Comparison of Energy Budget of Cockroach Nymph (Hemimetabolous) and Hornworm (Holometabolous) under Food Restriction
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Rearing and Food Supply
2.2. Measurement of Growth Rate
2.3. Measurement of Metabolic Rate
2.4. Data Analysis and Statistics
3. Results
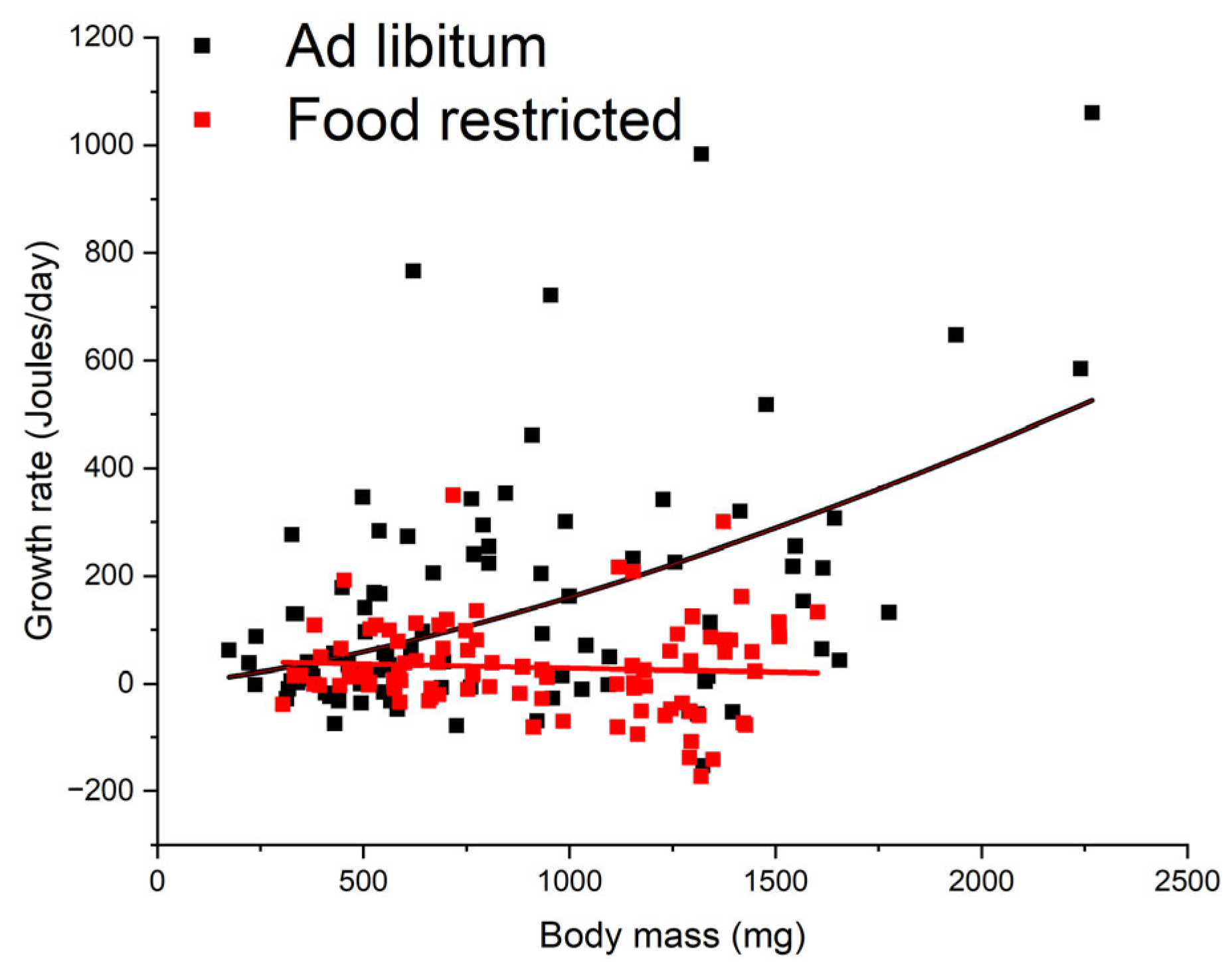
3.1. Growth Rate
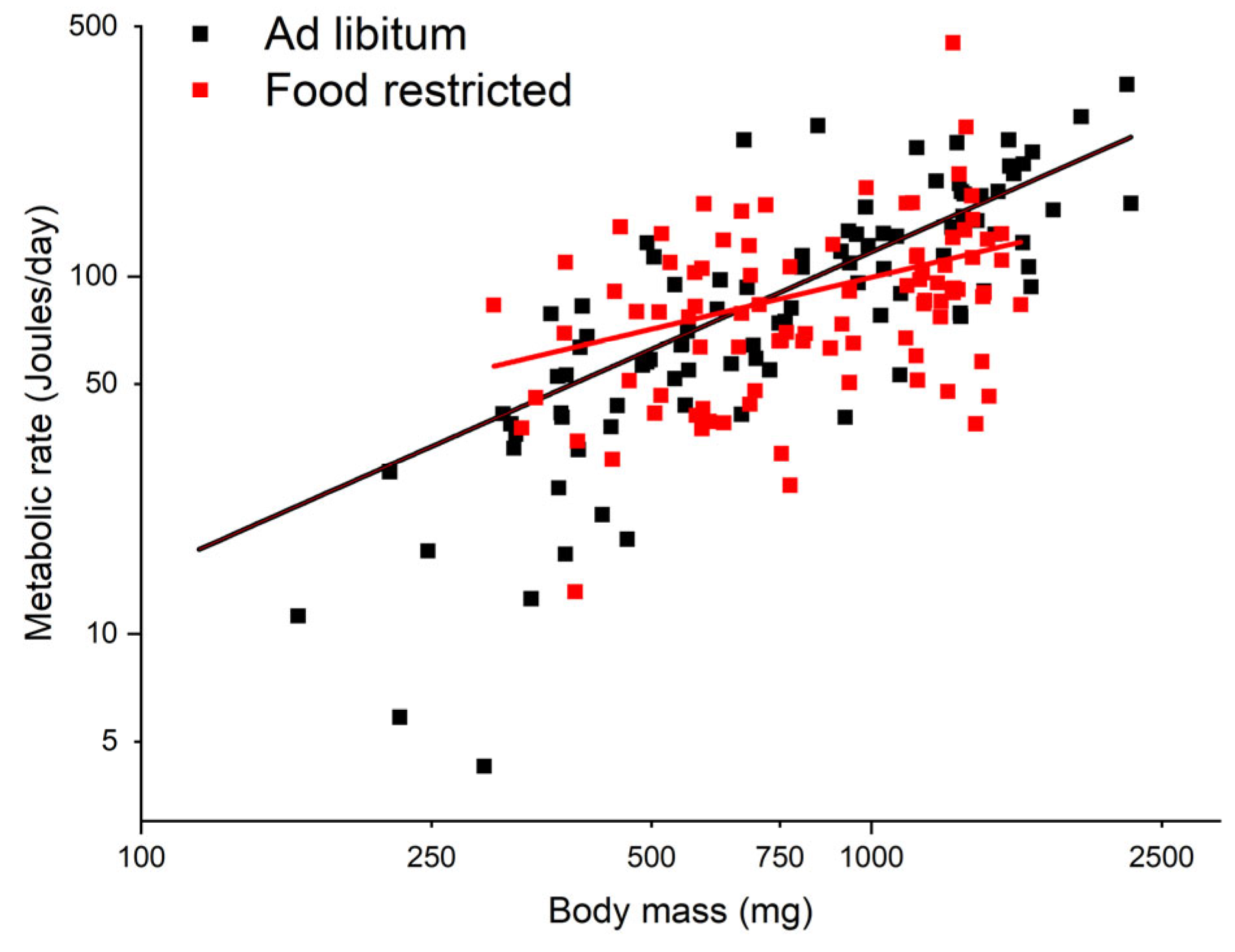
3.2. Metabolic Rate
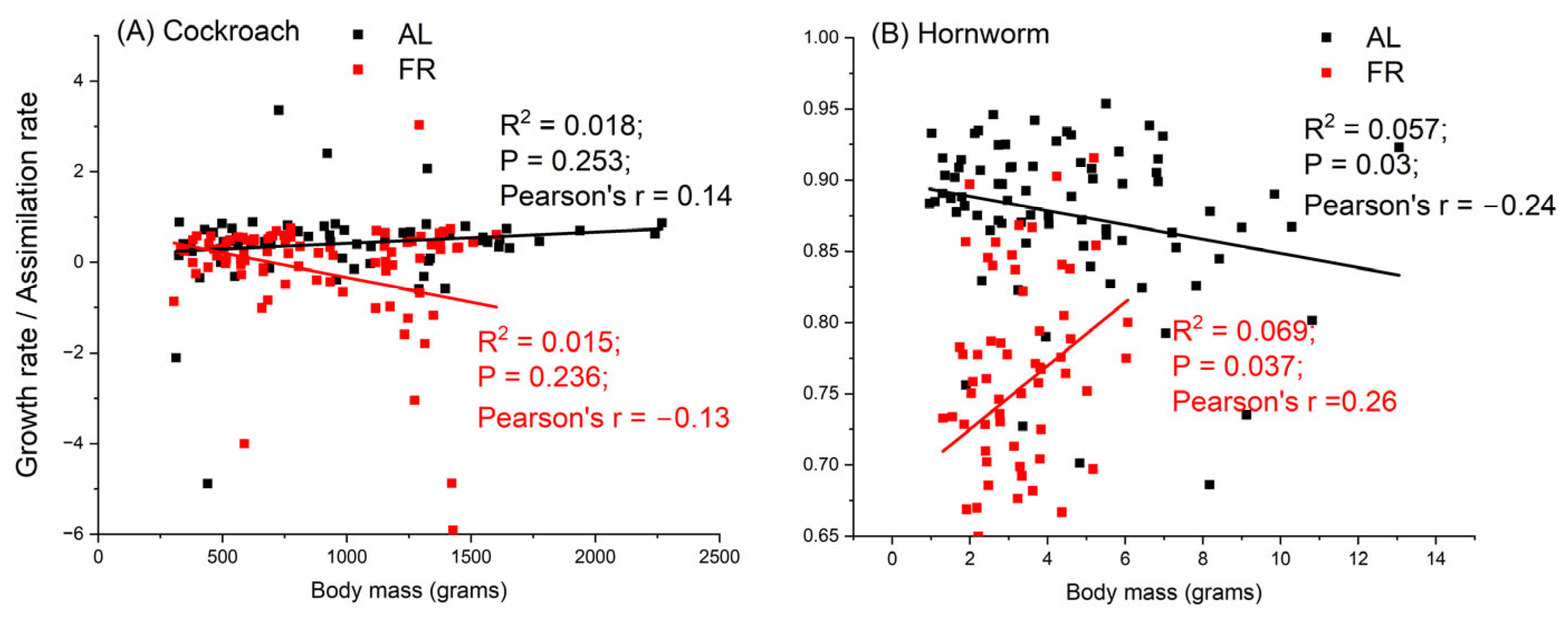
3.3. Assimilation Rate
3.4. Comparing Energy Budgets of Cockroaches and Hornworms
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kleiber, M. The Fire of Life: An Introduction to Animal Energetics; John Wiley & Sons Inc.: New York, NY, USA, 1961. [Google Scholar]
- Brody, S. Bioenergetics and Growth; Reinhold: New York, NY, USA, 1945. [Google Scholar]
- Hou, C.; Zuo, W.Y.; Moses, M.E.; Woodruff, W.H.; Brown, J.H.; West, G.B. Energy uptake and allocation during ontogeny. Science 2008, 322, 736–739. [Google Scholar] [CrossRef] [PubMed]
- Kooijman, S.A.L.M. Dynamic Energy and Mass Budgets in Biological Systems; Cambridge University Press: Cambridge, UK, 2000. [Google Scholar]
- Makarieva, A.; Gorshkov, V.G.; Li, B.L. Ontogenetic growth: Models and theory. Ecol. Model. 2004, 176, 15–26. [Google Scholar] [CrossRef]
- van der Meer, J. Metabolic theories in ecology. Trends Ecol. Evol. 2006, 21, 136–140. [Google Scholar] [CrossRef] [PubMed]
- West, G.B.; Brown, J.H.; Enquist, B.J. A general model for ontogenetic growth. Nature 2001, 413, 628–631. [Google Scholar] [CrossRef] [PubMed]
- Schoener, T.W. Theory of Feeding Strategies. Annu. Rev. Ecol. Syst. 1971, 2, 369–404. [Google Scholar] [CrossRef]
- Hou, C.; Bolt, K.M.; Bergman, A. A general model for ontogenetic growth under food restriction. Proc. R. Soc. B Biol. Sci. 2011, 278, 2881–2890. [Google Scholar] [CrossRef] [PubMed]
- Roff, D.A. Life History Evolution; Sinauer Associates: Sunderland, MA, USA, 2001. [Google Scholar]
