Differences in Male-Killing Rickettsia Bacteria between Lineages of the Invasive Gall-Causing Pest Leptocybe invasa
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Sample Processing and Extraction of L. invasa Total Genomic DNA
2.3. Identification of L. invasa Lineages
2.4. Extraction of Total Microbial DNA from L. invasa and Amplification of the 16S rRNA Gene Sequence
2.5. Data Optimization and Statistical Analysis
2.6. Phylogenetic Analysis of Rickettsia Endosymbionts from L. invasa Based on 16S rRNA Gene Sequences
2.7. Fluorescence Quantitative PCR Verification
3. Results
3.1. Identification of L. invasa Lineages
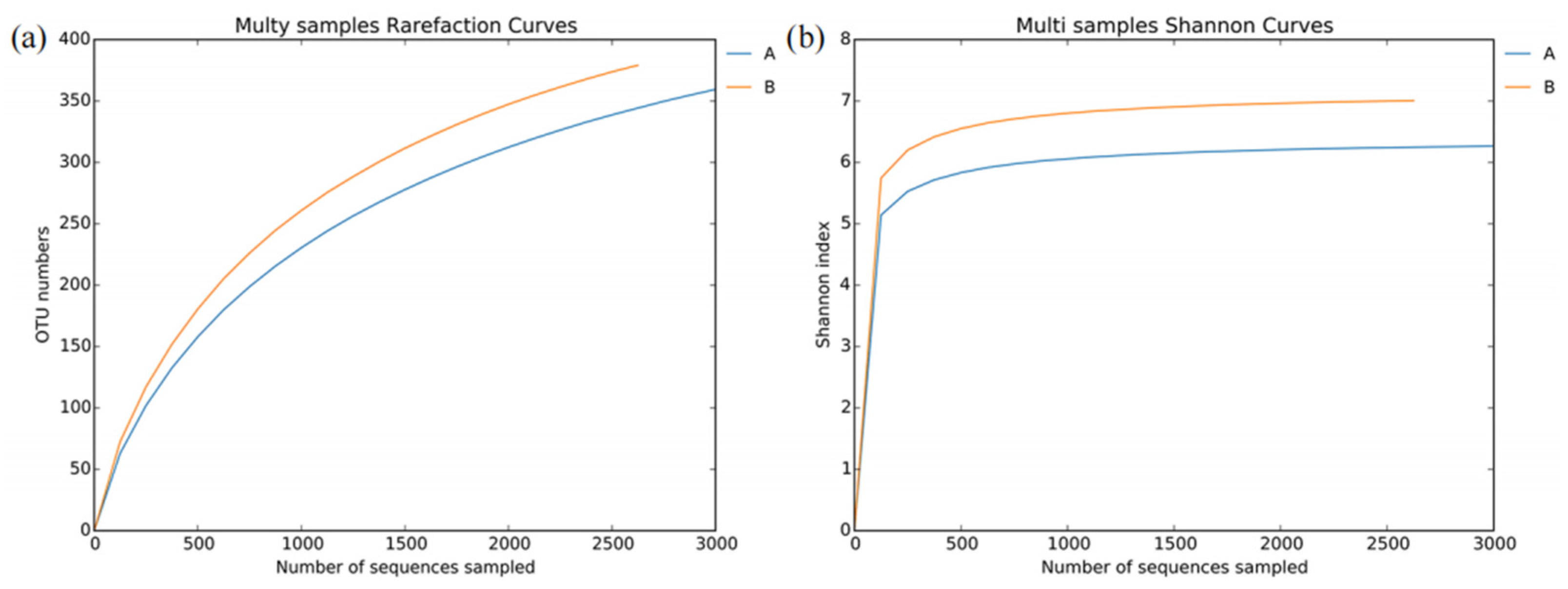
3.2. Bacterial Diversity and Abundance in L. invasa Lineages A and B
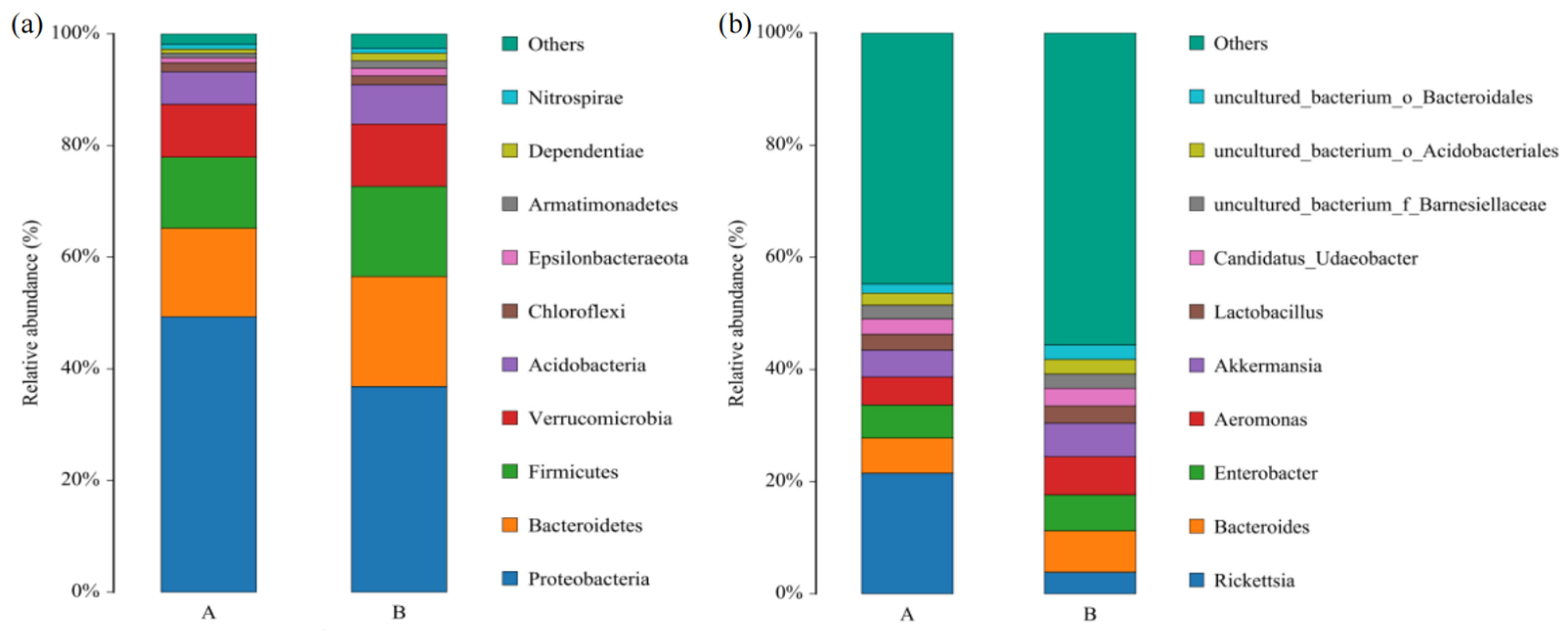
3.3. Bacterial Community Structure in L. invasa Lineages A and B
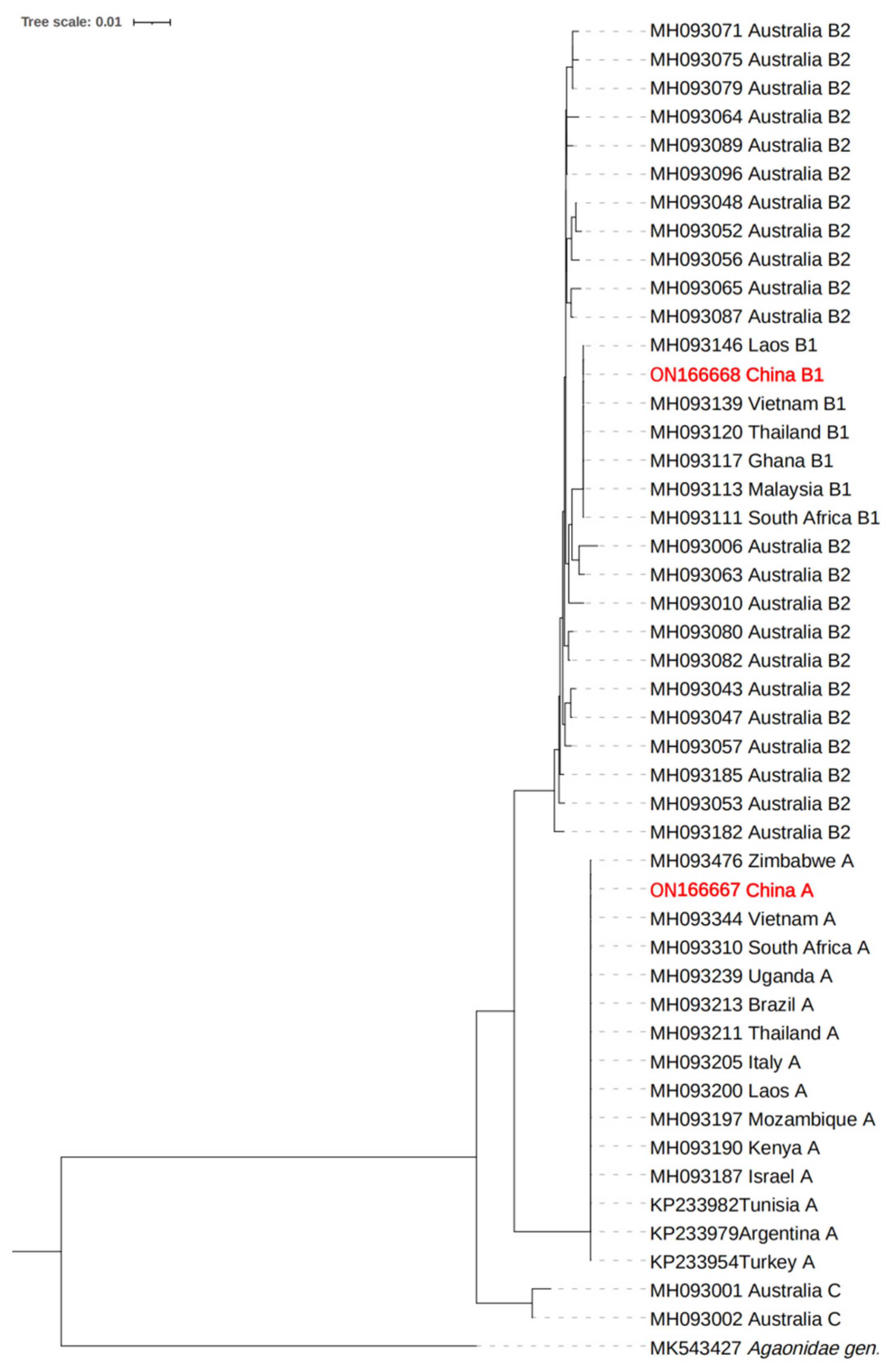
3.4. Phylogenetic Analysis of L. invasa Rickettsia Based on 16S rRNA Gene Sequence
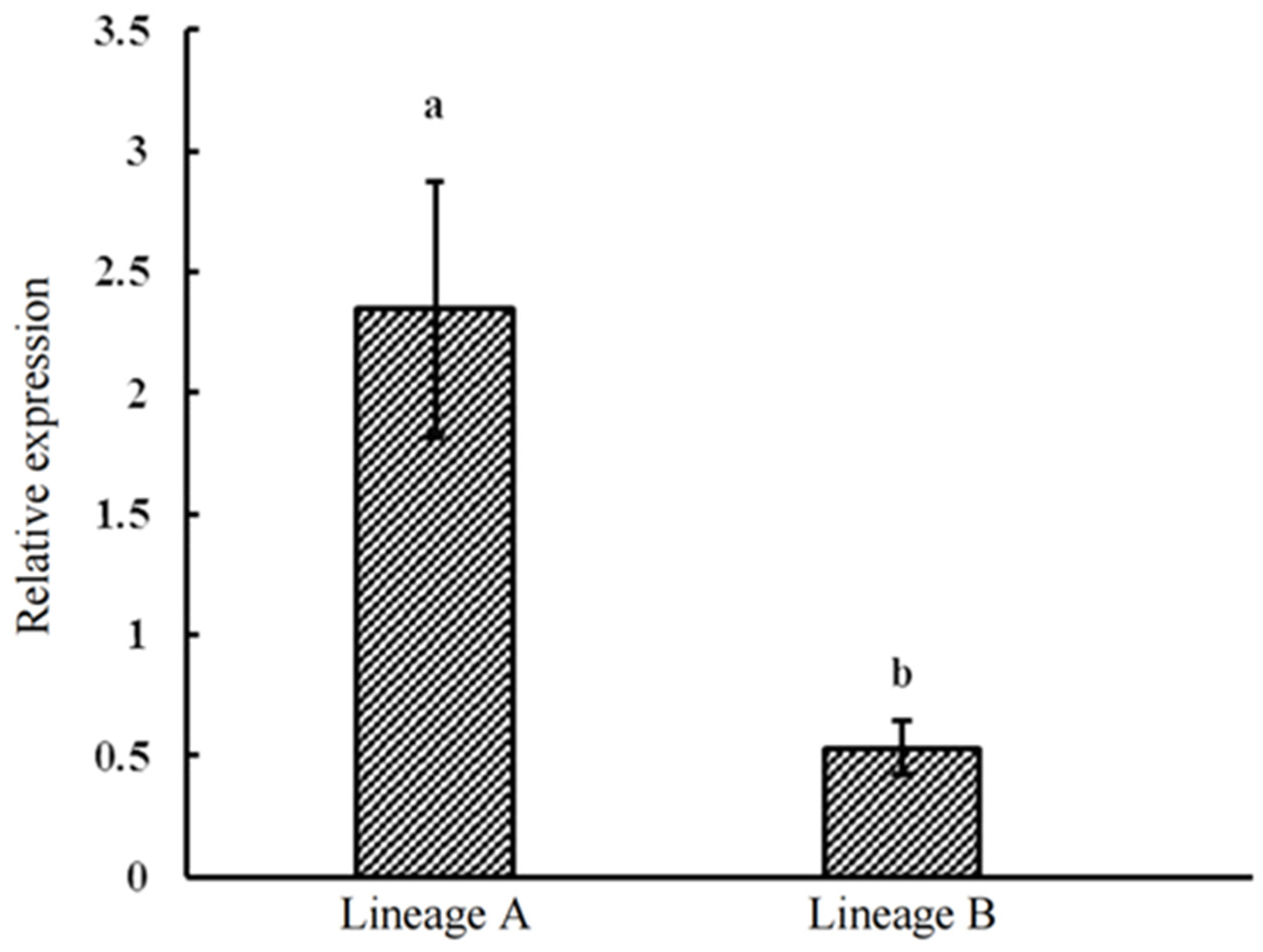
3.5. Fluorescent Quantitative PCR Validation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Moran, N.A.; McCutcheon, J.P.; Nakabachi, A. Genomics and evolution of heritable bacterial symbionts. Annu. Rev. Genet. 2008, 42, 165–190. [Google Scholar] [CrossRef] [PubMed]
- Oliver, K.M.; Degnan, P.H.; Burke, G.R.; Moran, N.A. Facultative symbionts in aphids and the horizontal transfer of ecologically important traits. Annu. Rev. Entomol. 2010, 55, 247–266. [Google Scholar] [CrossRef] [PubMed]
- Latorre, A.; Manzano-Marín, A. Dissecting genome reduction and trait loss in insect endosymbionts. Ann. N. Y. Acad. Sci. 2017, 1389, 52–75. [Google Scholar] [CrossRef] [PubMed]
