Demography and Population Projection of Tetranychus urticae (Tetranychidae) on Phaseolus vulgaris (Fabaceae) Colonized by Entomopathogenic Fungal Endophytes
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Fungal Isolate Preparation and Preparation of Conidia
2.2. Seed Inoculation with Entomopathogenic Fungi
2.3. Evaluation of Plant P. vulgaris Endophytic Colonization
2.4. Spider Mites
2.5. Life Table Study
2.6. Population Projection
2.7. Statistical Analyses
3. Results
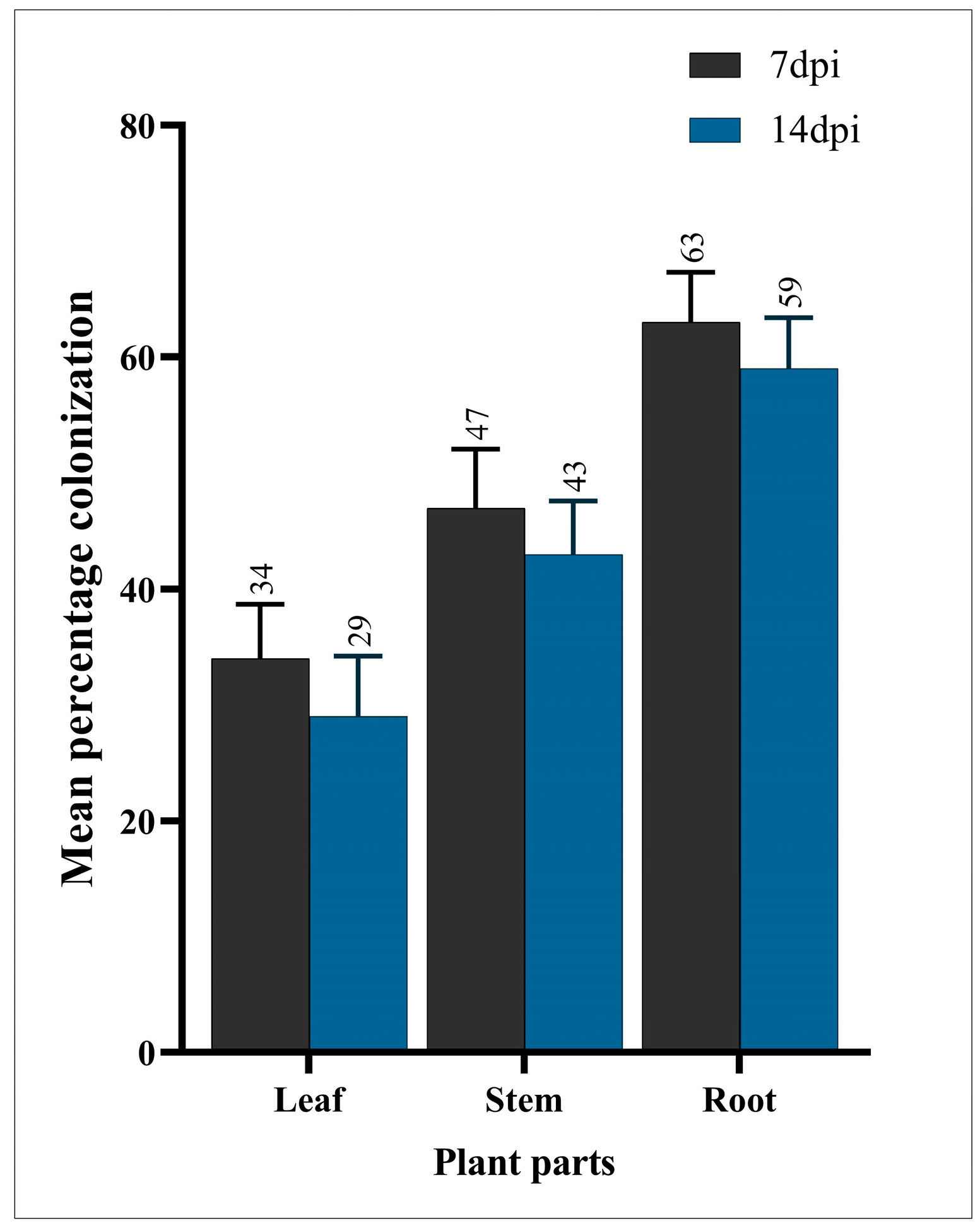
3.1. Endophytic Colonization
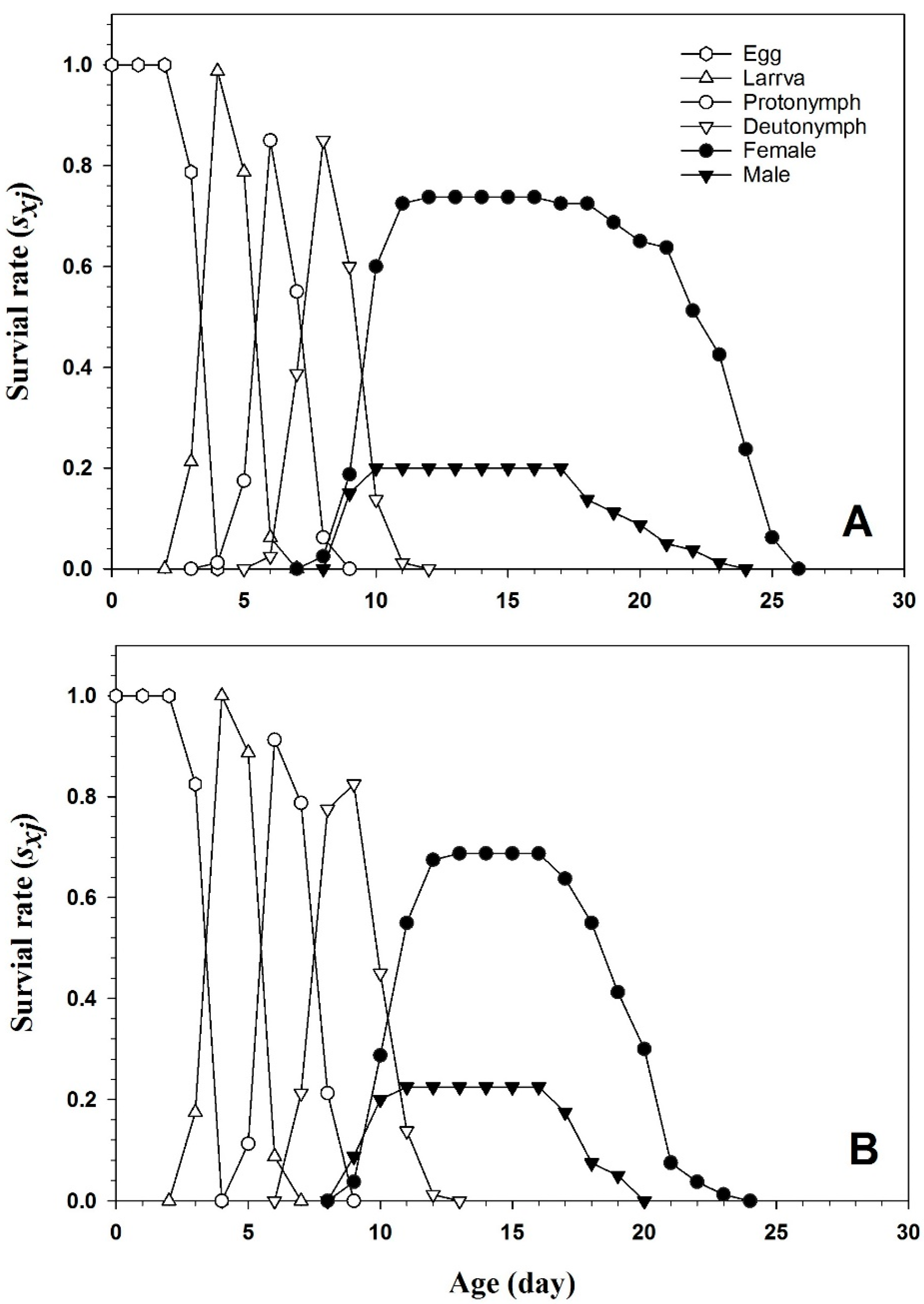
3.2. Key Life History Data of Tetranychus urticae
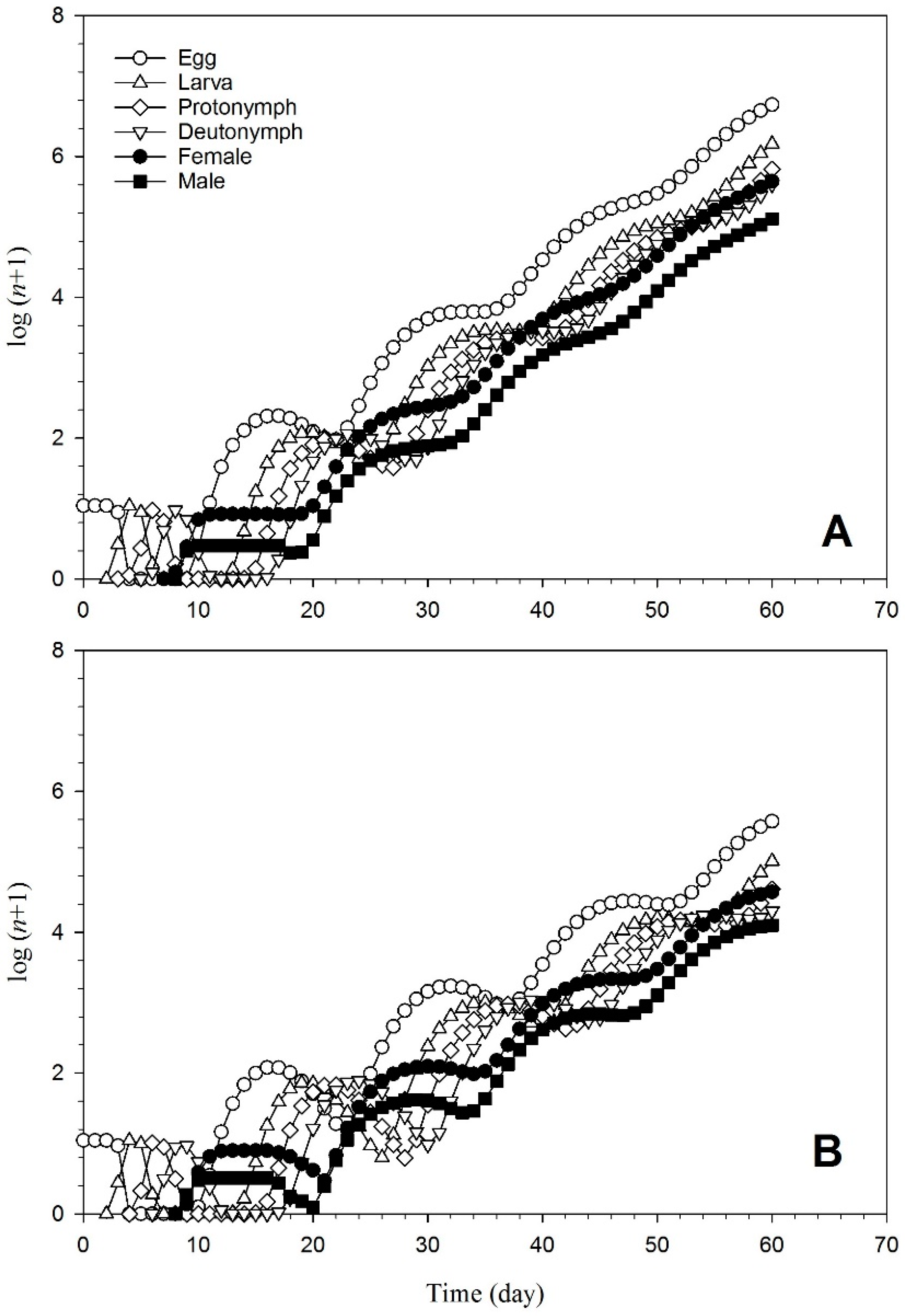
3.3. Demographic Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Esmaeel, R.E.; Basha, A.; Mostafa, E.; Abd El Mageed, A. Seasonal abundance of the two spotted spider mite, Tetranychus urticae koch on four cotton cultivars at Dakahlia Governorate, Egypt. Zagazig J. Agric. Res. 2018, 45, 1663–1674. [Google Scholar] [CrossRef]
- Dash, C.K.; Bamisile, B.S.; Keppanan, R.; Qasim, M.; Lin, Y.; Islam, S.U.; Hussain, M.; Wang, L. Endophytic entomopathogenic fungi enhance the growth of Phaseolus vulgaris L.(Fabaceae) and negatively affect the development and reproduction of Tetranychus urticae Koch (Acari: Tetranychidae). Microb. Pathog. 2018, 125, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Migeon, A. Spider Mites Web: A Comprehensive Database for the Tetranychidae. 2007. Available online: http://www.montpellier.inra.fr./CBGP/Spmweb/ (accessed on 23 April 2022).