- Jiao, L.; Amunugama, K.; Hayes, M.; Jennings, M.; Domingo, A.; Hou, C. Food restriction alters energy allocation strategy during growth in tobacco hornworms (Manduca sexta larvae). Sci. Nat. 2015, 102, 40–50. [Google Scholar] [CrossRef]
- Mangel, M.; Munch, S.B. A life-history perspective on short- and long-term consequences of compensatory growth. Am. Nat. 2005, 166, E155–E176. [Google Scholar] [CrossRef]
- Broekhuizen, N.; Gurney, W.S.C.; Jones, A.; Bryant, A.D. Modelling Compensatory Growth. Funct. Ecol. 1994, 8, 770–782. [Google Scholar] [CrossRef]
- Dmitriew, C.M. The evolution of growth trajectories: What limits growth rate? Biol. Rev. 2011, 86, 97–116. [Google Scholar] [CrossRef]
- Davidowitz, G.; D’Amico, L.J.; Nijhout, H.F. Critical weight in the development of insect body size. Evol. Dev. 2003, 5, 188–197. [Google Scholar] [CrossRef] [PubMed]
- Nijhout, H.F. A threshold size for metamorphosis in the tobacco hornworm, Manduca sexta (L.). Biol. Bull. 1975, 149, 214–225. [Google Scholar] [CrossRef] [PubMed]
- Cummins, K.W.; Wuycheck, J.C. Calorific equivalents for investigations in ecological energetics. Mitt. Int. Ver. Theor. Angew. Limnol. 1971, 18, 1–58. [Google Scholar]
- Robbins, C.T. Wildlife Feeding and Nutrition; Academic Press Inc.: Cambridge, MA, USA, 1983. [Google Scholar]
- Hayes, M.; Jiao, L.; Tsao, T.-h.; King, I.; Jennings, M.; Hou, C. High temperature slows down growth in tobacco hornworms (Manduca sexta larvae) under food restriction. Insect Sci. 2014; in press. [Google Scholar] [CrossRef] [PubMed]
- Lighton, J.R.B. Measuring Metabolic Rates: A Manual for Scientists; Oxford University Press: Oxford, MS, USA, 2018. [Google Scholar]
- Blaxter, K.L. Energy Metabolism in Animals and Man; Cambridge University Press: Cambridge, UK, 1989. [Google Scholar]
- Withers, P.C. Comparative Animal Physiology; Saunders College Pub: Fort Worth, TX, USA, 1992. [Google Scholar]