- Cardoza, Y.J.; Klepzig, K.D.; Raffa, K.F. Bacteria in oral secretions of an endophytic insect inhibit antagonistic fungi. Ecol. Entomol. 2006, 31, 636–645. [Google Scholar] [CrossRef]
- Douglas, A.E. Multiorganismal insects: Diversity and function of resident microorganisms. In: Berenbaum MR, edito. Annu. Rev. Entomol. 2015, 60, 17–34. [Google Scholar] [CrossRef]
- Feldhaar, H. Bacterial symbionts as mediators of ecologically important traits of insect hosts. Ecol. Entomol. 2011, 36, 533–543. [Google Scholar] [CrossRef]
- Oliver, K.M.; Degnan, P.H.; Hunter, M.S.; Moran, N.A. Bacteriophages encode factors required for protection in a symbiotic mutualism. Science 2009, 325, 992–994. [Google Scholar] [CrossRef]
- Baumann, P.; Baumann, L.; Lai, C.Y.; Rouhbakhsh, D.; Moran, N.A.; Clark, M.A. Genetics, physiology, and evolutionary relationships of the genus Buchnera: Intracellular symbionts of aphids. Annu. Rev. Microbiol. 1995, 49, 55–94. [Google Scholar] [CrossRef]
- Chiel, E.; Inbar, M.; Mozes-Daube, N.; White, J.A.; Hunter, M.S.; Zchori-Fein, E. Assessments of fitness effects by the facultative symbiont Rickettsia in the Sweetpotato Whitefly (Hemiptera: Aleyrodidae). Ann. Entomol. Soc. Am. 2009, 102, 413–418. [Google Scholar] [CrossRef]
- Himler, A.G.; Adachi-Hagimori, T.; Bergen, J.E.; Kozuch, A.; Kelly, S.E.; Tabashnik, B.E.; Chiel, E.; Duckworth, V.E.; Dennehy, T.J.; Zchori-Fein, E.; et al. Rapid spread of a bacterial symbiont in an invasive Whitefly is driven by fitness Benefits and Female Bias. Science 2011, 332, 254–256. [Google Scholar] [CrossRef]
