Simple Summary
Maternally transmitted Wolbachia is one of the most common endosymbionts in many arthropods, influencing various aspects of host physiology, reproduction, and fitness. While recent studies on Wolbachia-Drosophila systems have revealed its influence on host thermal choice behavior, its broader impact on host thermal preference (Tp) in non-model insects remains poorly understood. The polyphagous leaf-miner fly, Liriomyza huidobrensis (Blanchard), one of the most notorious pests of vegetable and flowers globally, harbors a range of bacterial symbionts, with Wolbachia being especially prevalent. This study aims to explore the effects of Wolbachia on the thermal preference of the invasive leaf-miner L. huidobrensis. Understanding the potential roles of Wolbachia in host thermal behavior is crucial for elucidating the co-evolution of invasive species and their endosymbiont Wolbachia in the context of global climate change and temperature extremes, and may offer insight for the development of pest control strategies utilizing Wolbachia.
Abstract
Heritable endosymbiont Wolbachia is prevalent among arthropods, serving multiple functions for their hosts. However, the role of Wolbachia in mediating thermal preference selection remains largely unexplored. In this study, we utilized a custom-built thermal gradient to evaluate the thermal preference (Tp) of 1367 individuals of the invasive leaf-miner Liriomyza huidobrensis with or without Wolbachia wLhui from Yunnan and Xinjiang populations. Under meticulously controlled conditions and with a vast sample size, we found no significant difference in the mean Tp between wLhui-infected and uninfected leaf miners from either population when host age and sex were not considered. Furthermore, generalized linear model (GLM) analysis revealed no significant correlation between average Tp and age, sex, or Wolbachia infection, nor interactions among these factors, except in the Xinjiang population, where Tp was strongly associated with host age. Finally, we discuss the ecological implications of these findings and propose future research directions on Wolbachia-mediated host Tp in the leaf miner. Overall, our findings do not provide evidence that Wolbachia significantly affects the thermal preference of L. huidobrensis. Further studies across different systems are needed to investigate the complex interactions between Wolbachia and insect thermal behavior.
1. Introduction
Maternally inherited Wolbachia is one of the most widespread intracellular endosymbionts in arthropods, infecting over 50% of insect species as well as numerous species of spider mites [1,2,3,4]. Wolbachia has wide-ranging effects on the ecology and evolution of its insect hosts, including reproductive manipulations [5,6], nutrient provision [7,8,9], thermotolerance modification [10,11], and pathogen resistance [12,13]. As our understanding of Wolbachia’s effects on the host phenotype grows, the development of Wolbachia-based strategies for the control of agricultural pests has made great progress [14,15]. Recent studies suggest Wolbachia can manipulate host behavior to mitigate environmental stress, which is crucial for maintaining stable symbiosis [10,16]. However, the strategies of employing Wolbachia to regulate host thermal preference under stress remain poorly understood.
Until recently, only a few studies on model Drosophila flies and non-model spider mites have demonstrated Wolbachia’s ability to influence host thermal preference behavior [17,18]. Our previous research showed that Wolbachia-infected spider mites Tetranychus truncatus preferred lower temperatures [18], consistent with findings in flies infected with Wolbachia strains such as wMel, wMelCS, and wMelPop [17,19,20]. However, comparative studies in flies yielded conflicting results, with wMau-infected flies favoring warmer temperatures [17], and some studies failing to identify significant impacts [21]. These variable effects suggest that Wolbachia-induced thermal preference may depend on host genetics, specific Wolbachia strains, and environmental factors [16,20], raising questions and generating debate about the influence of maternally inherited endosymbionts on insect thermal preference.
The invasive leaf-miner Liriomyza huidobrensis, a notorious pest of vegetable and flowers, has spread globally, causing substantial economic losses [22,23]. The Liriomyza huidobrensis was first reported in Yunnan, China in 1993 [24]. This pest species prefers cool environments and has rapidly expanded into multiple cool regions of China, including Xinjiang [24,25]. Our previous survey revealed that the endosymbiont Wolbachia is widespread in natural populations of the leaf-miner L. huidobrensis [26,27]. Since heritable symbionts in invasive insects may influence host adaption and expansion in various ways, including modulating host thermal preference [19,20,28], we hypothesize that the endosymbiont Wolbachia could manipulate host thermal preference, helping the insect cope with temperature stress during invasions.