- Honěk, A. Intraspecific variation in body size and fecundity in insects: A general relationship. Oikos 1993, 66, 483–492. [Google Scholar] [CrossRef]
- McCarter, R.J.; McGee, J.R. Transient Reduction of Metabolic-Rate by Food Restriction. Am. J. Physiol.-Endocrinol. Metab. 1989, 257, E175–E179. [Google Scholar] [CrossRef] [PubMed]
- McCarter, R.J.; Palmer, J. Energy-Metabolism and Aging—A Lifelong Study of Fischer-344 Rats. Am. J. Physiol.-Endocrinol. Metab. 1992, 263, E448–E452. [Google Scholar] [CrossRef]
- Naim, M.; Brand, J.G.; Kare, M.R.; Kaufmann, N.A.; Kratz, C.M. Effects of unpalatable diets and food restriction on feed efficiency in growing rats. Physiol. Behav. 1980, 25, 609–614. [Google Scholar] [CrossRef]
- Ocak, N.; Erener, G. The effects of restricted feeding and feed form on growth, carcass characteristics and days to first egg of Japanese quail (Coturnix coturnix japonica). Asian Australas. J. Anim. Sci. 2005, 18, 1479. [Google Scholar] [CrossRef]
- Benyi, K.; Habi, H. Effects of food restriction during the finishing period on the performance of broiler chickens. Br. Poultry Sci. 1998, 39, 423–425. [Google Scholar] [CrossRef]
- Kitaysky, A.S. Metabolic and developmental responses of alcid chicks to experimental variation in food intake. Physiol. Biochem. Zool. 1999, 72, 462–473. [Google Scholar] [CrossRef] [PubMed]
- Rønning, B.; Mortensen, A.S.; Moe, B.; Chastel, O.; Arukwe, A.; Bech, C. Food restriction in young Japanese quails: Effects on growth, metabolism, plasma thyroid hormones and mRNA species in the thyroid hormone signalling pathway. J. Exp. Biol. 2009, 212, 3060–3067. [Google Scholar] [CrossRef] [PubMed]
- Brzęk, P.; Konarzewski, M. Effect of food shortage on the physiology and competitive abilities of sand martin (Riparia riparia) nestlings. J. Exp. Biol. 2001, 204, 3065–3074. [Google Scholar] [CrossRef] [PubMed]
