–218. [Google Scholar] [CrossRef]
- Liu, J.; Zhao, K.J. Biology and control techniques of soybean aphid, Aphis glycines. Chin. Bull. Entomol. 2007, 44, 179–185. [Google Scholar]
- Wang, C.L.; Xiang, N.Y.; Zhang, G.S.; Zhu, H.F. Studies on the soybean aphid, Aphis glycines Matsumura. Acta Entomol. Sinica. 1962, 11, 31–44. [Google Scholar]
- Hill, C.B.; Li, Y.; Hartman, G.L. Resistance of Glycine species and various cultivated legumes to the soybean aphid (Homoptera: Aphididae). J. Econ. Entomol. 2004, 97, 1071–1077. [Google Scholar] [CrossRef]
- Tilmon, K.J.; Michel, A.; O’Neal, M.E. Aphid resistance is the future for soybean production, and has been since 2004: Efforts towards a wider use of host plant resistance in soybean. Curr. Opin. Insect Sci. 2021, 45, 53–58. [Google Scholar] [CrossRef]
- Wang, S.Y.; Bao, X.Z.; Sun, Y.J.; Chen, R.L.; Zhai, B.P. Study on effect of population dynamics of soybean aphid (Aphis glycines) on both growth and yield of soybean. Soyb. Sci. 1996, 15, 243–247. [Google Scholar]
- Hill, J.H.; Alleman, R.; Hogg, D.B.; Grau, C.R. First report of transmission of Soybean mosaic virus and Alfalfa mosaic virus by Aphis glycines in the New World. Plant Dis. 2001, 85, 561. [Google Scholar] [CrossRef][Green Version]
- Davis, J.A.; Radcliffe, E.B.; Ragsdale, D.W. Soybean aphid, Aphis glycines Matsumura, a new vector of Potato virus Y in potato. Am. J. Potato Res. 2005, 82, 197–201. [Google Scholar] [CrossRef]
- Voegtlin, D.J.; Halbert, S.E.; Qiao, G.X. A Guide to separating Aphis glycines Matsumura and morphologically similar species that share its hosts. Ann. Entomol. Soc. Am. 2004, 97, 227–232. [Google Scholar] [CrossRef]
- Tian, Z.Q.; Wang, S.J.; Bai, B.; Liu, J.; Zhao, K.J. A morphological study on autumnal morphs of Aphis glycines (Hemiptera: Aphididae). J. Asia-Pac. Entomol. 2018, 21, 731–736. [Google Scholar] [CrossRef]
- Li, H.; Liu, X.X.; Zhi, H.J.; Li, K.; Zhang, Q.W.; Li, Z.H. Morphological characteristics for instar identification of Aphis glycines (Hemiptera: Aphididae). Acta Entomol. Sinica. 2018, 61, 877–884. [Google Scholar]
- Liu, X.D.; Zhai, B.P.; Zhang, X.X.; Lu, Y. Differentiation in morphometrics and ecological adaptability of cotton and cucumber biotypes of the cotton aphid, Aphis gossypii (Homoptera: Aphididae). Acta Entomol. Sinica. 2004, 47, 768–773. [Google Scholar]
- Meihls, L.N.; Clark, T.L.; Bailey, W.C.; Ellersieck, M.R. Population growth of soybean aphid, Aphis glycines, under varying levels of predator exclusion. J. Insect Sci. 2010, 10, 1–18. [Google Scholar] [CrossRef]
- Liu, J.; Xu, W.J.; Wang, Q.Y.; Zhao, K.J. Insect predators in northeast China and their impacts on Aphis glycines. Can. Entomol. 2012, 144, 779–789. [Google Scholar] [CrossRef]
- Fan, Y.J.; Tian, Z.Q.; Wang, S.J.; Liu, J.; Zhao, K.J. Dynamics and correlation of Aphis glycines Matsumura and its natural enemies in Harbin. Soyb. Sci. 2017, 36, 104–107. [Google Scholar]
- Ragsdale, D.W.; Mccornack, B.P.; Venette, R.C.; Potter, B.D.; Macrae, I.V.; Hodgson, E.W.; O’Neal, M.E.; Johnson, K.D.; O’Neil, R.J.; Difonzo, C.D.; et al. Economic Threshold for Soybean Aphid (Hemiptera: Aphididae). J. Econ. Entomol. 2007, 100, 1258–1267. [Google Scholar] [CrossRef]
- Mccarville, M.T.; Kanobe, C.; Macintosh, G.C.; O’Neal, M. What is the economic threshold of soybean aphids (hemiptera: Aphididae) in enemy-free space? J. Econ. Entomol. 2011, 104, 845–852. [Google Scholar] [CrossRef]