- Bourtzis, K.; Dobson, S.L.; Xi, Z.Y.; Rasgon, J.L.; Calvitti, M.; Moreira, L.A.; Bossin, H.C.; Moretti, R.; Baton, L.A.; Hughes, G.L.; et al. Harnessing mosquito-Wolbachia symbiosis for vector and disease control. Acta Trop. 2014, 132, S150–S163. [Google Scholar] [CrossRef] [PubMed]
- Rainey, S.M.; Shah, P.; Kohl, A.; Dietrich, I. Understanding the Wolbachia-mediated inhibition of arboviruses in mosquitoes: Progress and challenges. J. Gen. Virol. 2014, 95, 517–530. [Google Scholar] [CrossRef] [PubMed]
- Shentu, X.P.; Li, D.T.; Xu, J.F.; She, L.; Yu, X.P. Effects of fungicides on the yeast-like symbiotes and their host, Nilaparvata lugens Stal (Hemiptera: Delphacidae). Pestic. Biochem. Physiol. 2016, 128, 16–21. [Google Scholar] [CrossRef]
- Zheng, X.Y.; Zhang, D.J.; Li, Y.J.; Yang, C.; Wu, Y.; Liang, X.; Liang, Y.K.; Pan, X.L.; Hu, L.C.; Sun, Q.; et al. Incompatible and sterile insect techniques combined eliminate mosquitoes. Nature 2019, 572, 56–61. [Google Scholar] [CrossRef]
- Dittrich-Schröder, G.; Hoareau, T.B.; Hurley, B.P.; Wingfield, M.J.; Lawson, S.; Nahrung, H.F.; Slippers, B. Population genetic analyses of complex global insect invasions in managed landscapes: A Leptocybe invasa (Hymenoptera) case study. Biol. Invasions 2018, 20, 2395–2420. [Google Scholar] [CrossRef]
- Mendel, Z.; Protasov, A.; Fisher, N.; La Salle, J. Taxonomy and biology of Leptocybe invasa gen. & sp. n. (Hymenoptera: Eulophidae), an invasive gall inducer on Eucalyptus. Aust. J. Entomol. 2004, 43, 101–113. [Google Scholar]
- Le, N.H.; Nahrung, H.F.; Griffiths, M.; Lawson, S.A. Invasive Leptocybe spp. and their natural enemies: Global movement of an insect fauna on eucalypts. Biol. Control. 2018, 125, 7–14. [Google Scholar] [CrossRef]
