MicroRNA Expression Prior to Biting in a Vector Mosquito Anticipates Physiological Processes Related to Energy Utilization, Reproduction and Immunity
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Small RNA Sequencing
3.2. MicroRNA Target Prediction
3.3. Quantitative Reverse-Transcription PCR
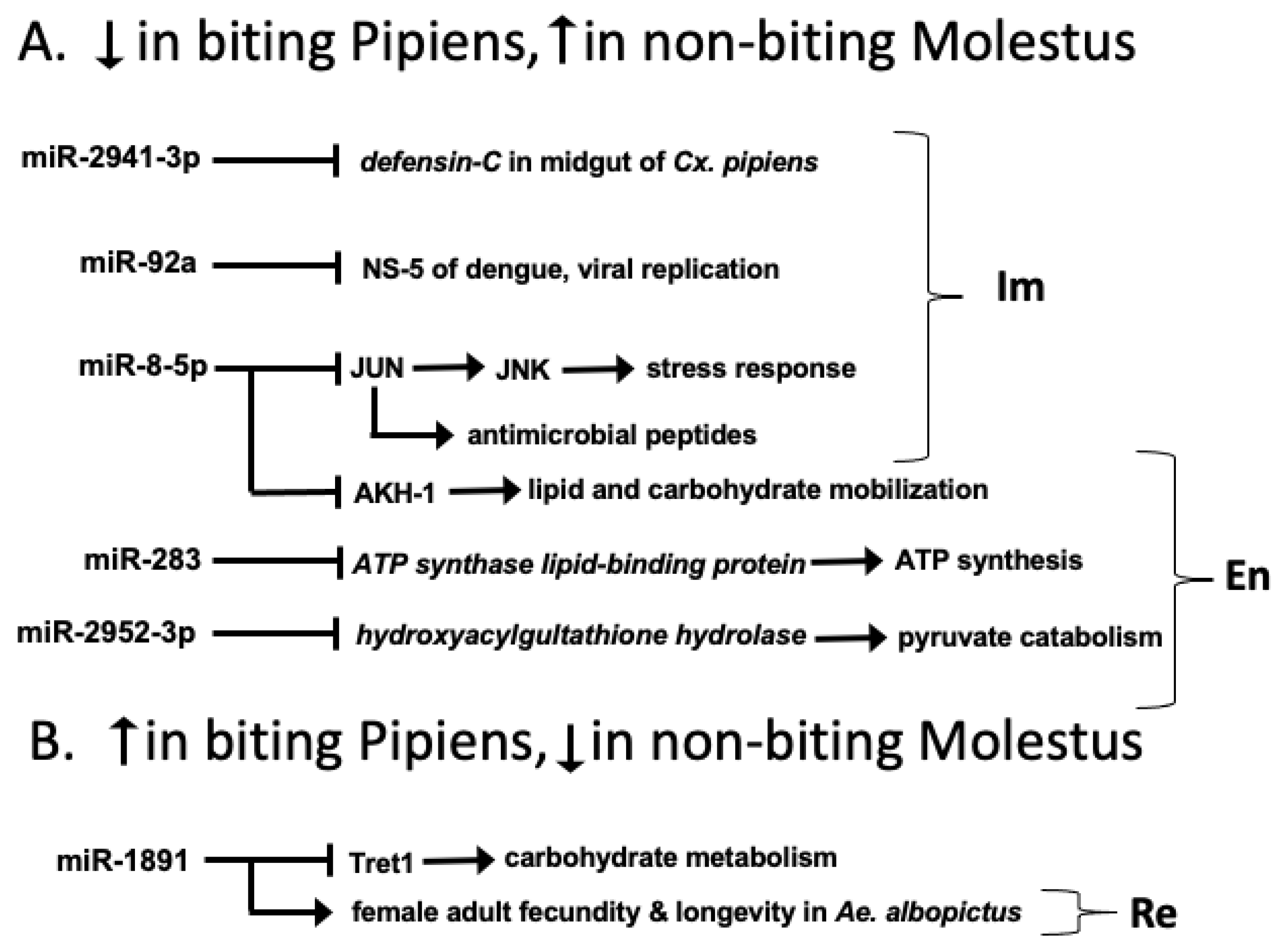
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Malaria Report 2021; World Health Organization: Geneva, Switzerland, 2021.