To test the hypothesis, we assessed the thermal preference of Wolbachia-infected and uninfected leaf miners using a custom-built thermal gradient [18]. We also examined the influence of sex, age, and Wolbachia infection state on host thermal preference using the generalized linear model (GLM) analysis. We aimed to (1) investigate the role of Wolbachia in manipulating host thermal preference, and (2) explore its association with host age, sex, and population.
2. Materials and Methods
2.1. Leaf-Miner Collection and Rearing
To examine the effect of Wolbachia on the thermal preference of L. huidobrensis, we selected two representative populations of L. huidobrensis: Yunnan, where it was first reported in China, and Xinjiang, where it has recently spread after invasion [24,25,27]. These two wild populations, naturally infected with Wolbachia, were collected in March 2023 from cowpea plants in Yunnan (25.72° N, 101.87° E) and in April 2023 from tomatoes in Xinjiang (41.55° N, 82.62° E) (Figure 1). Upon transition to the laboratory, the Wolbachia wLhui-infected lines from each population were established. The wLhui-uninfected lines were derived through three generations of tetracycline treatment (1 mg/mL), followed by six generations of recovery without tetracycline to mitigate potential side effects. The Wolbachia infection status of each strain was verified via PCR before the experiment, as detailed previously [7]. All leaf-miner lines were maintained on bean seedlings under controlled laboratory conditions: 25 ± 1 °C temperature, 60% relative humidity, and a 16 h light/8 h dark cycle.
Figure 1.
The sampling locations of the two leaf-miner populations. The template map, obtained from the Chinese National Basic Geographic Information Center (http://ngcc.sbsm.gov.cn) (accessed on 16 October 2020), was annotated using ArcGIS 10 Crack software.
2.2. Temperature Preference Measurement
We employed a custom-built thermal gradient apparatus [18] to assess the Tp of the leaf miners. This apparatus consisted of a 50 cm long aluminum bar with a temperature gradient (ranging from 10 to 40 °C) generated by water baths positioned at each end. Six grooves were etched into the aluminum bar, enabling leaf miners to move freely along the gradient without interference. To prevent escape, these grooves were covered with a removable Plexiglas lid.
Temperature readings along the gradient were obtained using K-type thermocouples. All experiments were conducted in a room maintained at a constant temperature of 25 °C and 40% relative humidity. To collect data, six individuals from each strain were placed at the midpoint of each groove within the apparatus. After allowing 30 min for the miners to freely adjust within their respective grooves, the temperatures at their resting positions were recorded. At least seventy individuals from each group were assessed to determine the host temperature preference.
2.3. Statistical Analysis
All statistical analyses were performed and visualized using either GraphPad Prism version 9.00 or SPSS statistics version 20.0 for Windows (SPSS Inc., Chicago, IL, USA). We used the generalized linear models (GLMs) to assess the effects of Wolbachia infection status, sex, and age (1-day-old and 3-day-old) on the Tp of the leaf-miner host. The Tp of each individual was considered as the dependent variable, with age, sex, and Wolbachia infection status as fixed factors. The significance of the fixed factors and their interactions on Tp were determined. Additionally, the Mann–Whitney U test was used to compare Tp between wLhui-infected and uninfected hosts within each sex or age group. The Mann–Whitney test was also applied to assess differences in Tp between wLhui-infected and uninfected hosts in each population, irrespective of host age and sex.
3. Results
3.1. Characteristics of the Study Population
We assessed the thermal performance (Tp) of 1367 individuals from leaf-miner populations in Yunnan and Xinjiang (Figure 1). The Yunnan population included 642 individuals, comprising both 1-day-old and 3-day-old miners, with 321 Wolbachia wLhui-infected and 321 Wolbachia wLhui-uninfected individuals. The Xinjiang population consisted of 725 individuals, also split between 1-day-old and 3-day-old miners, with 368 Wolbachia wLhui-infected and 357 Wolbachia wLhui-uninfected individuals.