- Boyle, S.M.; Salom, S.; Schultz, P.; Lopez, L.; Weber, D.C.; Kuhar, T.P. Augmentative biological control for squash bug (Hemiptera: Coreidae) using the egg parasitoid, Hadronotus pennsylvanicus (Hymenoptera: Scelionidae). Environ. Entomol. 2023, 52, 779–786. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.-L.; Lin, H.-Y.; Hou, J.; Feng, M.-G.; Ying, S.-H. The entomopathogenic fungus Beauveria bassiana employs autophagy as a persistence and recovery mechanism during conidial dormancy. mBio 2023, 14, e03049-22. [Google Scholar] [CrossRef] [PubMed]
- Jaber, L.R.; Ownley, B.H. Can we use entomopathogenic fungi as endophytes for dual biological control of insect pests and plant pathogens? Biol. Control 2018, 116, 36–45. [Google Scholar] [CrossRef]
- Jaronski, S.T. Mass production of entomopathogenic fungi—State of the art. Mass Prod. Benef. Org. 2023, 317–357. [Google Scholar] [CrossRef]
- Shahid, A.A.; Rao, Q.A.; Bakhsh, A.; Husnain, T. Entomopathogenic fungi as biological controllers: New insights into their virulence and pathogenicity. Arch. Biol. Sci. 2012, 64, 21–42. [Google Scholar] [CrossRef]
- Bamisile, B.S.; Siddiqui, J.A.; Akutse, K.S.; Ramos Aguila, L.C.; Xu, Y. General limitations to endophytic entomopathogenic fungi use as plant growth promoters, pests and pathogens biocontrol agents. Plants 2021, 10, 2119. [Google Scholar] [CrossRef]
- Van Loon, L. Plant responses to plant growth-promoting rhizobacteria. New Perspect. Approaches Plant Growth-Promot. Rhizobacteria Res. 2007, 119, 243–254. [Google Scholar]
- Glick, B.R. Plant growth-promoting bacteria: Mechanisms and applications. Scientifica 2012, 2012, 963401. [Google Scholar] [CrossRef]
- Thambugala, K.M.; Daranagama, D.A.; Phillips, A.J.; Kannangara, S.D.; Promputtha, I. Fungi vs. fungi in biocontrol: An overview of fungal antagonists applied against fungal plant pathogens. Front. Cell. Infect. Microbiol. 2020, 10, 604923. [Google Scholar] [CrossRef]