- Bhatt, S.; Gething, P.W.; Brady, O.J.; Messina, J.P.; Farlow, A.W.; Moyes, C.L.; Drake, J.M.; Brownstein, J.S.; Hoen, A.G.; Sankoh, O.; et al. The global distribution and burden of dengue. Nature 2013, 496, 504–507. [Google Scholar] [CrossRef]
- Edman, J.D.; Scott, T.W. Host defensive behavior and the feeding success of mosquitoes. Insect Sci. Its Appl. 1987, 8, 617–622. [Google Scholar] [CrossRef]
- de Carvalho, S.S.; Rodovalho, C.M.; Gaviraghi, A.; Mota, M.B.S.; Jablonka, W.; Rocha-Santos, C.; Nunes, R.D.; Sa-Guimaraes, T.d.E.; Oliveira, D.S.; Melo, A.C.A.; et al. Aedes aegypti post-emergence transcriptome: Unveiling the molecular basis for the hematophagic and gonotrophic capacitation. PLoS Negl. Trop. Dis. 2021, 15, e0008915. [Google Scholar] [CrossRef] [PubMed]
- Benoit, J.B.; Lopez-Martinez, G.; Patrick, K.R.; Phillips, Z.P.; Krause, T.B.; Denlinger, D.L. Drinking a hot blood meal elicits a protective heat shock response in mosquitoes. Proc. Natl. Acad. Sci. USA 2011, 108, 8026–8029. [Google Scholar] [CrossRef]
- Lahondere, C.; Lazzari, C.R. Mosquitoes Cool Down during Blood Feeding to Avoid Overheating. Curr. Biol. 2012, 22, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Pascoa, V.; Oliveira, P.L.; Dansa-Petretski, M.; Silva, J.R.; Alvarenga, P.H.; Jacobs-Lorena, M.; Lemos, F.J.A. Aedes aegypti peritrophic matrix and its interaction with heme during blood digestion. Insect Biochem. Mol. Biol. 2002, 32, 517–523. [Google Scholar] [CrossRef]
- Geiser, D.L.; Chavez, C.A.; Flores-Munguia, R.; Winzerling, J.J.; Pham, D.Q.D. Aedes aegypti ferritin—A cytotoxic protector against iron and oxidative challenge? Eur. J. Biochem. 2003, 270, 3667–3674. [Google Scholar] [CrossRef] [PubMed]
- Graca-Souza, A.V.; Maya-Monteiro, C.; Paiva-Silva, G.O.; Braz, G.R.C.; Paes, M.C.; Sorgine, M.H.F.; Oliveira, M.F.; Oliveira, P.L. Adaptations against heme toxicity in blood-feeding arthropods. Insect Biochem. Mol. Biol. 2006, 36, 322–335. [Google Scholar] [CrossRef]
- Devenport, M.; Alvarenga, P.H.; Shao, L.; Fujioka, H.; Bianconi, M.L.; Oliveira, P.L.; Jacobs-Lorena, M. Identification of the Aedes aegypti peritrophic matrix protein AeIMUCI as a heme-binding protein. Biochemistry 2006, 45, 9540–9549. [Google Scholar] [CrossRef]
- Esquivel, C.J.; Cassone, B.J.; Piermarini, P.M. Transcriptomic Evidence for a Dramatic Functional Transition of the Malpighian Tubules after a Blood Meal in the Asian Tiger Mosquito Aedes albopictus. PLoS Negl. Trop. Dis. 2014, 8, e2929. [Google Scholar] [CrossRef]
- Nikbakhtzadeh, M.R.; Buss, G.K.; Leal, W.S. Toxic Effect of Blood Feeding in Male Mosquitoes. Front. Physiol. 2016, 7, 4. [Google Scholar] [CrossRef] [PubMed]
- Bradshaw, W.E.; Burkhart, J.; Colbourne, J.K.; Borowczak, R.; Lopez, J.; Denlinger, D.L.; Reynolds, J.A.; Pfrender, M.E.; Holzapfel, C.M. Evolutionary transition from blood feeding to obligate nonbiting in a mosquito. Proc. Natl. Acad. Sci. USA 2018, 115, 1009–1014. [Google Scholar] [CrossRef] [PubMed]
- Siperstein, A.; Marzec, S.; Fritz, M.L.; Holzapfel, C.M.; Bradshaw, W.E.; Armbruster, P.A.; Meuti, M.E. Conserved molecular pathways underlying biting in two divergent mosquito genera. Evol. Appl. 2022, 15, 878–890. [Google Scholar] [CrossRef]