- Konarzewski, M.; Starck, J.M. Effects of food shortage and oversupply on energy utilization, histology, and function of the gut in nestling song thrushes (Turdus philomelos). Physiol. Biochem. Zool. 2000, 73, 416–427. [Google Scholar] [CrossRef] [PubMed]
- Moe, B.; Brunvoll, S.; Mork, D.; Brobakk, T.E.; Bech, C. Developmental plasticity of physiology and morphology in diet-restricted European shag nestlings (Phalacrocorax aristotelis). J. Exp. Biol. 2004, 207, 4067–4076. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Naya, D.E.; Bozinovic, F. The role of ecological interactions on the physiological flexibility of lizards. Funct. Ecol. 2006, 20, 601–608. [Google Scholar] [CrossRef]
- Trzcionka, M.; Withers, K.W.; Klingenspor, M.; Jastroch, M. The effects of fasting and cold exposure on metabolic rate and mitochondrial proton leak in liver and skeletal muscle of an amphibian, the cane toad Bufo marinus. J. Exp. Biol. 2008, 211, 1911–1918. [Google Scholar] [CrossRef] [PubMed]
- Devi, V.; Prabhakara Rao, Y.; Prasada Rao, D.G.V. Starvation as a stress factor influencing the metabolism of a tropical intertidal gastropod Morula granulata (Duclos). Proc. Anim. Sci. 1986, 95, 539–547. [Google Scholar] [CrossRef]
- Marsden, I.D.; Newell, R.C.; Ahsanullah, M. The effect of starvation on the metabolism of the shore crab, Carcinus maenas. Comp. Biochem. Physiol. Part A Physiol. 1973, 45, 195–213. [Google Scholar] [CrossRef]
- Hagerman, L. The oxygen consumption of Crangon vulgaris (Fabricius) (Crustacea, Natantia) in relation to salinity. Ophelia 1970, 7, 283–292. [Google Scholar] [CrossRef]
- Rossetto, M.; De Leo, G.; Bevacqua, D.; Micheli, F. Allometric scaling of mortality rates with body mass in abalones. Oecologia 2011, 168, 989–996. [Google Scholar] [CrossRef]
- Armitage, K.B.; Wall, T.J. The effects of body size, starvation and temperature acclimation on oxygen consumption of the crayfish Orconectes nais. Comp. Biochem. Physiol. Part A Physiol. 1982, 73, 63–68. [Google Scholar] [CrossRef]