- Beneduzi, A.; Ambrosini, A.; Passaglia, L.M. Plant growth-promoting rhizobacteria (PGPR): Their potential as antagonists and biocontrol agents. Genet. Mol. Biol. 2012, 35, 1044–1051. [Google Scholar] [CrossRef]
- Cocking, E.C. Endophytic colonization of plant roots by nitrogen-fixing bacteria. Plant Soil 2003, 252, 169–175. [Google Scholar] [CrossRef]
- Branine, M.; Bazzicalupo, A.; Branco, S. Biology and applications of endophytic insect-pathogenic fungi. PLoS Pathog. 2019, 15, e1007831. [Google Scholar] [CrossRef]
- Gurulingappa, P.; Sword, G.A.; Murdoch, G.; McGee, P.A. Colonization of crop plants by fungal entomopathogens and their effects on two insect pests when in planta. Biol. Control 2010, 55, 34–41. [Google Scholar] [CrossRef]
- Canassa, F.; Tall, S.; Moral, R.A.; de Lara, I.A.; Delalibera, I., Jr.; Meyling, N.V. Effects of bean seed treatment by the entomopathogenic fungi Metarhizium robertsii and Beauveria bassiana on plant growth, spider mite populations and behavior of predatory mites. Biol. Control 2019, 132, 199–208. [Google Scholar] [CrossRef]
- Zhu, H.; Fu, J.; Wang, H.; Bidochka, M.J.; Duan, M.; Xu, W.; Sui, L.; Ren, B.; Li, Q.; Zhang, Z. Fitness consequences of oviposition choice by an herbivorous insect on a host plant colonized by an endophytic entomopathogenic fungus. J. Pest Sci. 2023, 96, 745–758. [Google Scholar] [CrossRef]
- Tuan, S.J.; Lee, C.C.; Chi, H. Population and damage projection of Spodoptera litura (F.) on peanuts (Arachis hypogaea L.) under different conditions using the age-stage, two-sex life table. Pest Manag. Sci. 2014, 70, 805–813. [Google Scholar] [CrossRef]
- Humber, R.A. Fungi: Identification. In Manual of Techniques in Insect Pathology; Elsevier: Amsterdam, The Netherlands, 1997; pp. 153–185. [Google Scholar]