- Downes, J.A. The feeding habits of biting flies and their significance in classification. Annu. Rev. Entomol. 1958, 3, 249–266. [Google Scholar] [CrossRef]
- Foster, W.A. Mosquito sugar feeding and reproductive energetics. Annu. Rev. Entomol. 1995, 40, 443–474. [Google Scholar] [CrossRef]
- Miyagi, I.; Toma, T.; Okazawa, T.; Wong, S.F.; Leh, M.U.; Yong, H.S. Three new phytotelmata mosquitoes of the genus Topomyia (Diptera: Culicidae) from Katibas, Lanjak-Entimau, Sarawak, Malaysia. J. Sci. Technol. Trop. 2012, 8, 97–117. [Google Scholar]
- Rattanarithikul, R.; Harbach, R.E.; Harrison, B.A.; Panthusiri, P.; Coleman, R.E. Illustrated keys to the mosquitoes of Thailand V. Genera Orthopodomyia, Kimia, Malaya, Topomyia, Tripteroides, and Toxorhynchites. Southeast Asian J. Trop. Med. Public Health 2007, 38 (Suppl. S2), 1–65. [Google Scholar]
- Wahid, I.; Sunahara, T.; Mogi, M. The hypopharynx of male and female mosquitoes. Open Entomol. J. 2007, 1, 1–16. [Google Scholar] [CrossRef][Green Version]
- Zhou, X.; Rinker, D.C.; Pitts, R.J.; Rokas, A.; Zwiebel, L.J. Divergent and Conserved Elements Comprise the Chemoreceptive Repertoire of the Nonblood-Feeding Mosquito Toxorhynchites amboinensis. Genome Biol. Evol. 2014, 6, 2883–2896. [Google Scholar] [CrossRef]
- Wahid, I.; Sunahara, T.; Mogi, M. Maxillae and mandibles of male mosquitoes and female autogenous mosquitoes (Diptera: Culicidae). J. Med. Entomol. 2003, 40, 150–158. [Google Scholar] [CrossRef]
- Mogi, M. Distribution and overcrowding effects in mosquito larvae (Diptera, Culicidae) inhabiting taro axils in the Ryukyus, Japan. J. Med. Entomol. 1984, 21, 63–68. [Google Scholar] [CrossRef]
- Mogi, M.; Sembel, D.T. Predator-prey system structure in patchy and ephemeral phytotelmata: Aquatic communities in small aroid axils. Res. Popul. Ecol. 1996, 38, 95–103. [Google Scholar] [CrossRef]
- Noreuil, A.; Fritz, M.L. Differential Gene Expression in the Heads of Behaviorally Divergent Culex pipiens Mosquitoes. Insects 2021, 12, 271. [Google Scholar] [CrossRef]
- Strickman, D.; Fonseca, D.M. Autogeny in Culex pipiens Complex Mosquitoes from the San Francisco Bay Area. Am. J. Trop. Med. Hyg. 2012, 87, 719–726. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Haba, Y.; McBride, L. Origin and status of Culex pipiens mosquito ecotypes. Curr. Biol. 2022, 32, R237–R246. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.S.; Kim, S.; Cotten, M.A.; Sim, C. Transcript Assembly and Quantification by RNA-Seq Reveals Significant Differences in Gene Expression and Genetic Variants in Mosquitoes of the Culex pipiens (Diptera: Culicidae) Complex. J. Med. Entomol. 2021, 58, 139–145. [Google Scholar] [CrossRef]
- Hussain, M.; Frentiu, F.D.; Moreira, L.A.; O’Neill, S.L.; Asgari, S. Wolbachia uses host microRNAs to manipulate host gene expression and facilitate colonization of the dengue vector Aedes aegypti. Proc. Natl. Acad. Sci. USA 2011, 108, 9250–9255. [Google Scholar] [CrossRef]
- Fabian, M.R.; Sonenberg, N.; Filipowicz, W. Regulation of mRNA Translation and Stability by microRNAs. Annu. Rev. Biochem. 2010, 79, 351–379. [Google Scholar] [CrossRef]
- Roberts, A.P.; Lewis, A.P.; Jopling, C.L. The role of microRNAs in viral infection. Prog. Mol. Biol. Transl. Sicence 2011, 102, 101–139. [Google Scholar] [CrossRef]
- Vasudevan, S. Posttranscriptional Upregulation by MicroRNAs. Wiley Interdiscip. Rev.-Rna 2012, 3, 311–330. [Google Scholar] [CrossRef]
