Fungal Communities Associated with Siricid Wood Wasps: Focus on Sirex juvencus, Urocerus gigas, and Tremex fuscicornis
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Sites and Sampling
2.2. Fungal Culturing from Wood Wasps
2.3. DNA Extraction, Amplification, and Sequencing
2.4. Bioinformatics
2.5. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Slippers, B.; de Groot, P.; Wingfield, M.J. The Sirex Woodwasp and Its Fungal Symbiont: Research and Management of a Worldwide Invasive Pest; Springer: Dordrecht, The Netherlands, 2012. [Google Scholar]
- Hurley, B.P.; Slippers, B.; Wingfield, M.J. A comparison of control results for the alien invasive woodwasp, Sirex noctilio, in the southern hemisphere. Agric. For. Entomol. 2007, 9, 159–171. [Google Scholar] [CrossRef]
- Spradbery, J.P.; Kirk, A.A. Experimental studies on the responses of European siricid woodwasps to host trees. Ann. Appl. Biol. 1981, 98, 179–185. [Google Scholar] [CrossRef]
- Talbot, P.H.B. The Sirex-Amylostereum-Pinus association. Annu. Rev. Phytopathol. 1977, 15, 41–54. [Google Scholar] [CrossRef]
- Kukor, J.J.; Martin, M.M. Acquisition of digestive enzymes by thesiricid woodwasps from their fungal symbiont. Science 1983, 220, 1161–1163. [Google Scholar] [CrossRef]
- Madden, J.L.; Coutts, M.P. The role of fungi in the biology and ecology of woodwasps (Hymenoptera: Siricidae). In Insect–Fungus Symbiosis; Batra, L.R., Ed.; John Wiley: New York, NY, USA, 1979; Volume 16, pp. 165–174. [Google Scholar]
- Martin, M.M. Acquired enzymes in the siricid woodwasp Sirex cyaneus. In Invertebrate–Microbial Interactions. Ingested Fungal Enzymes in Arthropod Biology; Cornell University Press: Ithaca, NY, USA, 1987; Volume 17, pp. 37–48. [Google Scholar]
- Morgan, F. Bionomics of Siricidae. Annu. Rev. Entomol. 1968, 13, 239–256. [Google Scholar] [CrossRef]
- Gilmour, J.W. The life cycle of the fungal symbiont of Sirex noctilio. N. Z. J. For. 1965, 10, 80–89. [Google Scholar]
- King, J.M. Same aspects of the biology of the fungal symbiont of Sirex noctilio. Aust. J. Bot. 1966, 14, 25–30. [Google Scholar] [CrossRef]
- Francke-Grosmann, H. Über das Zusammenleben von Holzwespen (Siricidae) mit Pilzen. Z. Angew. Entomol. 1939, 25, 647–680. [Google Scholar] [CrossRef]
- Hudson, H.J. Fungal Biology; CUP Archive: Cambridge, UK, 1992. [Google Scholar]
- Ministry of Environment, State Forest Service. Lithuanian Statistical Yearbook of Forestry 2022; VšĮ Lulutė; Ministry of Environment, State Forest Service: Vilnius, Lithuania, 2019; 184p.