- Verity, P.G. Grazing, respiration, excretion, and growth rates of tintinnids. Limnol. Oceanogr. 1985, 30, 1268–1282. [Google Scholar] [CrossRef]
- Bohrer, R.; Lampert, W. Simultaneous measurement of the effect of food concentration on assimilation and respiration in Daphnia magna Straus. Funct. Ecol. 1988, 463–471. [Google Scholar] [CrossRef]
- Schiemer, F. Food dependence and energetics of freeliving nematodes. Oecologia 1982, 54, 108–121. [Google Scholar] [CrossRef]
- Roark, A.M.; Bjorndal, K.A. Metabolic rate depression is induced by caloric restriction and correlates with rate of development and lifespan in a parthenogenetic insect. Exp. Gerontol. 2009, 44, 413–419. [Google Scholar] [CrossRef]
- Hou, C.; Amunugama, K. On the complex relationship between energy expenditure and longevity: Reconciling the contradictory empirical results with a simple theoretical model. Mech. Ageing Dev. 2015, 149, 50–64. [Google Scholar] [CrossRef]
- Hou, C.; Bolt, K.; Bergman, A. A general life history theory for effects of caloric restriction on health Maintenance. BMC Syst. Biol. 2011, 5, 78–90. [Google Scholar] [CrossRef]
- Hou, C.; Bolt, K.M.; Bergman, A. Energetic basis of correlation between catch-up growth, health maintenance, and aging. J. Gerontol. A Biol. Sci. Med. Sci. 2011, 66A, 627–638. [Google Scholar] [CrossRef]
- Hou, C. The energy trade-off between growth and longevity. Mech. Ageing Dev. 2013, 134, 373–380. [Google Scholar] [CrossRef]
- Kleiber, M. Body Size and Metabolic Rate. Physiol. Rev. 1947, 27, 511–541. [Google Scholar] [CrossRef] [PubMed]
- Hemmingsen, A.M. Energy metabolism as related to body size and respiratory surfaces, and its evolution. Rep. Steno Meml. Hosp. Nord. Insul. Lab. 1960, 9, 6–110. [Google Scholar]
- Peters, R.H. The Ecological Implications of Body Size; Cambridge University Press: Cambridge, UK, 1983. [Google Scholar]
- Brown, J.H.; Gillooly, J.F.; Allen, A.P.; Savage, V.M.; West, G.B. Toward a metabolic theory of ecology. Ecology 2004, 85, 1771–1789. [Google Scholar] [CrossRef]