- Bartel, D.P. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef]
- Lewis, B.P.; Burge, C.B.; Bartel, D.P. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell 2005, 120, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Baek, D.; Villen, J.; Shin, C.; Camargo, F.D.; Gygi, S.P.; Bartel, D.P. The impact of microRNAs on protein output. Nature 2008, 455, 64–71. [Google Scholar] [CrossRef]
- Cai, Y.; Yu, X.; Hu, S.; Yu, J. A brief review on the mechanisms of miRNA regulation. Genom. Proteom. Bioinform. 2009, 7, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Lucas, K.J.; Zhao, B.; Liu, S.; Raikhel, A.S. Regulation of physiological processes by microRNAs in insects. Curr. Opin. Insect Sci. 2015, 11, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Bryant, B.; Macdonald, W.; Raikhel, A.S. microRNA miR-275 is indispensable for blood digestion and egg development in the mosquito Aedes aegypti. Proc. Natl. Acad. Sci. USA 2010, 107, 22391–22398. [Google Scholar] [CrossRef]
- Lucas, K.J.; Roy, S.; Ha, J.; Gervaise, A.L.; Kokoza, V.A.; Raikhel, A.S. MicroRNA-8 targets the Wingless signaling pathway in the female mosquito fat body to regulate reproductive processes. Proc. Natl. Acad. Sci. USA 2015, 112, 1440–1445. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, B.; Roy, S.; Saha, T.T.; Kokoza, V.A.; Li, M.; Raikhel, A.S. microRNA-309 targets the Homeobox gene SIX4 and controls ovarian development in the mosquito Aedes aegypti. Proc. Natl. Acad. Sci. USA 2016, 113, E4828–E4836. [Google Scholar] [CrossRef]
- Marzec, S. Culex Biting miRNA Workflow. Available online: https://github.com/srmarzec/Culex_Biting_miRNA/blob/main/MasterNotes. (accessed on 25 April 2022).
- Hong, S.; Guo, Q.; Wang, W.J.; Hu, S.L.; Fang, F.J.; Lv, Y.; Yu, J.; Zou, F.F.; Lei, Z.T.; Ma, K.; et al. Identification of differentially expressed microRNAs in Culex pipiens and their potential roles in pyrethroid resistance. Insect Biochem. Mol. Biol. 2014, 55, 39–50. [Google Scholar] [CrossRef]
- Friedlaender, M.R.; Mackowiak, S.D.; Li, N.; Chen, W.; Rajewsky, N. miRDeep2 accurately identifies known and hundreds of novel microRNA genes in seven animal clades. Nucleic Acids Res. 2012, 40, 37–52. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- Hausser, J.; Zavolan, M. Identification and consequences of miRNA-target interactions-beyond repression of gene expression (vol 15, pg 599, 2014). Nat. Rev. Genet. 2014, 15, 702. [Google Scholar] [CrossRef]
- Enright, A.J.; John, B.; Gaul, U.; Tuschl, T.; Sander, C.; Marks, D.S. MicroRNA targets in Drosophila. Genome Biol. 2003, 5, R1. [Google Scholar] [CrossRef]
- Krueger, J.; Rehmsmeier, M. RNAhybrid: microRNA target prediction easy, fast and flexible. Nucleic Acids Res. 2006, 34, W451–W454. [Google Scholar] [CrossRef]
- Yen, P.-S.; Chen, C.-H.; Sreenu, V.; Kohl, A.; Failloux, A.-B. Assessing the Potential Interactions between Cellular miRNA and Arboviral Genomic RNA in the Yellow Fever Mosquito, Aedes aegypti. Viruses 2019, 11, 540. [Google Scholar] [CrossRef] [PubMed]
- Puthiyakunnon, S.; Yao, Y.; Li, Y.; Gu, J.; Peng, H.; Chen, X. Functional characterization of three MicroRNAs of the Asian Tiger Mosquito, Aedes albopictus. Parasites Vectors 2013, 6, 230. [Google Scholar] [CrossRef]