- Vasiliauskas, R.; Stenlid, J.; Thomsen, I.M. Clonality and genetic variation in Amylostereum areolatum and A. chailletii from northern Europe. New Phytol. 1998, 139, 751–758. [Google Scholar] [CrossRef]
- Wermelinger, B.; Thomsen, I.M. The woodwasp Sirex noctilio and its associated fungus Amylostereum areolatum in Europe. In The Sirex Woodwasp and Its Fungal Symbiont: Research and Management of a Worldwide Invasive Pest; Springer: Dordrecht, The Netherlands, 2011; pp. 65–80. [Google Scholar]
- Hausner, G.; Iranpour, M.; Kim, J.J.; Breuil, C.; Davis, C.N.; Gibb, E.A.; Hopkin, A.A. Fungi vectored by the introduced bark beetle Tomicus piniperda in Ontario, Canada, and comments on the taxonomy of Leptographium lundbergii, Leptographium terebrantis, Leptographium truncatum, and Leptographium wingfieldii. Botany 2005, 83, 1222–1237. [Google Scholar] [CrossRef]
- Persson, Y.; Vasaitis, R.; Långström, B.; Öhrn, P.; Ihrmark, K.; Stenlid, J. Fungi vectored by the bark beetle Ips typographus following hibernation under the bark of standing trees and in the forest litter. Microb. Ecol. 2009, 58, 651–659. Available online: https://www.jstor.org/stable/40343509 (accessed on 15 November 2023). [CrossRef] [PubMed]
- Menkis, A.; Lynikienė, J.; Marčiulynas, A.; Gedminas, A.; Povilaitienė, A. The great spruce bark beetle (Dendroctonus micans Kug.) (Coleoptera: Scolytidae) in Lithuania: Occurrence, phenology, morphology and communities of associated fungi. Bull. Entom. Res. 2017, 107, 431–438. [Google Scholar] [CrossRef] [PubMed]
- Pažoutova, S.; Šrutka, P. Symbiotic relationship between Cerrena unicolor and the hortnail Tremex fuscicornis recorded in the Czech Republic. Czech Mycol. 2007, 59, 83–90. [Google Scholar] [CrossRef]
- Šrutka, P.; Pažoutova, S.; Kolarik, M. Daldinia decipiens and Entonaema cinnabarina as fungal symbionts of Xiphydria wood wasps. Mycol. Res. 2007, 111, 224–231. [Google Scholar] [CrossRef]
- Thomsen, I.M.; Hardini, S. Fungal symbionts of siricid woodwasps: Isolation techniques and identification. For. Pathol. 2011, 41, 325–333. [Google Scholar] [CrossRef]
- Atlas, R.M. Handbook of Microbiological Media, 2nd ed.; CRC Press: Boca Raton, FL, USA, 1997; p. 1706. [Google Scholar]
- Menkis, A.; Marčiulynas, A.; Gedminas, A.; Lynikienė, J.; Povilaitienė, A. High-throughput sequencing reveals drastic changes in fungal communities in the phyllosphere of Norway spruce (Picea abies) following invasion of the spruce bud scale (Physokermes piceae). Microb. Ecol. 2015, 70, 904–911. [Google Scholar] [CrossRef]
- Ihrmark, K.; Bodeker, I.T.M.; Cruz-Martinez, K.; Friberg, H.; Kubartova, A.; Schenck, J.; Strid, Y.; Stenlid, J.; Brandstrom-Durling, M.; Clemmensen, K.E.; et al. New primers to amplify the fungal ITS2—Evaluation by 454-sequencing of artificial and natural communities. FEMS Microbiol. Ecol. 2012, 82, 666–677. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press: San Diego, CA, USA, 1990; pp. 315–322. [Google Scholar]
- Magurran, A.E. Ecological Diversity and Its Measurement; Princeton University Press: Princeton, NJ, USA, 1988; p. 192. [Google Scholar]
- Sokal, R.R.; Rohlf, F.J. Biometry: The Principles and Practice of Statistics in Biological Research, 3rd ed.; W.H. Freeman and Co.: New York, NY, USA, 1995; p. 887. [Google Scholar]
- Shannon, C.E. A mathematical theory of communication. Bell Syst. Technol. J. 1948, 27, 379–423. [Google Scholar] [CrossRef]
- ter Braak, C.J.F.; Smilauer, P. Canoco Reference Manual and User’s Guide to Canoco for Windows: Software for Canonical Community Ordination, Version 4; Microcomputer Power: Ithaca, NY, USA, 1998; p. 351. [Google Scholar]
- Oksanen, J.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.; O’Hara, B.; Simpson, G.; Solymos, P.; Stevens, H.; Wagner, H. Vegan: Community Ecology Package, R Package Version 2.5–3. 2018. Available online: https://cran.r-project.org (accessed on 14 August 2023).