3.2. Wolbachia Infection Has No Effect on Tp in the Yunnan Leaf-Miner Population
When disregarding host age and sex, there was no significant difference in mean Tp between wLhui-infected and uninfected leaf miners in Yunnan populations (Mann–Whitney U test, p = 0.138; Figure 2A). The average Tp of wLhui-infected leaf miners (Tp = 19.30 °C) was slightly lower than that of wLhui-uninfected individuals (Tp = 20.20 °C), representing a difference of approximately 0.9 °C (Figure 2A).
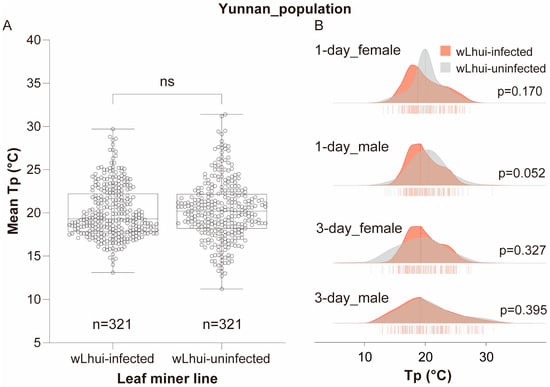
Figure 2.
Thermal preference (Tp) of Wolbachia wLhui-infected and uninfected leaf miners in the Yunnan populations. (A) Boxplots illustrating Tp in the two leaf-miner lines without differentiating by host age and sex. The statistical significance between the two lines was assessed using the Mann–Whitney U test. ns, not significant. (B) Ridgeline plots depicting Tp in 1-day-old or 3-day-old female and male leaf miners, both infected and uninfected with wLhui. The Mann–Whitney test was used to assess significant differences between the two lines at the same age and sex.
When comparing the average Tp of wLhui-infected and uninfected leaf miners of the same age and sex, no significant differences were observed between the two lines (p > 0.05 for all cases; Figure 2B). Both lines of leaf miners exhibited a broad range of Tp values, spanning from 11.2 °C to 31.4 °C, with considerable overlap in the Tp distributions of the two leaf-miner lines (Figure 2B).
Furthermore, generalized linear model (GLM) analysis revealed no significant effects of Wolbachia (χ2 = 1.037, p = 0.308), age (χ2 = 0.444, p = 0.505), sex (χ2 = 1.918, p = 0.166), or their interactions (p > 0.05 for all cases) on host Tp (Table 1).

Table 1.
Generalized linear model (GLM) testing for the effects of host age, sex, Wolbachia, and their interaction on the thermal preference (Tp) in two leaf-miner populations.
3.3. Wolbachia Infection Does Not Alter Tp in the Xinjiang Leaf-Miner Population
In the Xinjiang population, the average Tp values of wLhui-infected and uninfected leaf miners were nearly identical (wLhui-infected = 19.70 °C, wLhui-uninfected = 19.60 °C), with no significant statistical difference (Mann–Whitney U test, p = 0.469; Figure 3A). Similar results were observed when comparing infected and uninfected leaf miners of the same age and sex (p > 0.05 for all cases; Figure 3B). The Tp of the two leaf-miner lines in the Xinjiang population ranged from 12.8 °C to 29.6 °C, with some overlap in the Tp distributions (Figure 3B).
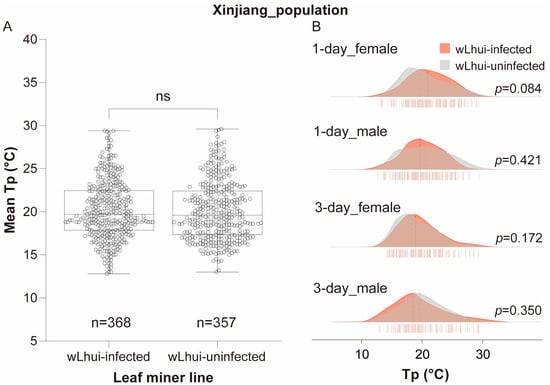
Figure 3.