- Chi, H. Life-table analysis incorporating both sexes and variable development rates among individuals. Environ. Entomol. 1988, 17, 26–34. [Google Scholar] [CrossRef]
- Chi, H.; Liu, H. Two new methods for the study of insect population ecology. Bull. Inst. Zool. Acad. Sin 1985, 24, 225–240. [Google Scholar]
- Chi, H.; You, M.; Atlıhan, R.; Smith, C.L.; Kavousi, A.; Özgökçe, M.S.; Güncan, A.; Tuan, S.-J.; Fu, J.-W.; Xu, Y.-Y.; et al. Age-Stage, two-sex life table: An introduction to theory, data analysis, and application. Entomol. Gen. 2020, 40, 103–124. [Google Scholar] [CrossRef]
- Hanife, G.; Saran, C. Age-Stage, Two-Sex Life Table of The Diamondback Moth, Plutella xylostella (Linnaeus, 1758) (Lepidoptera: Plutellidae) on Different Brassicaeous Plants. Türk Tarım Ve Doğa Bilim. Derg. 2021, 8, 615–628. [Google Scholar]
- Kirby, K.N.; Gerlanc, D. BootES: An R package for bootstrap confidence intervals on effect sizes. Behav. Res. Methods 2013, 45, 905–927. [Google Scholar] [CrossRef]
- Rosana, A.R.R.; Pokorny, S.; Klutsch, J.G.; Ibarra-Romero, C.; Sanichar, R.; Engelhardt, D.; van Belkum, M.J.; Erbilgin, N.; Bohlmann, J.; Carroll, A.L. Selection of entomopathogenic fungus Beauveria bassiana (Deuteromycotina: Hyphomycetes) for the biocontrol of Dendroctonus ponderosae (Coleoptera: Curculionidae, Scolytinae) in Western Canada. Appl. Microbiol. Biotechnol. 2021, 105, 2541–2557. [Google Scholar] [CrossRef]
- Gull, A.; Lone, A.A.; Wani, N.U.I. Biotic and Abiotic Stresses in Plants; IntechOpen Limited: London, UK, 2019; pp. 1–19. [Google Scholar] [CrossRef]
- Subbarayalu Mohankumar, S.M.; Natarajan Balakrishnan, N.B.; Ramasamy Samiyappan, R.S. Biotechnological and molecular approaches in the management of pests and diseases of crop plants. In Integrated Pest Management: Principles and Practice; CABI: Wallingford, UK, 2012; pp. 92–118. [Google Scholar]