- Neumann, F.G.; Morey, J.L. Influence of natural enemies on the Sirex wood wasp in herbicide-treated trap trees of radiata pine in north-eastern Victoria. Aust. For. 1984, 47, 218–224. [Google Scholar] [CrossRef]
- Bedding, R.A. Controlling the pine-killing woodwasp, Sirex noctilio, with nematodes. In Use of Microbes for Control and Eradication of Invasive Arthropods; Springer: Dordrecht, The Netherlands, 2009; pp. 213–235. [Google Scholar]
- Nielsen, C.; Williams, D.W.; Hajek, A.E. Putative source of the invasive Sirex noctilio fungal symbiont, Amylostereum areolatum, in the eastern United States and its association with native siricid woodwasps. Mycol. Res. 2009, 113, 1242–1253. [Google Scholar] [CrossRef]
- Eichhorn, O. “Siricoidea”. In Die Forstschädlinge Europas. Band 4: Hautflügler und Zweiflügler; Wolfgang, S., Ed.; Paul Parey: Hamburg, Germany, 1982; pp. 196–231. (In German) [Google Scholar]
- Boddy, L. Fungal community ecology and wood decomposition processes in angiosperms: From standing tree to complete decay of coarse woody debris. Ecol. Bull. 2001, 49, 43–56. Available online: http://www.jstor.org/stable/20113263 (accessed on 18 November 2023).
- Parisi, F.; Pioli, S.; Lombardi, F.; Fravolini, G.; Marchetti, M.; Tognetti, R. Linking deadwood traits with saproxylic invertebrates and fungi in European forests-a review. iForest 2018, 11, 423. [Google Scholar] [CrossRef]
- Domsch, K.H.; Gams, W.; Anderson, T.H. Compendium of Soil Fungi; IHW-Verlag: Eching, Germany, 2007; p. 672. [Google Scholar]
- Kirk, P.M.; Cannon, P.F.; Minter, D.W.; Stalpers, J.A. Dictionary of the Fungi; CABI: Wallingford, UK, 2008; p. 310. [Google Scholar]
- Menkis, A.; Vasiliauskas, R.; Taylor, A.F.S.; Stenström, E.; Stenlid, J.; Finlay, R. Fungi in decayed roots of conifer seedlings from forest nurseries, afforested clearcuts and abandoned farmland. Plant Pathol. 2006, 55, 117–129. [Google Scholar] [CrossRef]