Thermal preference (Tp) of leaf miners infected with or without Wolbachia wLhui from the Xinjiang populations. (A) Boxplots illustrating Tp in wLhui-infected and uninfected leaf-miner lines, without considering host age or sex. Statistical significance between the two lines was assessed using the Mann–Whitney U test. ‘ns’ indicates no significant difference. (B) Ridgeline plots depicting Tp in 1-day-old or 3-day-old female and male leaf miners, infected or uninfected with wLhui, from the Xinjiang population. The Mann–Whitney U test was used to assess significant differences between the two lines at the same age and sex.
4. Discussion
The role of the endosymbiont Wolbachia in mediating the temperature preference (Tp) in insect hosts remains a topic of ongoing debate. In this study, we examined, for the first time, the relationship between the temperature preference of L. huidobrensis and Wolbachia infection under controlled laboratory conditions. Our findings provide no evidence that Wolbachia significantly affects the Tp of leaf miners in either the Yunnan or Xinjiang populations.
4.1. Divergent Influences of Wolbachia on Host Tp in Various Insect Species
Tp can vary significantly between populations of the same species [29,30]. In our study, we observed that Tp was strongly related to host age in the Xinjiang leaf-miner population but not in the Yunnan population, suggesting Tp may vary between these two populations. Nevertheless, we consistently found that Wolbachia had only subtle effects on Tp in both leaf-miner populations. Our results align with previous findings that show Wolbachia does not significantly impact host Tp [21], though they contradict other studies that report notable influences of Wolbachia on host Tp [16,17,19,20]. This suggests that Wolbachia-mediated host Tp can vary across different insect–endosymbiont systems.
It is widely recognized that the effect size and direction of Wolbachia-mediated host Tp are strongly influenced by several factors, including host background and genotypes, Wolbachia strains and titers, and environmental conditions [16,17]. Notably, Hague et al. [17] proposed that the effects of Wolbachia on Tp may exhibit a phylogenetic pattern; most Drosophila hosts infected with A-group Wolbachia strains prefer cooler temperatures, whereas species infected with divergent B-group Wolbachia strains prefer warmer temperatures, compared to uninfected genotypes [17]. Despite the fact that Wolbachia wLhui within the leaf-miner L. huidobrensis belongs to the A supergroup, we found no significant effect of Wolbachia on Tp. Therefore, the universal impact of A-group Wolbachia strains on insect host Tp remains uncertain and warrants further investigation. In addition to Wolbachia traits, the potential role of host traits and environmental factors in Wolbachia-mediated Tp in leaf miners are still unclear [16]. Additionally, the mechanisms underlying Wolbachia-induced changes in host Tp are largely unknown. Hague et al. [17] speculated that the differences in Tp between infected and uninfected insects might arise from the conflicting physiological requirements between Wolbachia and their hosts under temperature stress. The divergent influences of Wolbachia on host Tp may depend on specific co-evolutionary dynamics between Wolbachia and their hosts, resulting from trade-offs in thermal adaptation and balancing selection [18].
4.2. The Ecological Significance of Wolbachia-Mediated Host Tp
Small temperature fluctuations can significantly alter host–symbiont interactions [31,32]. These interactions, mediated by Wolbachia, play essential roles in host and Wolbachia ecology and evolution, including symbiosis maintenance, Wolbachia spread, and host adaptation to new environments [1,2,3,10,16]. For many invasive insects, Wolbachia-induced behavioral changes may confer a fitness advantage in novel environments, potentially accelerating population expansion and facilitating Wolbachia’s spread through host populations [28,33]. Given that we found no significant influence of Wolbachia on host Tp, we speculate that the rapid spread of L. huidobrensis may be not linked to Wolbachia-mediated thermal behavioral regulation. Instead, our recent work suggests that Wolbachia modifies host–cell metabolite profiles in response to short-term temperature stress, which may in turn affect the fitness and adaptive capacity of invasive L. huidobrensis [34].
In terms of practical applications, Wolbachia is utilized to control human diseases and agricultural pests through strategies such as the population replacement strategy (PRS) and the incompatible insect technique (IIT) [2,35]. Both strategies rely on the release of Wolbachia-infected individuals. Considering Wolbachia’s susceptibility to temperature [34], its potential positive regulation of host Tp in natural settings could mitigate the selection pressure from the thermal environment, thereby enhancing symbiosis stability and expanding its applications. Although we observed only subtle effects of Wolbachia on thermal preference, it could confer a fitness advantage in other ways, such as modifying host physiological responses to unsuitable temperature conditions [34], which could also enhance symbiosis stability. Given that Wolbachia induces complete cytoplasmic incompatibility in many leaf-miner species, there is great potential for using Wolbachia-induced incompatible insect techniques to control these pests [36,37].