- Zheng, X.L.; Li, J.; Yang, Z.D.; Xian, Z.H.; Wei, J.G.; Lei, C.L.; Wang, X.P.; Lu, W. A review of invasive biology, prevalence and management of Leptocybe invasa Fisher & La Salle (Hymenoptera: Eulophidae: Tetrastichinae). Afr. Entomol. 2014, 22, 68–79. [Google Scholar]
- Wu, Y.J.; Jiang, X.J.; Li, D.W.; Luo, J.T.; Zhou, G.F.; Chang, M.S.; Yang, Z.Q. Leptocybe invasa, a New invasive forest pest making galls on twigs and leaves of eucalyptus trees in China (Hymenoptera: Eulophidae). Sci. Silvae Sin. 2009, 45, 161–163+182–183. [Google Scholar]
- Zheng, X.L.; Huang, Z.Y.; Li, J.; Yang, Z.D.; Yang, X.H.; Lu, W. Reproductive biology of Leptocybe invasa Fisher & La Salle (Hymenoptera: Eulophidae). Neotrop. Entomol. 2018, 47, 19–25. [Google Scholar]
- Zhang, H.; Song, J.Y.; Zhao, H.X.; Li, M.; Han, W.H. Predicting the distribution of the invasive species Leptocybe invasa: Combining MaxEnt and geodetector models. Insects 2021, 12, 92. [Google Scholar] [CrossRef] [PubMed]
- Schröder, M.L.; Slippers, B.; Wingfield, M.J.; Hurley, B.P. Invasion history and management of Eucalyptus snout beetles in the Gonipterus scutellatus species complex. J. Pest. Sci. 2020, 93, 11. [Google Scholar] [CrossRef]
- Chu, D.; Guo, D.; Pan, H.P.; Zhang, Y.J.; Wan, F.H. Cryptic invasion of alien species: Types and effects. Acta Entomol. Sin. 2012, 55, 218–225. [Google Scholar]
- Tsai, C.L.; Lee, H.C.; Cho, G.; Liao, Y.C.; Yang, M.M.; Yeh, W.B. Invasive and quarantine risks of Cacopsylla chinensis (Hemiptera: Psyllidae) in East Asia: Hybridization or gene flow between differentiated lineages. J. Econ. Entomol. 2020, 113, 2890–2899. [Google Scholar] [CrossRef]