- Wingfield, M.J.; Hammerbacher, A.; Ganley, R.J.; Steenkamp, E.T.; Gordon, T.R.; Wingfield, B.D.; Coutinho, T.A. Pitch canker caused by Fusarium circinatum—A growing threat to pine plantations and forests worldwide. Australas. Plant Pathol. 2008, 37, 319–334. [Google Scholar] [CrossRef]
- Moya-Elizondo, E.A.; Arismendi, N.; Montalva, C.; Doussoulin, H. First report of Fusarium sporotrichioides causing foliar spots on forage corn in Chile. Plant Dis. 2013, 97, 1113. [Google Scholar] [CrossRef] [PubMed]
- Ocamb, C.M.; Juzwik, J.; Martin, F.B. Fusarium spp. and Pinus strobus seedlings: Root disease pathogens and taxa associated with seed. New For. 2002, 24, 67–79. [Google Scholar] [CrossRef]
- Bezos, D.; Martínez-Álvarez, P.; Sanz-Ros, A.V.; Martín-García, J.; Fernandez, M.M.; Diez, J.J. Fungal Communities Associated with Bark Beetles in Pinus radiata Plantations in Northern Spain Affected by Pine Pitch Canker, with Special Focus on Fusarium Species. Forests 2018, 9, 698. [Google Scholar] [CrossRef]
- Ivanová, H.; Hrehová, Ľ.; Pristaš, P. First confirmed report on Fusarium sporotrichioides on Pinus ponderosa var. jeffreyi in Slovakia. Plant Protect. Sci. 2016, 52, 250–253. [Google Scholar] [CrossRef]
- Müller, M.M.; Hantula, J.; Wingfield, M.; Drenkhan, R. Diplodia sapinea found on Scots pine in Finland. For. Pathol. 2019, 49, e12483. [Google Scholar] [CrossRef]
- Luchi, N.; Oliveira Longa, C.M.; Danti, R.; Capretti, P.; Maresi, G. Diplodia sapinea: The main fungal species involved in the colonization of pine shoots in Italy. For. Pathol. 2014, 44, 372–381. [Google Scholar] [CrossRef]
- Brasier, C.M.; Webber, J.F. Is there evidence for post-epidemic attenuation in the Dutch elm disease pathogen Ophiostoma novo-ulmi? Plant Pathol. 2019, 68, 921–929. [Google Scholar] [CrossRef]