- Al Khoury, C. Can colonization by an endophytic fungus transform a plant into a challenging host for insect herbivores? Fungal Biol. 2021, 125, 1009–1016. [Google Scholar] [CrossRef]
- White, N.; Bale, J.S.; Hayward, S.A. Life-history changes in the cold tolerance of the two-spot spider mite Tetranychus urticae: Applications in pest control and establishment risk assessment. Physiol. Entomol. 2018, 43, 334–345. [Google Scholar] [CrossRef]
- Macuphe, N. The Effect of the Entomopathogenic Fungus Beauveria bassiana on Growth, Physiological Responses and Control of Aphid (Myzus persicae) Infestation on Lactuca sativa L. Ph.D. Thesis, Cape Peninsula University of Technology, Cape Town, South Africa, 2020. [Google Scholar]
- Akello, J.; Dubois, T.; Gold, C.S.; Coyne, D.; Nakavuma, J.; Paparu, P. Beauveria bassiana (Balsamo) Vuillemin as an endophyte in tissue culture banana (Musa spp.). J. Invertebr. Pathol. 2007, 96, 34–42. [Google Scholar] [CrossRef]
- Athman, S.Y. Host-Endophyte-Pest Interactions of Endophytic Fusarium oxysporum Antagonistic to Radopholus similis in Banana (Musa spp.). Ph.D. Thesis, University of Pretoria, Pretoria, South Africa, 2006. [Google Scholar]
- Petrini, O. Fungal endophytes of tree leaves. In Microbial Ecology of Leaves; Springer: Berlin/Heidelberg, Germany, 1991; pp. 179–197. [Google Scholar]
- Riquelme, M.; Aguirre, J.; Bartnicki-García, S.; Braus, G.H.; Feldbrügge, M.; Fleig, U.; Hansberg, W.; Herrera-Estrella, A.; Kämper, J.; Kück, U. Fungal morphogenesis, from the polarized growth of hyphae to complex reproduction and infection structures. Microbiol. Mol. Biol. Rev. 2018, 82, e00068-17. [Google Scholar] [CrossRef]