Understanding Wolbachia’s potential role in influencing host thermal behavior is essential for unraveling the invasion dynamics of the leaf miner and lays the groundwork for future Wolbachia-based pest control strategies. Previous studies have shown that leaf miners often associate with multiple endosymbionts, with infection patterns that can vary spatially and temporally [26,38]. While this study focused on two representative populations of L. huidobrensis to examine the role of Wolbachia in host thermal behavior, distinct Wolbachia strains may exist in different populations [36], potentially influencing host responses to thermal changes. Nonetheless, our understanding of Wolbachia-mediated thermal preference in invasive leaf miners remains limited. Future studies should encompass a broader range of leaf miner–Wolbachia systems to explore the intricate interactions between Wolbachia and leaf-miner thermal behavior.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/insects15100784/s1, Table S1: Thermal preference (Tp) of leaf miners infected with or without Wolbachia.
Author Contributions
Y.Z.: conceptualization, data curation, formal analysis, funding acquisition, methodology, project administration, validation, writing—original draft, writing—review and editing; X.W.: data curation, investigation; S.W.: formal analysis, investigation; Z.S.: software, methodology, visualization, writing—original draft; Y.D.: conceptualization, supervision, writing—review and editing. All authors have agreed to be held accountable for the work performed herein. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Natural Science Foundation of Jiangsu Province, grant number BK20231330.
Data Availability Statement
The data are provided in Table S1 of the electronic Supplementary Materials.
Acknowledgments
We are grateful to Xiao-Feng Xue of Nanjing University, China and three anonymous reviewers for providing comments that improved this paper.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Werren, J.H.; Baldo, L.; Clark, M.E. Wolbachia: Master manipulators of invertebrate biology. Nat. Rev. Microbiol. 2008, 6, 741–751. [Google Scholar] [CrossRef] [PubMed]
- Kaur, R.; Shropshire, J.D.; Cross, K.L.; Leigh, B.; Mansueto, A.J.; Stewart, V.; Bordenstein, S.R.; Bordenstein, S.R. Living in the endosymbiotic world of Wolbachia: A centennial review. Cell Host Microbe 2021, 29, 879–893. [Google Scholar] [CrossRef]
- Porter, J.; Sullivan, W. The cellular lives of Wolbachia. Nat. Rev. Microbiol. 2023, 21, 750–766. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.X.; Song, Y.L.; Zhang, Y.K.; Hoffmann, A.A.; Zhou, J.C.; Sun, J.T.; Hong, X.Y. Incidence of facultative bacterial endosymbionts in spider mites associated with local environments and host plants. Appl. Environ. Microb. 2018, 84, e02546-17. [Google Scholar] [CrossRef] [PubMed]
- Adams, K.L.; Abernathy, D.G.; Willett, B.C.; Selland, E.K.; Itoe, M.A.; Catteruccia, F. Wolbachia cifB induces cytoplasmic incompatibility in the malaria mosquito vector. Nat. Microbiol. 2021, 6, 1575–1582. [Google Scholar] [CrossRef]
- Stouthamer, R.; Breeuwer, J.A.; Hurst, G.D. Wolbachia pipientis: Microbial manipulator of arthropod reproduction. Annu. Rev. Microbiol. 1999, 53, 71–102. [Google Scholar] [CrossRef]
- Douglas, A.E. The microbial dimension in insect nutritional ecology. Funct. Ecol. 2009, 23, 38–47. [Google Scholar] [CrossRef]