- Linnakoski, R.; De Beer, Z.W.; Rousi, M.; Solheim, H.; Wingfield, M.J. Ophiostoma denticiliatum sp. nov. and other Ophiostoma species associated with the birch bark beetle in southern Norway. Mol. Phylogeny Evol. Fungi 2009, 23, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Stadler, M.; Baumgartner, M.; Wollweber, H.; Ju, Y.M.; Rogers, J. Daldinia decipiens sp. nov and notes on some other European Daldinia spp. inhabiting Betulaceae. Mycotaxon 2001, 80, 167–177. Available online: http://ir.sinica.edu.tw/handle/201000000A/28387 (accessed on 20 November 2023).
- Johannesson, H.; Gustafsson, M.; Stenlid, J. Local population structure of the wood decay ascomycete Daldinia loculata. Mycologia 2001, 93, 440–446. [Google Scholar] [CrossRef]
- Liston, A.D. Increasing abundance of Xiphydria longicollis Geoffroy, linked to poor health of mature broadleaved trees in Germany and England (Hymenoptera: Xiphydriidae). Sawfly News Daibersdorf 1997, 1, 26–29. [Google Scholar]
- Kraus, M. Zur Verbreitung und Lebensweise der Eichensch-wertwespe Xiphydria longicollis Geoffroy, 1785. In Bayern Hym.: Symphyta, Fam. Xiphydriidae. Galathea 1997, 13, 89–106. [Google Scholar]
- Lou, Q.Z.; Lu, M.; Sun, J.H. Yeast diversity associated with invasive Dendroctonus valens killing Pinus tabuliformis in China using culturing and molecular methods. Microb. Ecol. 2014, 68, 397–415. [Google Scholar] [CrossRef]
- Davis, T.S. The ecology of yeasts in the bark beetle holobiont: A century of research revisited. Microb. Ecol. 2015, 69, 723–732. [Google Scholar] [CrossRef]
- Veselská, T.; Švec, K.; Kostovčík, M.; Peral-Aranega, E.; Garcia-Fraile, P.; Křížková, B.; Kolařík, M. Proportions oftaxa belonging to the gut core microbiome change throughout the life cycle and season of the bark beetle Ips typographus. FEMS Microbiol. Ecol. 2023, 99, fiad072. [Google Scholar] [CrossRef]