- Wang, Z.; Li, M.; Ju, W.; Ye, W.; Xue, L.; Boufford, D.E.; Gao, X.; Yue, B.; Liu, Y.; Pierce, N.E. The entomophagous caterpillar fungus Ophiocordyceps sinensis is consumed by its lepidopteran host as a plant endophyte. Fungal Ecol. 2020, 47, 100989. [Google Scholar] [CrossRef]
- Torres-Rodriguez, J.A.; Reyes-Pérez, J.J.; Quiñones-Aguilar, E.E.; Hernandez-Montiel, L.G. Actinomycete potential as biocontrol agent of phytopathogenic fungi: Mechanisms, source, and applications. Plants 2022, 11, 3201. [Google Scholar] [CrossRef]
- Grabka, R.; d’Entremont, T.W.; Adams, S.J.; Walker, A.K.; Tanney, J.B.; Abbasi, P.A.; Ali, S. Fungal endophytes and their role in agricultural plant protection against pests and pathogens. Plants 2022, 11, 384. [Google Scholar] [CrossRef] [PubMed]
- Birch, L. The intrinsic rate of natural increase of an insect population. J. Anim. Ecol. 1948, 17, 15–26. [Google Scholar] [CrossRef]
- Medeiros, R.; Ramalho, F.; Lemos, W.; Zanuncio, J. Age-dependent fecundity and life-fertility tables for Podisus nigrispinus (Dallas) (Het., Pentatomidae). J. Appl. Entomol. 2000, 124, 319–324. [Google Scholar] [CrossRef]




| Statistics | Untreated Plants (50) | Colonized Plants (50) |
|---|---|---|
| Development duration (d) | ||
| Egg | 3.78 ± 0.04 a | 3.82 ± 0.04 a |
| Larva | 2.08 ± 0.04 a | 2.15 ± 0.03 a |
| Protonymph | 1.76 ± 0.04 b | 2.02 ± 0.06 a |
| Deutonymph | 2.14 ± 0.04 b | 2.45 ± 0.06 a |
| Total pre-adult stage | 9.77 ± 0.08 b | 10.49 ± 0.10 a |
| Preadult survival rate (sa) | 0.93 ± 0.001 a | 0.91 ± 0.001 a |
| Adult longevity (d) | 12.88 ± 0.25 a | 9.05 ± 0.13 b |
| APOP (d) | 0.84 ± 0.001 a | 0.81 ± 0.001 a |
| TPOP (d) | 11.56 ± 0.13 a | 10.76 ± 0.11 b |
| Total life span (d) | 22.65 ± 0.27 a | 19.54 ± 0.19 b |
| Fecundity (F) (eggs) | 57.67 ± 1.45 a | 28.01 ± 0.73 b |