- Hosokawa, T.; Koga, R.; Kikuchi, Y.; Meng, X.Y.; Fukatsu, T. Wolbachia as a bacteriocyte-associated nutritional mutualist. Proc. Natl. Acad. Sci. USA 2010, 107, 769–774. [Google Scholar] [CrossRef]
- Ju, J.F.; Bing, X.L.; Zhao, D.S.; Guo, Y.; Xi, Z.; Hoffmann, A.A.; Zhang, K.J.; Huang, H.J.; Gong, J.T.; Zhang, X.; et al. Wolbachia supplement biotin and riboflavin to enhance reproduction in planthoppers. ISME J. 2020, 14, 676–687. [Google Scholar] [CrossRef]
- Hague, M.T.; Shropshire, J.D.; Caldwell, C.N.; Statz, J.P.; Stanek, K.A.; Conner, W.R.; Cooper, B.S. Temperature effects on cellular host-microbe interactions explain continent-wide endosymbiont prevalence. Curr. Biol. 2022, 32, 878–888. [Google Scholar] [CrossRef]
- Ferguson, L.F.; Ross, P.A.; van Heerwaarden, B. Wolbachia infection negatively impacts Drosophila simulans heat tolerance in a strain-and trait-specific manner. Environ. Microbiol. 2024, 26, e16609. [Google Scholar] [CrossRef] [PubMed]
- Hedges, L.M.; Brownlie, J.C.; O’Neill, S.L.; Johnson, K.N. Wolbachia and virus protection in insects. Science 2008, 322, 702. [Google Scholar] [CrossRef]
- Teixeira, L.; Ferreira, Á.; Ashburner, M. The bacterial symbiont Wolbachia induces resistance to RNA viral infections in Drosophila melanogaster. PLoS Biol. 2008, 6, e1000002. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.T.; Li, Y.; Li, T.P.; Liang, Y.; Hu, L.; Zhang, D.; Zhou, C.Y.; Yang, C.; Zhang, X.; Zha, S.S.; et al. Stable introduction of plant-virus-inhibiting Wolbachia into planthoppers for rice protection. Curr. Biol. 2020, 30, 4837–4845. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.T.; Li, T.P.; Wang, M.K.; Hong, X.Y. Wolbachia-based strategies for control of agricultural pests. Curr. Opin. Insect Sci. 2023, 57, 101039. [Google Scholar] [CrossRef]
- Strunov, A.; Schönherr, C.; Kapun, M. Wolbachia effects on thermal preference of natural Drosophila melanogaster are influenced by host genetic background, Wolbachia type, and bacterial titer. Environ. Microbiol. 2024, 26, e16579. [Google Scholar] [CrossRef]
- Hague, M.T.J.; Caldwell, C.N.; Cooper, B.S. Pervasive effects of Wolbachia on host temperature preference. mBio 2020, 11, e01768-20. [Google Scholar] [CrossRef]
- Zhu, Y.X.; Song, Z.R.; Zhang, Y.Y.; Hoffmann, A.A.; Hong, X.Y. Spider mites singly infected with either Wolbachia or Spiroplasma have reduced thermal tolerance. Front. Microbiol. 2021, 12, 706321. [Google Scholar] [CrossRef]
- Arnold, P.A.; Levin, S.C.; Stevanovic, A.L.; Johnson, K.N. Wolbachia-infected Drosophila prefer cooler temperatures. Ecol. Entomol. 2019, 44, 287–290. [Google Scholar] [CrossRef]
- Truitt, A.M.; Kapun, M.; Kaur, R.; Miller, W.J. Wolbachia modifies thermal preference in Drosophila melanogaster. Environ. Microbiol. 2019, 21, 3259–3268. [Google Scholar] [CrossRef]
- Strunov, A.; Schoenherr, C.; Kapun, M. Wolbachia has subtle effects on thermal preference in highly inbred Drosophila melanogaster which vary with life stage and environmental conditions. Sci. Rep. 2023, 13, 13792. [Google Scholar] [CrossRef] [PubMed]
- Weintraub, P.G.; Scheffer, S.J.; Visser, D.; Valladares, G.; Soares Correa, A.; Shepard, B.M.; Rauf, A.; Murphy, S.T.; Mujica, N.; MacVean, C.; et al. The invasive Liriomyza huidobrensis (Diptera: Agromyzidae): Understanding its pest status and management globally. J. Insect Sci. 2017, 17, 28. [Google Scholar] [CrossRef] [PubMed]