- Linnakoski, R.; Lasarov, I.; Veteli, P.; Tikkanen, O.P.; Viiri, H.; Jyske, T.; Wingfield, M.J. Filamentous fungi and yeasts associated with mites phoretic on Ips typographus in eastern Finland. Forests 2021, 12, 743. [Google Scholar] [CrossRef]
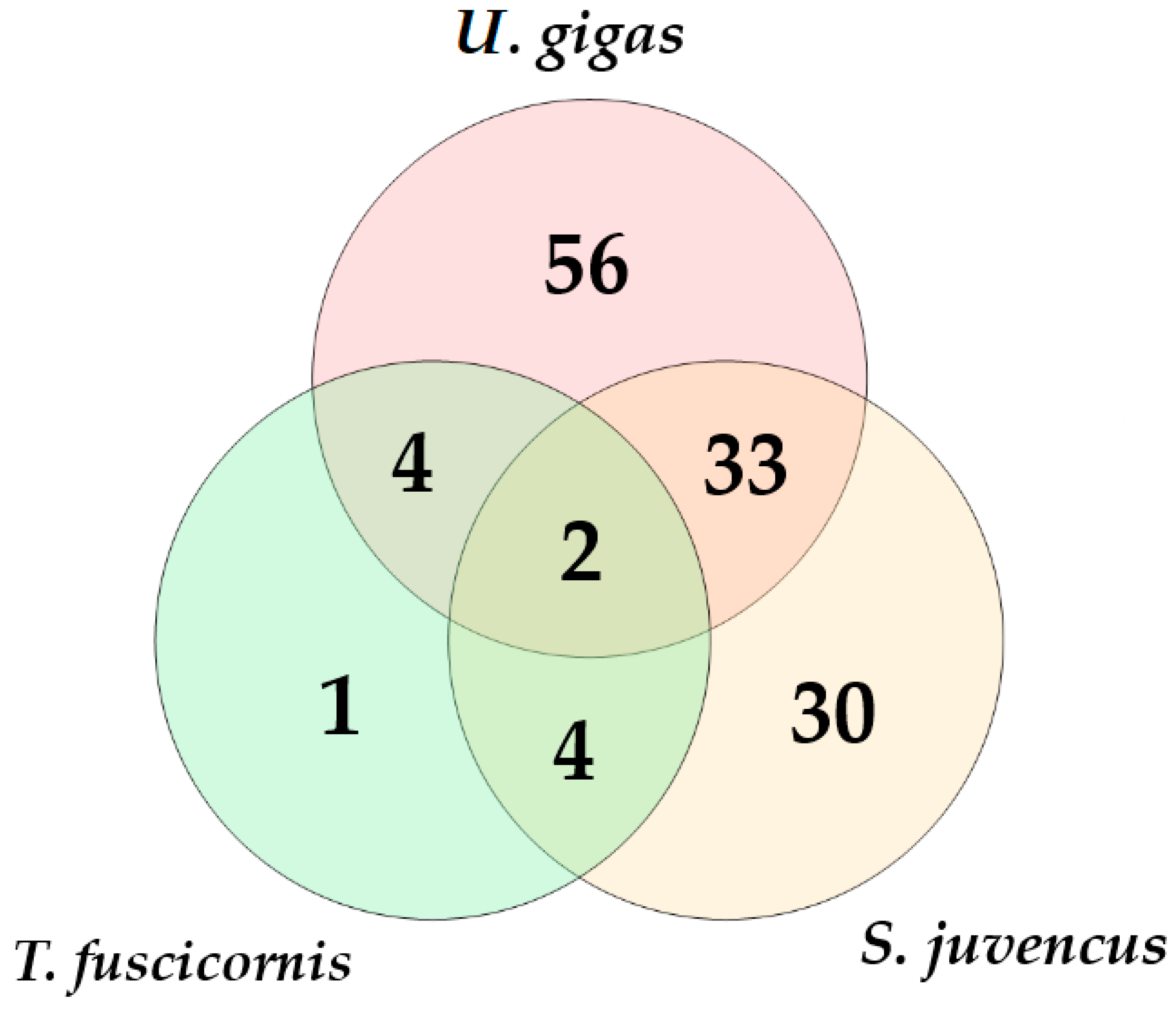


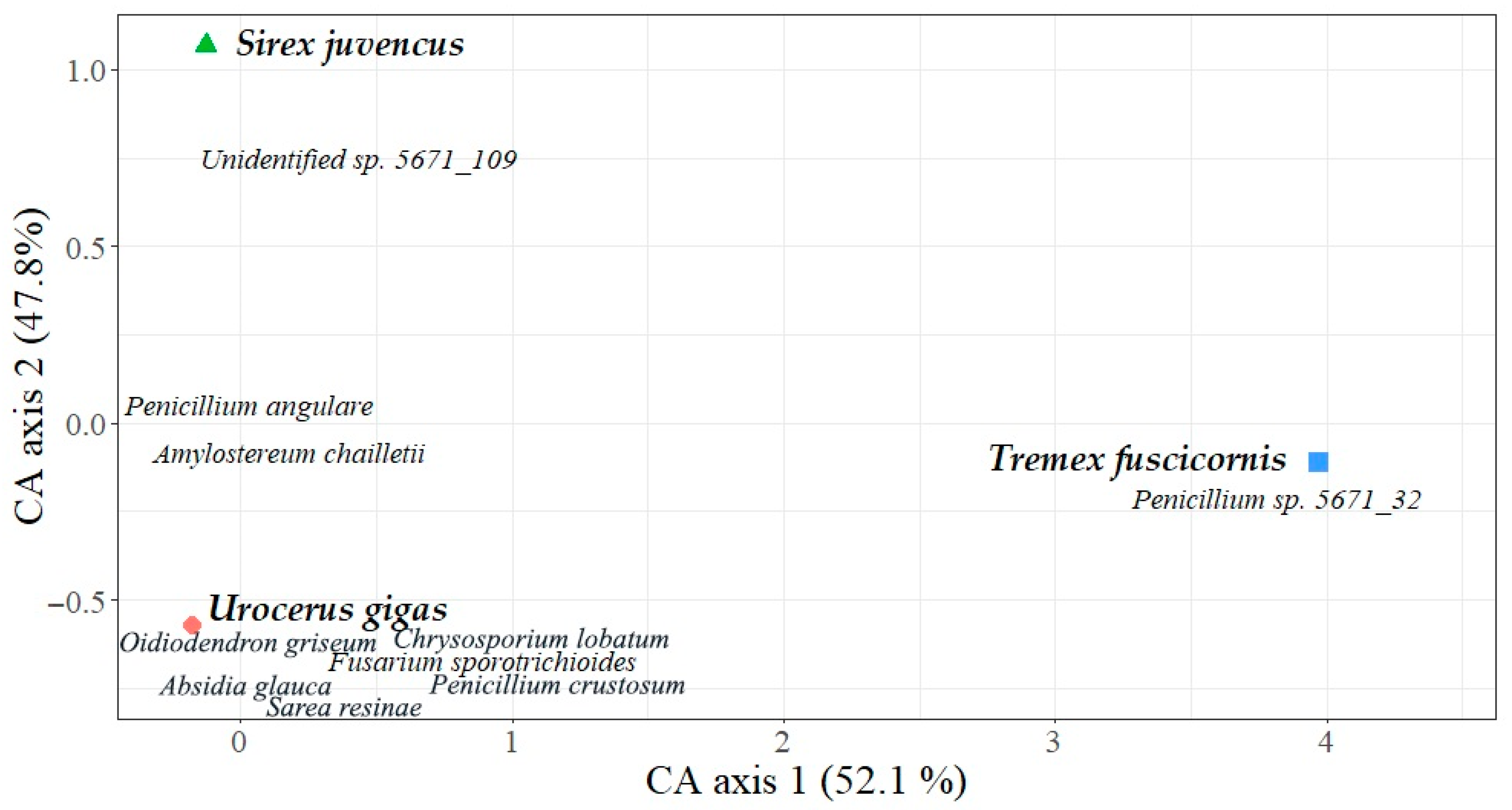
| Species | Stage | No. of Individuals | No. of Reads | Average read Length, bp | No. of Fungal OTUs | Shannon Diversity Index |
|---|---|---|---|---|---|---|
| Sirex juvencus | Adult female | 17 | 1390 | 327 | 66 | 2.42 |
| Urocerus gigas | Adult female | 19 | 58,543 | 323 | 93 | 1.28 |
| Tremex fuscicornis | Larvae | 24 | 293 | 338 | 10 | 0.32 |
| All | 60 | 59,797 | 330 | 127 |
| Phylum * | Species | GenBank Reference | Compared, bp; Similarity, % | Urocerus gigas, % | Sirex juvencus, % | Tremex fuscicornis, % | All, % |
|---|---|---|---|---|---|---|---|
| A | Fusarium sporotrichioides | MN452643 | 303/306 (99) | 64.92 | - | - | 63.11 |
| B | Amylostereum chailletii | MH857239 | 336/343 (98) | 15.17 | 7.69 | 0.34 | 14.92 |
| A | Penicillium crustosum | MT316358 | 319/323 (99) | 7.84 | 6.48 | - | 7.77 |
| A | Microascus sp. 5671_3 | NR_155397 | 324/329 (98) | 5.10 | - | - | 4.96 |
| A | Pithoascus ater | NR_132951 | 328/333 (98) | 2.14 | - | - | 2.08 |
| A | Cyberlindnera sp. 5671_6 | MK394132 | 326/348 (94) | 1.15 | - | - | 1.12 |
| A | Unidentified sp. 5671_7 | MG827482 | 330/330 (100) | 0.03 | 45.80 | - | 1.08 |
| A | Wickerhamomyces canadensis | KY105899 | 330/337 (98) | 0.59 | - | - | 0.58 |
| A | Daldinia decipiens | LC376942 | 323/327 (99) | 0.002 | - | 94.54 | 0.46 |
| A | Ogataea sp. 5671_10 | KY104428 | 341/344 (99) | 0.45 | - | - | 0.44 |
| A | Unidentified sp. 5671_13 | MT535771 | 326/334 (98) | 0.45 | - | - | 0.44 |
| A | Kuraishia molischiana | KY103919 | 337/340 (99) | 0.43 | - | - | 0.42 |