- Nugnes, F.; Gebiola, M.; Monti, M.M.; Gualtieri, L.; Giorgini, M.; Wang, J.G.; Bernardo, U. Genetic diversity of the invasive gall wasp Leptocybe invasa (Hymenoptera: Eulophidae) and of its Rickettsia endosymbiont, and associated Sex-Ratio differences. PLoS ONE 2015, 10, e124660. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Wang, H.T.; Guo, C.H.; Yang, Z.D.; Zhou, J.; Wang, X.; Ding, Z.R. EST-SSR Development and cryptic species Identification for the invasive gall-pest Leptocybe invasa (Hymenoptera: Eulophidae). Sci. Silvae Sin. 2021, 57, 140–151. [Google Scholar]
- Peng, X.; Wang, H.T.; Guo, C.H.; Hu, P.; Xu, L.; Zhou, J.; Ding, Z.R.; Yang, Z.D. Genetic diversity analysis of the invasive gall pest Leptocybe invasa (Hymenoptera: Eulophidae) from China. PLoS ONE 2021, 16, e258610. [Google Scholar] [CrossRef]
- Guo, C.H.; Peng, X.; Zheng, X.L.; Wang, X.Y.; Wang, R.R.; Huang, Z.Y.; Yang, Z.D. Comparison of bacterial diversity and abundance between sexes of Leptocybe invasa Fisher & La Salle (Hymenoptera: Eulophidae) from China. PeerJ 2020, 8, e8411. [Google Scholar]
- Liu, Y.P.; Xu, L.T.; Zhang, Z.Q.; Huang, Z.Y.; Fang, D.X.; Zheng, X.L.; Yang, Z.D.; Lu, M. Isolation, identification, and analysis of potential functions of culturable bacteria associated with an invasive gall wasp, Leptocybe invasa. Microb. Ecol. 2021, 83, 151–166. [Google Scholar] [CrossRef]
- Guo, C.H.; Peng, X.; Wang, H.T.; Zheng, X.L.; Hu, P.; Zhou, J.; Ding, Z.R.; Wang, X.; Yang, Z.D. Bacterial diversity of Leptocybe invasa Fisher & La Salle (Hymenoptera: Eulophidae) from different geographical conditions in China. Arch. Insect Biochem. Physiol. 2021, 108, e21847. [Google Scholar]
- Folmer, O.; Black, M.; Hoeh, W.; Lutz, R.; Vrijenhoek, R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotechnol. 1994, 3, 294–299. [Google Scholar] [PubMed]