- Diagne, C.; Leroy, B.; Vaissière, A.C.; Gozlan, R.E.; Roiz, D.; Jarić, I.; Salles, J.M.; Bradshaw, C.J.A.; Courchamp, F. High and rising economic costs of biological invasions worldwide. Nature 2021, 592, 571–576. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Kang, L. Variation in cold hardiness of Liriomyza huidobrensis (Diptera: Agromyzidae) along latitudinal gradients. Environ. Entomol. 2004, 33, 155–164. [Google Scholar] [CrossRef]
- Gao, Y.L.; Reitz, S.R.; Xing, Z.L.; Ferguson, S.; Lei, Z.R. A decade of a leafminer invasion in China: Lessons learned. Pest Manag. Sci. 2017, 73, 1775–1779. [Google Scholar] [CrossRef]
- Zhu, Y.X.; Chang, Y.W.; Wen, T.; Yang, R.; Wang, Y.C.; Wang, X.Y.; Lu, M.M.; Du, Y.Z. Species identity dominates over environment in driving bacterial community assembly in wild invasive leaf miners. Microbiol. Spectr. 2022, 10, e00266-22. [Google Scholar] [CrossRef]
- Zhu, Y.X.; Wang, X.Y.; Yang, T.Y.; Zhang, H.H.; Li, T.P.; Du, Y.Z. Mechanisms of bacterial and fungal community assembly in leaf miners during transition from natural to laboratory environments. Front. Microbiol. 2024, 15, 1424568. [Google Scholar] [CrossRef]
- Lu, M.; Hulcr, J.; Sun, J. The role of symbiotic microbes in insect invasions. Annu. Rev. Ecol. Evol. Syst. 2016, 47, 487–505. [Google Scholar] [CrossRef]
- Matute, D.R.; Novak, C.J.; Coyne, J.A. Temperature-based extrinsic reproductive isolation in two species of Drosophila. Evolution 2009, 63, 595–612. [Google Scholar] [CrossRef]
- Rajpurohit, S.; Schmidt, P.S. Measuring thermal behavior in smaller insects: A case study in Drosophila melanogaster demonstrates effects of sex, geographic origin, and rearing temperature on adult behavior. Fly 2016, 10, 149–161. [Google Scholar] [CrossRef]
- Corbin, C.; Heyworth, E.R.; Ferrari, J.; Hurst, G.D. Heritable symbionts in a world of varying temperature. Heredity 2017, 118, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Iltis, C.; Tougeron, K.; Hance, T.; Louâpre, P.; Foray, V. A perspective on insect–microbe holobionts facing thermal fluctuations in a climate-change context. Environ. Microbiol. 2022, 24, 18–29. [Google Scholar] [CrossRef] [PubMed]
- Lemoine, M.M.; Engl, T.; Kaltenpoth, M. Microbial symbionts expanding or constraining abiotic niche space in insects. Curr. Opin. Insect Sci. 2020, 39, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.X.; Zhang, Y.Y.; Wang, X.Y.; Yin, Y.; Du, Y.Z. Wolbachia modify host cell metabolite profiles in response to short-term temperature stress. Environ. Microbiol. Rep. 2024, 16, e70013. [Google Scholar] [CrossRef]
- Ross, P.A.; Turelli, M.; Hoffmann, A.A. Evolutionary ecology of Wolbachia releases for disease control. Annu. Rev. Genet. 2019, 53, 93–116. [Google Scholar] [CrossRef]
- Tagami, Y.; Doi, M.; Sugiyama, K.; Tatara, A.; Saito, T. Survey of leafminers and their parasitoids to find endosymbionts for improvement of biological control. Biol. Control 2006, 38, 210–216. [Google Scholar] [CrossRef]
- Pramono, A.K.; Hidayanti, A.K.; Tagami, Y.; Ando, H. Bacterial community and genome analysis of cytoplasmic incompatibility-inducing Wolbachia in American serpentine leafminer, Liriomyza trifolii. Front. Microbiol. 2024, 15, 1304401. [Google Scholar] [CrossRef]
- Xu, X.; Ridland, P.M.; Umina, P.A.; Gill, A.; Ross, P.A.; Pirtle, E.; Hoffmann, A.A. High incidence of related Wolbachia across unrelated leaf-mining Diptera. Insects 2021, 12, 788. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).