| A | Wickerhamomyces bisporus | KY105897 | 338/345 (98) | 0.23 | - | - | 0.22 |
| A | Yamadazyma scolyti | HE612108 | 344/347 (99) | 0.20 | 0.22 | - | 0.20 |
| A | Acrostalagmus luteoalbus | MT528981 | 335/338 (99) | 0.17 | - | - | 0.16 |
| A | Unidentified sp. 5671_25 | KP897542 | 308/309 (99) | 0.007 | 5.18 | - | 0.13 |
| A | Fusarium sp. 5671_18 | OQ306292 | 319/323 (99) | 0.12 | - | - | 0.12 |
| A | Lophium arboricola | MK159395 | 311/316 (98) | 0.007 | 4.17 | - | 0.10 |
| A | Scoliciosporum umbrinum | KX133008 | 303/306 (99) | 0.005 | 4.25 | - | 0.10 |
| A | Fusarium sp. 5671_22 | MT558569 | 303/307 (99) | 0.10 | - | - | 0.10 |
| All of 20 OTUs | 99.12 | 73.74 | 94.88 | 98.52 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marčiulynas, A.; Lynikienė, J.; Gedminas, A.; Povilaitienė, A.; Menkis, A. Fungal Communities Associated with Siricid Wood Wasps: Focus on Sirex juvencus, Urocerus gigas, and Tremex fuscicornis. Insects 2024, 15, 49. https://doi.org/10.3390/insects15010049
Marčiulynas A, Lynikienė J, Gedminas A, Povilaitienė A, Menkis A. Fungal Communities Associated with Siricid Wood Wasps: Focus on Sirex juvencus, Urocerus gigas, and Tremex fuscicornis. Insects. 2024; 15(1):49. https://doi.org/10.3390/insects15010049
Chicago/Turabian StyleMarčiulynas, Adas, Jūratė Lynikienė, Artūras Gedminas, Aistė Povilaitienė, and Audrius Menkis. 2024. "Fungal Communities Associated with Siricid Wood Wasps: Focus on Sirex juvencus, Urocerus gigas, and Tremex fuscicornis" Insects 15, no. 1: 49. https://doi.org/10.3390/insects15010049
APA StyleMarčiulynas, A., Lynikienė, J., Gedminas, A., Povilaitienė, A., & Menkis, A. (2024). Fungal Communities Associated with Siricid Wood Wasps: Focus on Sirex juvencus, Urocerus gigas, and Tremex fuscicornis. Insects, 15(1), 49. https://doi.org/10.3390/insects15010049