- Singer, E.; Bushnell, B.; Coleman-Derr, D.; Bowman, B.; Bowers, R.M.; Levy, A.; Gies, E.A.; Cheng, J.F.; Copeland, A.; Klenk, H.P.; et al. High-resolution phylogenetic microbial community profiling. ISME J. 2016, 10, 2020–2032. [Google Scholar] [CrossRef] [PubMed]
- Earl, J.P.; Adappa, N.D.; Krol, J.; Bhat, A.S.; Balashov, S.; Ehrlich, R.L.; Palmer, J.N.; Workman, A.D.; Blasetti, M.; Sen, B.; et al. Species-level bacterial community profiling of the healthy sinonasal microbiome using Pacific Biosciences sequencing of full-length 16S rRNA genes. Microbiome 2018, 6, 190. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.C. Study on the Biology, Risk Analysis and Control Strategy of Leptocybe invasa Fisher & LaSalle. Ph.D. Thesis, Fujian Agriculture and Forestry University, Fuzhou, China, 2017. [Google Scholar]
- Liu, X.D.; Zhang, Y.C. Infection pattern and dynamics of endosymbionts in aphids and their effects on population differentiation of hosts. J. Nanjing Agric. Univ. 2018, 41, 209–217. [Google Scholar]
- Gauthier, J.P.; Outreman, Y.; Mieuzet, L.; Simon, J.C.; Duperron, S. Bacterial communities associated with Host-Adapted populations of Pea Aphids revealed by deep sequencing of 16S Ribosomal DNA. PLoS ONE 2015, 10, e120664. [Google Scholar] [CrossRef] [PubMed]
- Skaljac, M.; Zanic, K.; Ban, S.G.; Kontsedalov, S.; Ghanim, M. Co-infection and localization of secondary symbionts in two whitefly species. BMC Microbiol. 2010, 10, 142. [Google Scholar] [CrossRef] [PubMed]
- Chu, D.; Cong, B.; Zhang, Y.J.; Xu, B.Y.; Wu, Q.J.; Zhu, G.R. Detection and phylogenetic analysis of Wolbachia in different Bemisia tabaci biotypes. Acta Entomol. Sin. 2005, 48, 518–525. [Google Scholar]