- Savage, V.M.; Gillooly, J.F.; Woodruff, W.H.; West, G.B.; Allen, A.P.; Enquist, B.J.; Brown, J.H. The predominance of quarter-power scaling in biology. Funct. Ecol. 2004, 18, 257–282. [Google Scholar] [CrossRef]
- West, G.B.; Brown, J.H.; Enquist, B.J. A general model for the origin of allometric scaling laws in biology. Science 1997, 276, 122–126. [Google Scholar] [CrossRef]
- Savage, V.M.; Deeds, E.J.; Fontana, W. Sizing Up Allometric Scaling Theory. PLoS Comput. Biol. 2008, 4, e1000171. [Google Scholar] [CrossRef]
- Moses, M.E.; Hou, C.; Woodruff, W.H.; West, G.B.; Nekola, J.C.; Zuo, W.; Brown, J.H. Revisiting a Model of Ontogenetic Growth: Estimating Model Parameters from Theory and Data. Am. Nat. 2008, 171, 632–645. [Google Scholar] [CrossRef]
- Glazier, D.S. Beyond the “3/4 power law”: Variation in the intra- and interspecific scaling of metabolic rate in animals. Biol. Rev. 2005, 80, 611. [Google Scholar] [CrossRef]
- Blossman-Myer, B.L.; Burggren, W.W. Metabolic Allometry during Development and Metamorphosis of the Silkworm Bombyx mori: Analyses, Patterns, and Mechanisms. Physiol. Biochem. Zool. 2010, 83, 215–231. [Google Scholar] [CrossRef]
- Moran, D.; Wells, R.M.G. Ontogenetic scaling of fish metabolism in the mouse-to-elephant mass magnitude range. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2007, 148, 611–620. [Google Scholar] [CrossRef]
- Yagi, M.; Kanda, T.; Takeda, T.; Ishimatsu, A.; Oikawa, S. Ontogenetic phase shifts in metabolism: Links to development and anti-predator adaptation. Proc. R. Soc. B Biol. Sci. 2010, 277, 2793–2801. [Google Scholar] [CrossRef] [PubMed]
- Sears, K.E.; Kerkhoff, A.J.; Messerman, A.; Itagaki, H. Ontogenetic Scaling of Metabolism, Growth, and Assimilation: Testing Metabolic Scaling Theory with Manduca sexta Larvae. Physiol. Biochem. Zool. 2012, 85, 159–173. [Google Scholar] [CrossRef] [PubMed]