| Oviposition days (d) | 11.10 ± 0.23 a | 7.50 ± 0.19 b |
| Parameters | Untreated Plants (50) | Colonized Plants (50) |
|---|---|---|
| Intrinsic rate of increase, r (d−1) | 0.245 ± 0.005 a | 0.196 ± 0.005 b |
| Finite rate of increase, λ (d−1) | 1.27 ± 0.006 a | 1.21 ± 0.007 b |
| Net fertility rate, R0 | 42.53 ± 3.03 a | 19.26 ± 1.54 b |
| Mean generation time, T (d) | 15.27 ± 0.12 a | 15.04 ± 0.14 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hong, P.; Dash, C.K.; Ghafar, M.A.; Haq, I.U.; Lu, L.; Zhou, C.; Wu, Q.; Wang, L. Demography and Population Projection of Tetranychus urticae (Tetranychidae) on Phaseolus vulgaris (Fabaceae) Colonized by Entomopathogenic Fungal Endophytes. Insects 2024, 15, 73. https://doi.org/10.3390/insects15010073
Hong P, Dash CK, Ghafar MA, Haq IU, Lu L, Zhou C, Wu Q, Wang L. Demography and Population Projection of Tetranychus urticae (Tetranychidae) on Phaseolus vulgaris (Fabaceae) Colonized by Entomopathogenic Fungal Endophytes. Insects. 2024; 15(1):73. https://doi.org/10.3390/insects15010073
Chicago/Turabian StyleHong, Pengxiang, Chandra Kanta Dash, Muhammad Adeel Ghafar, Inzamam Ul Haq, Liuyang Lu, Chenghua Zhou, Qing Wu, and Liande Wang. 2024. "Demography and Population Projection of Tetranychus urticae (Tetranychidae) on Phaseolus vulgaris (Fabaceae) Colonized by Entomopathogenic Fungal Endophytes" Insects 15, no. 1: 73. https://doi.org/10.3390/insects15010073
APA StyleHong, P., Dash, C. K., Ghafar, M. A., Haq, I. U., Lu, L., Zhou, C., Wu, Q., & Wang, L. (2024). Demography and Population Projection of Tetranychus urticae (Tetranychidae) on Phaseolus vulgaris (Fabaceae) Colonized by Entomopathogenic Fungal Endophytes. Insects, 15(1), 73. https://doi.org/10.3390/insects15010073