- An, P.; Xu, Y.P.; Wu, J.M.; Zheng, R.E.; Yu, X.P. Vertical transmission of insect symbionts. Acta Entomol. Sin. 2019, 62, 877–884. [Google Scholar]
- Shan, H.W.; Liu, Y.Q.; Luan, J.B.; Liu, S.S. New insights into the transovarial transmission of the symbiont Rickettsia in whiteflies. Sci. China Life Sci. 2021, 64, 1174–1186. [Google Scholar] [CrossRef]
- Ahmed, M.Z.; Li, S.J.; Xue, X.; Yin, X.J.; Ren, S.X.; Jiggins, F.M.; Greeff, J.M.; Qiu, B.L.; Hurst, G. The intracellular bacterium Wolbachia uses parasitoid wasps as phoretic vectors for efficient horizontal transmission. PLoS Pathog. 2015, 11, e1004672. [Google Scholar] [CrossRef]
- Liu, Y.; Fan, Z.Y.; Li, Y.; Peng, J.; Chen, X.Y.; Qiu, B.L. Plant-mediated horizontal transmission of insect endosymbionts and their biological effects on recipients. Chin. J. Appl. Entomol. 2020, 57, 272–283. [Google Scholar]
- Martinez, A.J.; Weldon, S.R.; Oliver, K.M. Effects of parasitism on aphid nutritional and protective symbioses. Mol. Ecol. 2014, 23, 1594–1607. [Google Scholar] [CrossRef] [PubMed]
- Mouton, L.; Henri, H.; Charif, D.; Boulétreau, M.; Vavre, F. Interaction between host genotype and environmental conditions affects bacterial density in Wolbachia symbiosis. Biol. Lett. 2007, 3, 210–213. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Parkinson, J.F.; Gobin, B.; Hughes, W.O.H. Heritability of symbiont density reveals distinct regulatory mechanisms in a tripartite symbiosis. Ecol. Evol. 2016, 6, 2053–2060. [Google Scholar] [CrossRef] [PubMed]
- Giorgini, M.; Bernardo, U.; Monti, M.M.; Nappo, A.G.; Gebiola, M. Rickettsia Symbionts Cause Parthenogenetic Reproduction in the Parasitoid Wasp Pnigalio soemius (Hymenoptera: Eulophidae). Appl. Environ. Microbiol. 2010, 76, 2589–2599. [Google Scholar] [CrossRef]