- Greenlee, K.J.; Harrison, J.F. Respiratory changes throughout ontogeny in the tobacco hornworm caterpillar, Manduca sexta. J. Exp. Biol. 2005, 208, 1385–1392. [Google Scholar] [CrossRef] [PubMed]
- Speakman, J.; McQueenie, J. Limits to sustained metabolic rate: The link between food intake, basal metabolic rate, and morphology in reproducing mice, Mus musculus. Physiol. Zool. 1996, 69, 746–769. [Google Scholar] [CrossRef]
- Killen, S.S.; Atkinson, D.; Glazier, D.S. The intraspecific scaling of metabolic rate with body mass in fishes depends on lifestyle and temperature. Ecol. Lett. 2010, 13, 184–193. [Google Scholar] [CrossRef]
- Glazier, D.S.; Butler, E.M.; Lombardi, S.A.; Deptola, T.J.; Reese, A.J.; Satterthwaite, E.V. Ecological effects on metabolic scaling: Amphipod responses to fish predators in freshwater springs. Ecol. Monogr. 2011, 81, 599–618. [Google Scholar] [CrossRef]
- DeLong, J.P.; Hanley, T.C.; Vasseur, D.A. Competition and the density dependence of metabolic rates. J. Anim. Ecol. 2014, 83, 51–58. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Green, C.J.; Hou, C. Comparison of Energy Budget of Cockroach Nymph (Hemimetabolous) and Hornworm (Holometabolous) under Food Restriction. Insects 2024, 15, 36. https://doi.org/10.3390/insects15010036
Green CJ, Hou C. Comparison of Energy Budget of Cockroach Nymph (Hemimetabolous) and Hornworm (Holometabolous) under Food Restriction. Insects. 2024; 15(1):36. https://doi.org/10.3390/insects15010036
Chicago/Turabian StyleGreen, Charles J., and Chen Hou. 2024. "Comparison of Energy Budget of Cockroach Nymph (Hemimetabolous) and Hornworm (Holometabolous) under Food Restriction" Insects 15, no. 1: 36. https://doi.org/10.3390/insects15010036
APA StyleGreen, C. J., & Hou, C. (2024). Comparison of Energy Budget of Cockroach Nymph (Hemimetabolous) and Hornworm (Holometabolous) under Food Restriction. Insects, 15(1), 36. https://doi.org/10.3390/insects15010036