- Hanson, A.A.; Menger-Anderson, J.; Silverstein, C.; Potter, B.D.; MacRae, I.V.; Hodgson, W.E.; Koch, R.L. Evidence for soybean aphid (Hemiptera: Aphididae) resistance to pyrethroid insecticides in the upper midwestern United States. J. Econ. Entomol. 2017, 110, 2235–2246. [Google Scholar] [CrossRef]
- Regan, K.; Ordosch, D.; Glover, K.D.; Tilmon, K.J.; Szczepaniec, A. Effects of a pyrethroid and two neonicotinoid insecticides on population dynamics of key pests of soybean and abundance of their natural enemies. Crop Prot. 2017, 98, 24–32. [Google Scholar] [CrossRef]
- Koch, R.L.; Queiroz da Silva, O.; Aita, R.C.; Hodgson, E.W.; Potter, B.D.; Nyoike, T.; Ellers-Kirk, C.D. Efficacy of afidopyropen against soybean aphid (Hemiptera: Aphididae) and toxicity to natural enemies. Pest Manag. Sci. 2020, 76, 375–383. [Google Scholar] [CrossRef]
- Rossman, D.R.; Byrne, A.M.; Chilvers, M.I. Profitability and efficacy of soybean seed treatment in Michigan. Crop Prot. 2018, 114, 44–52. [Google Scholar] [CrossRef]
- Yan, S.; Qian, J.; Cai, C.; Ma, Z.Z.; Li, J.H.; Yin, M.Z.; Ren, B.Y.; Shen, J. Spray method application of transdermal dsRNA delivery system for efficient gene silencing and pest control on soybean aphid Aphis glycines. J. Pest Sci. 2020, 93, 449–459. [Google Scholar] [CrossRef]
- Hirano, K.; Honda, K.I.; Miyai, S.I. Effects of temperature on development, longevity and reproduction of the soybean aphid, Aphis glycines (Homoptera: Aphididae). Appl. Entomol. Zool. 1996, 31, 178–180. [Google Scholar] [CrossRef]
- McCornack, B.P.; Ragsdale, D.W.; Venette, R.C. Demography of soybean aphid (Homoptera: Aphididae) at summer temperatures. J. Econ. Entomol. 2004, 97, 854–861. [Google Scholar] [CrossRef]
- Tian, Z.Q.; Wang, S.J.; Bai, B.; Gao, B.; Liu, J. Effects of temperature on survival, development, and reproduction of Aphis glycines (Hemiptera: Aphididae) autumnal morphs. Fla. Entomol. 2020, 103, 236–242. [Google Scholar] [CrossRef]
- Huo, D.B.; Tian, Z.Q.; Liu, D.L.; Wu, C.R.; Wang, L.; Liu, J. Adaptability of two soybean aphid species to high diurnal temperatures. Environ. Entomol. 2022, 51, 1241–1248. [Google Scholar] [CrossRef]
- Chen, X.H.; Fan, Y.J.; Zhang, W.; Tian, Z.Q.; Liu, J.; Zhao, J.K. Soybean aphid, Aphis glycines (Hemiptera: Aphididae), developmental and reproductive capacity on white clover, Trifolium repens (Rosales: Leguminosae), in northeast China. Appl. Entomol. Zool. 2017, 52, 491–495. [Google Scholar] [CrossRef]
- Liu, D.L.; Wu, C.R.; Wang, Q.; Liu, D.H.; Tian, Z.Q.; Liu, J. Effects of heat wave on development, reproduction, and morph differentiation of Aphis glycines (Hemiptera: Aphididae). Environ. Entomol. 2023, 52, 939–948. [Google Scholar] [CrossRef]
- Liu, J.; Liu, Z.; Wu, C.R.; Liu, D.L.; Huo, D.B.; Sun, W.P. Performance in survival, development and reproduction of Aphis glycines Matsumura virginoparae at high temperatures. J. Northeast Agri. Univ. 2023, 30, 20–27. [Google Scholar]
- Bai, B.; Tian, Z.Q.; Gao, B.; Liu, Z.; Wang, L.; Liu, J. Performance of Aphis glycines Matsumura reared under different methods. Fla. Entomol. 2022, 105, 211–218. [Google Scholar] [CrossRef]
- Chi, H.; Liu, H. Two new methods for the study of insect population ecology. Bull. Inst. Zool. Acad. Sin. 1985, 24, 225–240. [Google Scholar]
- Chi, H. Life-table analysis incorporating both sexes and variable development rates among individuals. Environ. Entomol. 1988, 17, 26–34. [Google Scholar] [CrossRef]
- Chi, H. TWOSEX-MSChart: A computer program for the population projection based on age-stage, two-sex life table. 2019. Available online: http://140.120.197.173/ecology/prod02.htm (accessed on 7 June 2019).
- Chi, H.; Güncan, A.; Kavousi, A.; Gharakhani, G.; Atlihan, R.; Ögöç, M.S.; Shirazi, J.; Amir-Maafi, M.; Maroufpoor, M.; Taghizadeh, R. TWOSEX-MSChart: The key tool for life table research and education. Entomol. Gen. 2022, 42, 845–849. [Google Scholar] [CrossRef]
- Efron, B.; Tibshirani, R.J. In Monographs on statistics and applied probability. In An Introduction to the Bootstrap; Chapman Hall/CRC Press: Boca Raton, FL, USA, 1993; pp. 49–54. [Google Scholar]
- Wei, M.F.; Chi, H.; Guo, Y.F.; Li, X.W.; Zhao, L.L.; Ma, R.Y. Demography of Cacopsylla chinensis (Hemiptera: Psyllidae) reared on four cultivars of Pyrus bretschneideri (Rosales: Rosaceae) and P. communis pears with estimations of confidence intervals of specific life table statistics. J. Econ. Entomol. 2020, 113, 2343–2353. [Google Scholar] [CrossRef]
- Gao, S.X.; Liu, D.G.; Chen, H.; Meng, X.X. Fitness traits and underlying genetic variation related to host plant specialization in the aphid Sitobion avenae. Insect Sci. 2014, 21, 352–362. [Google Scholar] [CrossRef]
- Guldemond, J.A.; Wouter, T.T.; Peter, W.F. Host races of Aphis gossypii (Homoptera: Aphididae) on cucumber and chrysanthemum. Environ. Entomol. 1994, 23, 1235–1240. [Google Scholar] [CrossRef]
- Liu, X.D.; Zhai, B.P.; Zhang, X.X. Specialized host-plant performance of the cotton aphid is altered by experience. Ecol. Res. 2008, 23, 919–925. [Google Scholar] [CrossRef]
- Xu, L.; Xu, G.Q.; Liu, P.B.; Chen, Y.; Wang, X.Y.; Zhao, T.H. Effects of temperature on development and reproduction of Aphis glycines Matsumura. Chin. J Oil Crop Sci. 2011, 33, 189–192. [Google Scholar]
- Liu, J.; Bai, B.; Gao, B.; Liu, Z. Effects of constant high temperature on survival, development and reproduction of Aphis glycines Matsumura. J. Northeast Agri. Univ. 2020, 27, 19–27. [Google Scholar]
- Yang, S. Study of the Ecological Adaptability in the Different Geographical Populations of Soybean Aphid. Master’s Thesis, Northeast Agricultural University, Harbin, China, 2009. [Google Scholar]
- Oka, Y.; Kagami-Yashima, C.; Kagawa, K.; Sonoda, S.; Murai, T. Clonal variation of sexual morph production in response to temperature and photoperiod in soybean aphid, Aphis glycines (Hemiptera: Aphididae). App. Entomol. Zool. 2018, 53, 509–517. [Google Scholar] [CrossRef]
- Wang, S.J. Induction Law of Aphis glycines Matsumura Gynoparae and Males at Different Temperatures and Photoperiods. Master’s Thesis, Northeast Agricultural University, Harbin, China, 2019. [Google Scholar]
- Pan, H.S.; Zhang, G.H.; Xu, N.P. A preliminary analysis of climate warming in Heilongjiang province since the 1980s. Climatic. Environ. Res. 2003, 3, 348–355. [Google Scholar]
- Kofsky, J.; Zhang, H.; Song, B.H. The untapped genetic reservoir: The past, current, and future applications of the wild soybean (Glycine soja). Front. Plant Sci. 2018, 9, 949. [Google Scholar] [CrossRef]
- Zhao, T.J.; Gai, J.Y. The origin and evolution of cultivated soybean [Glycine max (L.) Merr.]. Sci. Agr. Sin. 2004, 37, 954–962. [Google Scholar]
| Temperature (°C) | Nymph Stage Duration (Day) | Adult Lifespan (Day) | ||
|---|---|---|---|---|
| AgFS Fed on Wild Soybean | AgFW Fed on Soybean | AgFS Fed on Wild Soybean | AgFW Fed on Soybean | |
| 17 | 10.06 ± 0.09 a | 11.20 ± 0.11 a * | 28.22 ± 1.93 ab | 38.70 ± 2.18 a * |
| 20 | 7.06 ± 0.10 b | 8.15 ± 0.11 b * | 30.47 ± 1.72 a | 39.85 ± 1.46 a * |
| 23 | 6.57 ± 0.12 c | 6.24 ± 0.09 c * | 24.63 ± 1.68 b | 24.18 ± 0.99 b |
| 26 | 5.54 ± 0.09 d | 6.12 ± 0.14 c * | 20.50 ± 1.27 c | 23.78 ± 0.79 b * |
| 29 | 4.60 ± 0.09 e | 5.22 ± 0.09 d * | 8.51 ± 1.03 d | 16.38 ± 0.83 c * |
| 32 | 5.24 ± 0.11 f | 5.11 ± 0.07 d | 9.10 ± 0.87 d | 10.81 ± 0.60 d |
| Temperature (°C) | Adult Fecundity (Offspring/Female) | Intrinsic Rate of Increase (Day−1) | ||
|---|---|---|---|---|
| AgFS Fed on Wild Soybean | AgFW Fed on Soybean | AgFS Fed on Wild Soybean | AgFW Fed on Soybean | |
| 17 | 42.30 ± 2.10 c | 44.20 ± 2.02 b | 0.2321 ± 0.0024 c | 0.1989 ± 0.0019 d * |
| 20 | 51.32 ± 2.01 a | 45.28 ± 1.18 b * | 0.3236 ± 0.0059 b | 0.2772 ± 0.0047 c * |
| 23 | 44.80 ± 2.18 bc | 45.12 ± 1.77 ab | 0.3375 ± 0.0078 b | 0.3592 ± 0.0049 b * |
| 26 | 48.06 ± 1.91 ab | 49.22 ± 1.42 a | 0.4182 ± 0.0066 a | 0.3743 ± 0.0071 ab * |
| 29 | 14.96 ± 1.56 d | 32.22 ± 1.99 c * | 0.3459 ± 0.0126 b | 0.3884 ± 0.0099 a * |
| 32 | 6.12 ± 0.62 e | 9.08 ± 0.80 d * | 0.1983 ± 0.0150 d | 0.2579 ± 0.0165 c * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, B.; Yang, K.; Tian, Y.; Bai, B.; Tian, Z.; Liu, J. Adaptability of the Soybean Aphid Aphis glycines (Hemiptera: Aphididae) to Temperature and Photoperiod in a Laboratory Experiment. Insects 2024, 15, 816. https://doi.org/10.3390/insects15100816
Gao B, Yang K, Tian Y, Bai B, Tian Z, Liu J. Adaptability of the Soybean Aphid Aphis glycines (Hemiptera: Aphididae) to Temperature and Photoperiod in a Laboratory Experiment. Insects. 2024; 15(10):816. https://doi.org/10.3390/insects15100816
Chicago/Turabian StyleGao, Bo, Kaice Yang, Yifan Tian, Bing Bai, Zhenqi Tian, and Jian Liu. 2024. "Adaptability of the Soybean Aphid Aphis glycines (Hemiptera: Aphididae) to Temperature and Photoperiod in a Laboratory Experiment" Insects 15, no. 10: 816. https://doi.org/10.3390/insects15100816
APA StyleGao, B., Yang, K., Tian, Y., Bai, B., Tian, Z., & Liu, J. (2024). Adaptability of the Soybean Aphid Aphis glycines (Hemiptera: Aphididae) to Temperature and Photoperiod in a Laboratory Experiment. Insects, 15(10), 816. https://doi.org/10.3390/insects15100816