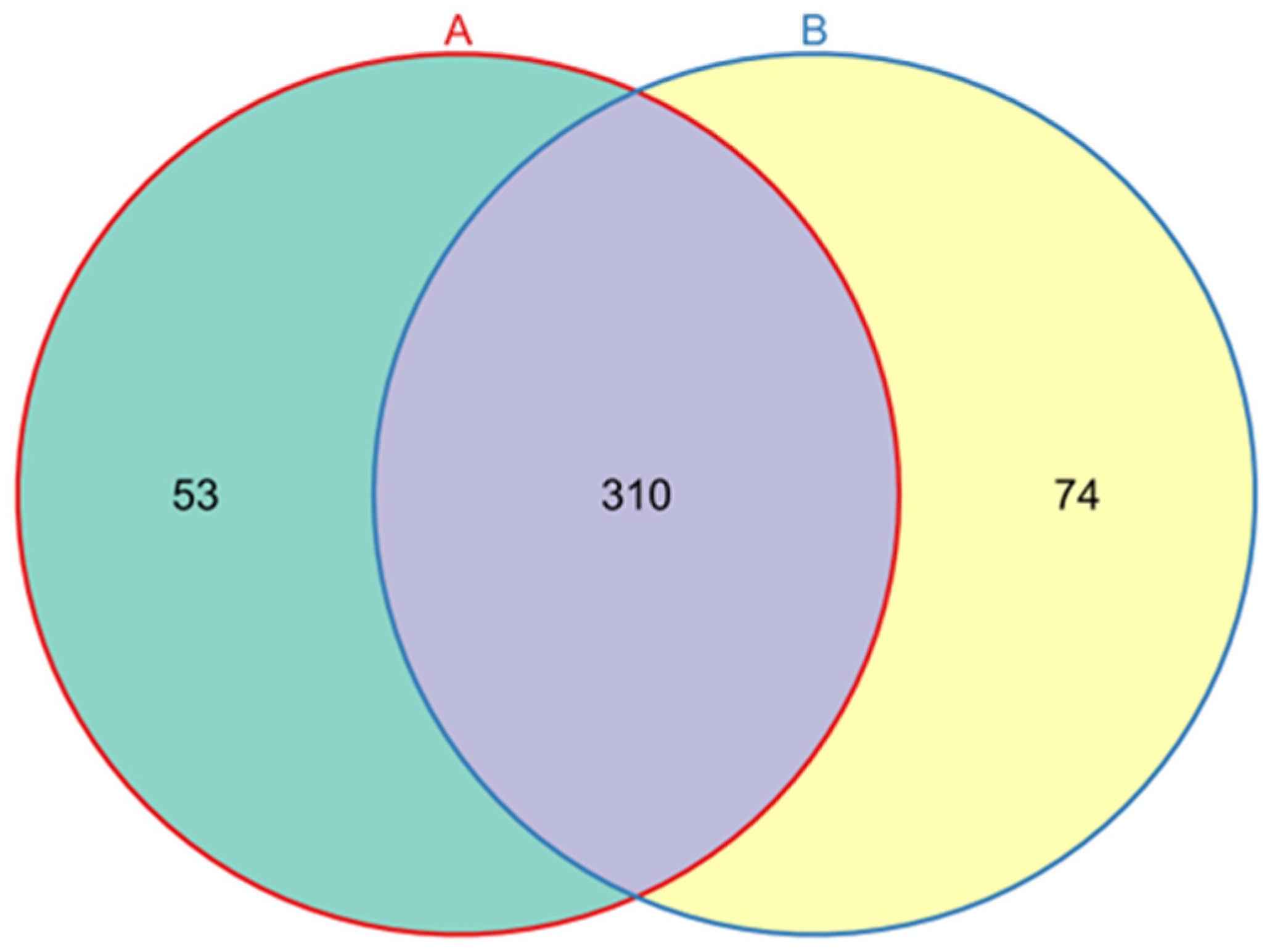

| Lineage Type | Phylum | Class | Order | Family | Genus | Species |
|---|---|---|---|---|---|---|
| Lineage A | 18 | 29 | 71 | 112 | 185 | 226 |
| Lineage B | 20 | 31 | 76 | 125 | 204 | 240 |
| Lineage A-specific | 0 | 1 | 1 | 4 | 13 | 21 |
| Lineage B-specific | 2 | 3 | 6 | 17 | 32 | 35 |
| Lineage in common | 18 | 28 | 70 | 108 | 172 | 205 |
| Total | 20 | 32 | 77 | 129 | 217 | 261 |
| Lineage Type | ACE | Chao 1 | Shannon | Simpson | Coverage |
|---|---|---|---|---|---|
| Lineage A | 460.7090 | 447.7917 | 6.269 | 0.9419 | 0.9641 |
| Lineage B | 474.4288 | 462.2692 | 7.013 | 0.9803 | 0.9595 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, X.; Wang, H.; Yang, Z. Differences in Male-Killing Rickettsia Bacteria between Lineages of the Invasive Gall-Causing Pest Leptocybe invasa. Insects 2023, 14, 757. https://doi.org/10.3390/insects14090757
Peng X, Wang H, Yang Z. Differences in Male-Killing Rickettsia Bacteria between Lineages of the Invasive Gall-Causing Pest Leptocybe invasa. Insects. 2023; 14(9):757. https://doi.org/10.3390/insects14090757
Chicago/Turabian StylePeng, Xin, Hantang Wang, and Zhende Yang. 2023. "Differences in Male-Killing Rickettsia Bacteria between Lineages of the Invasive Gall-Causing Pest Leptocybe invasa" Insects 14, no. 9: 757. https://doi.org/10.3390/insects14090757
APA StylePeng, X., Wang, H., & Yang, Z. (2023). Differences in Male-Killing Rickettsia Bacteria between Lineages of the Invasive Gall-Causing Pest Leptocybe invasa. Insects, 14(9), 757. https://doi.org/10.3390/insects14090757