- Nouzova, M.; Etebari, K.; Noriega, F.G.; Asgari, S. A comparative analysis of corpora allata-corpora cardiaca microRNA 14 repertoires revealed significant changes during mosquito metamorphosis. Insect Biochem. Mol. Biol. 2018, 96, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Monsanto-Hearne, V.; Asad, S.; Asgari, S.; Johnson, K.N. Drosophila microRNA modulates viral replication by targeting a homologue of mammalian cJun. J. Gen. Virol. 2017, 98, 1904–1912. [Google Scholar] [CrossRef]
- Meuti, M.E.; Bautista-Jimenez, R.; Reynolds, J.A. Evidence that microRNAs are part of the molecular toolkit regulating adult reproductive diapause in the mosquito, Culex pipiens. PLoS ONE 2018, 13, e0203015. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative C-T method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
- Clements, A.N. The Biology of Mosquitoes. Vol. 1. Development, Nutrition, and Reproduction; Chapman and Hall: London, UK, 1992; p. 509. [Google Scholar]
- Bednarova, A.; Kodrik, D.; Krishnan, N. Unique roles of glucagon and glucagon-like peptides: Parallels in understanding the functions of adipokinetic hormones in stress responses in insects. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2013, 164, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Vanderjagt, D.L. Glyoxalase-II—molecular characteristics, kinetics and mechanism. Biochem. Soc. Trans. 1993, 21, 522–527. [Google Scholar] [CrossRef] [PubMed]
- Lehane, M.J.; Aksoy, S.; Levashina, E. Immune responses and parasite transmission in blood-feeding insects. Trends Parasitol. 2004, 20, 433–439. [Google Scholar] [CrossRef]
- Bryant, W.B.; Michel, K. Blood feeding induces hemocyte proliferation and activation in the African malaria mosquito, Anopheles gambiae Giles. J. Exp. Biol. 2014, 217, 1238–1245. [Google Scholar] [CrossRef]
- Lowenberger, C. Innate immune response of Aedes aegypti. Insect Biochem. Mol. Biol. 2001, 31, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Tian, M.; Guo, Q.; Ma, L.; Zhou, D.; Shen, B.; Sun, Y.; Zhu, C. MiR-932 Regulates Pyrethroid Resistance in Culex pipiens pallens (Diptera: Culicidae). J. Med. Entomol. 2016, 53, 1205–1210. [Google Scholar] [CrossRef]
- Bartholomay, L.C.; Fuchs, J.F.; Cheng, L.L.; Beck, E.T.; Vizioli, J.; Lowenberger, C.; Christensen, B.M. Reassessing the role of defensin in the innate immune response of the mosquito, Aedes aegypti. Insect Mol. Biol. 2004, 13, 125–132. [Google Scholar] [CrossRef]
- Mendez, Y.; Pacheco, C.; Herrera, F. Inhibition of defensin and cecropin responses to dengue virus 1 infection in Aedes aegypti. Biomedica 2021, 41, 161–167. [Google Scholar] [CrossRef]
- Perkins, K.K.; Dailey, G.M.; Tjian, R. Novel JUN-related AND FOS-related proteins in Drosophila are functionally homologous to enhancer factor AP-1. EMBO J. 1988, 7, 4265–4273. [Google Scholar] [CrossRef]
- Sluss, H.K.; Han, Z.Q.; Barrett, T.; Davis, R.J.; Ip, Y.T. A JNK signal transduction pathway that mediates morphogenesis and an immune response in Drosophila. Genes Dev. 1996, 10, 2745–2758. [Google Scholar] [CrossRef]
- Soory, A.M.; Ratnaparkhi, G.M. SUMOylation of Jun fine-tunes the Drosophila gut immune response. PLoS Pathog. 2022, 18, e1010356. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.J.; Pan, Y.H.; