The Effect of Spinosad on the Oak Lace Bug Corythucha arcuata (Hemiptera: Tingidae)—A Preliminary Study Performed Under Laboratory Conditions
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
Data Analyses
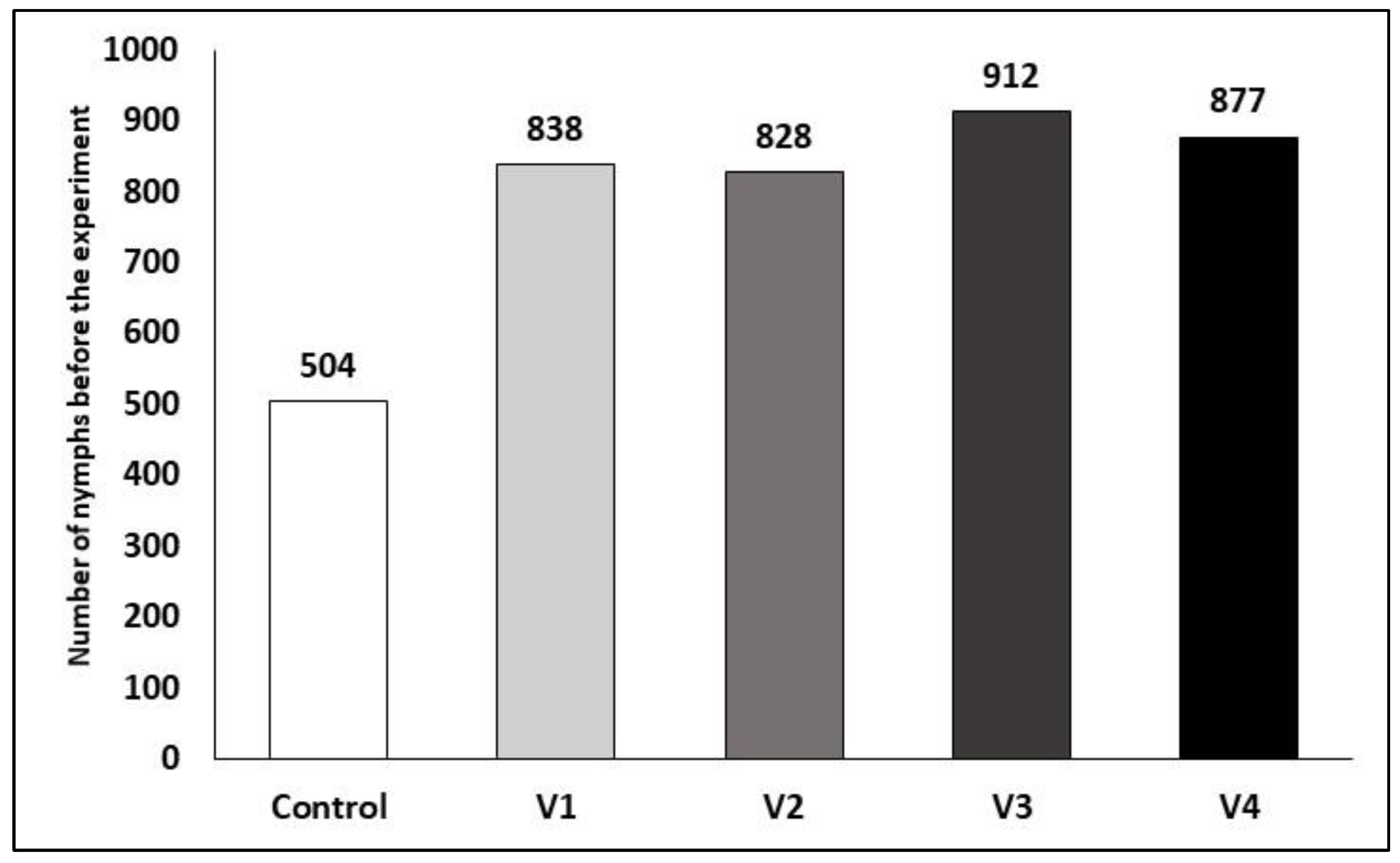
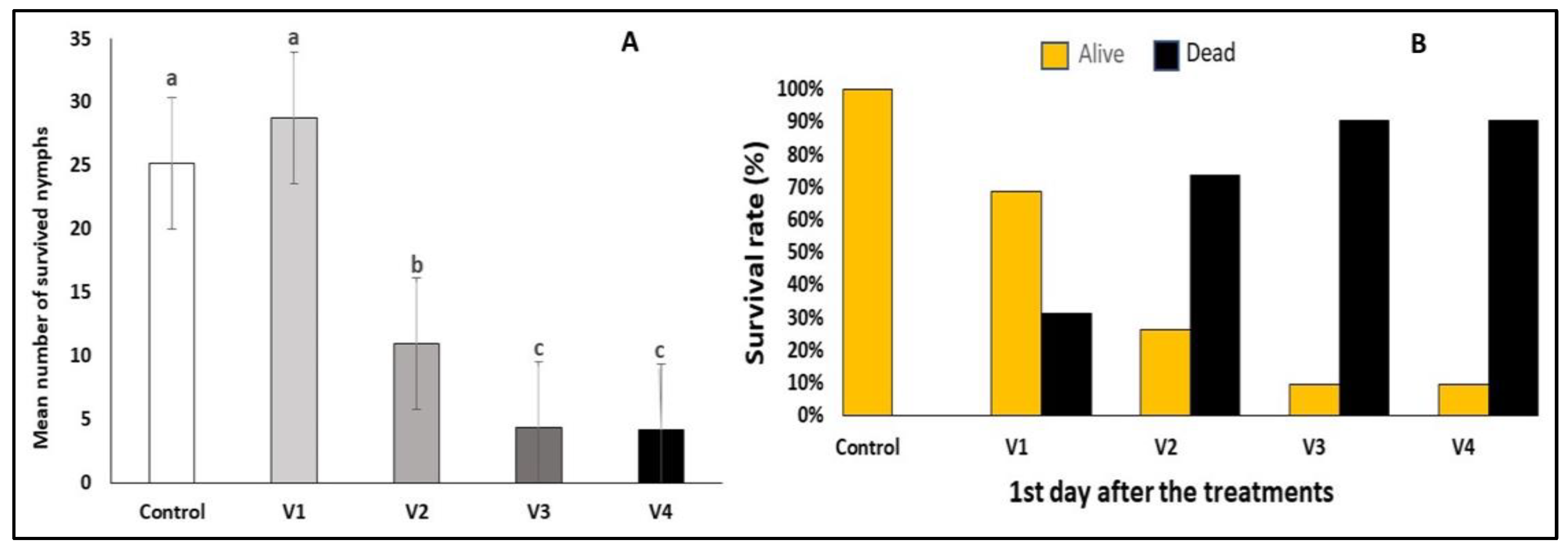
3. Results
3.1. Efficacy of the Treatments After One Day
3.2. Efficacy of the Treatments After Three Days
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kern, A.; Marjanović, H.; Csóka, G.; Móricz, N.; Pernek, M.; Hirka, A.; Matošević, D.; Paulin, M.; Kovač, G. Detecting the Oak Lace Bug Infestation in Oak Forests Using MODIS and Meteorological Data. Agric. For. Meteorol. 2021, 306, 108436. [Google Scholar] [CrossRef]
- Paulin, M.J.; Eötvös, C.B.; Zabransky, P.; Csóka, G.; Schebeck, M. Cold Tolerance of the Invasive Oak Lace Bug, Corythucha arcuata. Agric. For. Entomol. 2023, 25, 612–621. [Google Scholar] [CrossRef]
- Bălăcenoiu, F.; Nețoiu, C.; Tomescu, R.; Simon, D.C.; Buzatu, A.; Toma, D.; Petrițan, I.C. Chemical Control of Corythucha arcuata (Say, 1832), an Invasive Alien Species, in Oak Forests. Forests 2021, 12, 770. [Google Scholar] [CrossRef]
- Macháčová, M.; Nakládal, O.; Samek, M.; Baťa, D.; Zumr, V.; Pešková, V. Oak Decline Caused by Biotic and Abiotic Factors in Central Europe: A Case Study from the Czech Republic. Forests 2022, 13, 1223. [Google Scholar] [CrossRef]
- Bălăcenoiu, F.; Toma, D.; Nețoiu, C. From Field Data to Practical Knowledge: Investigating the Bioecology of the Oak Lace Bug—An Invasive Insect Species in Europe. Insects 2023, 14, 882. [Google Scholar] [CrossRef] [PubMed]
- Kovač, M.; Linde, A.; Lacković, N.; Bollmann, F.; Pernek, M. Natural Infestation of Entomopathogenic Fungus Beauveria Pseudobassiana on Overwintering Corythucha arcuata (Say) (Hemiptera: Tingidae) and Its Efficacy under Laboratory Conditions. For. Ecol. Manag. 2021, 491, 119193. [Google Scholar] [CrossRef]
- Bălăcenoiu, F.; Japelj, A.; Bernardinelli, I.; Castagneyrol, B.; Csóka, G.; Glavendekić, M.; Hoch, G.; Hrašovec, B.; Ostoic, S.K.; Paulin, M.; et al. Corythucha arcuata (Say, 1832) (Hemiptera, Tingidae) in Its Invasive Range in Europe: Perception, Knowledge and Willingness to Act in Foresters and Citizens. NeoBiota 2021, 69, 133–153. [Google Scholar] [CrossRef]
- Mutun, S.; Ceyhan, Z.; Sözen, C. Invasion by the Oak Lace Bug, Corythucha arcuata (Say) (Heteroptera: Tingidae), in Turkey. Turk. J. Zool. 2009, 33, 263–268. [Google Scholar] [CrossRef]
- Paulin, M.; Hirka, A.; Eötvös, C.B.; Gáspár, C.; Fürjes-Mikó, Á.; Csóka, G. Known and Predicted Impacts of the Invasive Oak Lace Bug (Corythucha arcuata) in European Oak Ecosystems—A Review. Folia Oecol. 2020, 47, 131–139. [Google Scholar] [CrossRef]
- Yao, S.; Yang, Y.; Xue, Y.; Zhao, W.; Liu, X.; Du, M.; Yin, X.; Guan, R.; Wei, J.; An, S. New Insights on the Effects of Spinosad on the Development of Helicoverpa armigera. Ecotoxicol. Environ. Saf. 2021, 221, 112452. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, H.; Fan, Y.; Yang, Y.; Gao, J.; Xu, W.; Xu, Z.; Li, Z.; Tao, L. Cytotoxic Effects of Bio-Pesticide Spinosad on Human Lung A549 cells. Chemosphere 2019, 230, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Henderson, C.F.; Tilton, E.W. Tests with Acaricides against the Brown Wheat Mite. J. Econ. Entomol. 1955, 48, 157–161. [Google Scholar] [CrossRef]
- Rojht, H.; Meško, A.; Vidrih, M.; Trdan, S. Insecticidal Activity of Four Different Substances against Larvae and Adults of Sycamore Lace Bug (Corythucha Ciliata [Say], Heteroptera, Tingidae). Acta Agric. Slov. 2009, 93, 31–36. [Google Scholar] [CrossRef]
- Crawley, M.J. Herbivory: The Dynamics of Animal-Plant Interaction, 1st ed.; University of California Press: Berkeley, CA, USA; Oxford, MS, USA, 1983; ISBN 978-0-632-00808-7. [Google Scholar]
- Kennedy, C.E.J.; Southwood, T.R.E. The Number of Species of Insects Associated with British Trees: A Re-Analysis. J. Anim. Ecol. 1984, 53, 455–478. [Google Scholar] [CrossRef]
- Csóka, G.; Ambrus, A. Erdei Fa-És Cserjefajok Szerepe a Herbivor Rovarok Fajgazdagságának Fenntartásában. In Az Erdőgazdálkodás Hatása az Erdők Biológiai Sokféleségére; Duna-Ipoly Nemzeti Park Igazgatósága: Budapest, Hungary, 2016; pp. 155–192. [Google Scholar]
- Shapiro-Ilan, D.I.; Mizell, R.F. Laboratory Virulence of Entomopathogenic Nematodes to Two Ornamental Plant Pests, Corythucha Ciliata (Hemiptera: Tingidae) and Stethobaris Nemesis (Coleoptera: Curculionidae). Fla. Entomol. 2012, 95, 922–927. [Google Scholar] [CrossRef]
- Julià, I.; Morton, A.; Roca, M.; Garcia-del-Pino, F. Evaluation of Three Entomopathogenic Nematode Species against Nymphs and Adults of the Sycamore Lace Bug, Corythucha Ciliata. BioControl 2020, 65, 623–633. [Google Scholar] [CrossRef]


| Variants | Commercial Name | Active Ingredient | Dosage |
|---|---|---|---|
| Control | - | - | - |
| V1 | Laser 240 SC | spinosad 240 g/L | 2 mL/6 L water |
| V2 | Laser 240 SC | spinosad 240 g/L | 2 mL/4 L water |
| V3 | Laser 240 SC | spinosad 240 g/L | 2 mL/2 L water |
| V4 | Karate Zeon 5 CS | lambda-cyhalothrin 50 g/L | 2 mL/10 L water |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fora, C.G.; Csorba, A.B.; Balog, A. The Effect of Spinosad on the Oak Lace Bug Corythucha arcuata (Hemiptera: Tingidae)—A Preliminary Study Performed Under Laboratory Conditions. Insects 2024, 15, 815. https://doi.org/10.3390/insects15100815
Fora CG, Csorba AB, Balog A. The Effect of Spinosad on the Oak Lace Bug Corythucha arcuata (Hemiptera: Tingidae)—A Preliminary Study Performed Under Laboratory Conditions. Insects. 2024; 15(10):815. https://doi.org/10.3390/insects15100815
Chicago/Turabian StyleFora, Ciprian George, Artúr Botond Csorba, and Adalbert Balog. 2024. "The Effect of Spinosad on the Oak Lace Bug Corythucha arcuata (Hemiptera: Tingidae)—A Preliminary Study Performed Under Laboratory Conditions" Insects 15, no. 10: 815. https://doi.org/10.3390/insects15100815
APA StyleFora, C. G., Csorba, A. B., & Balog, A. (2024). The Effect of Spinosad on the Oak Lace Bug Corythucha arcuata (Hemiptera: Tingidae)—A Preliminary Study Performed Under Laboratory Conditions. Insects, 15(10), 815. https://doi.org/10.3390/insects15100815









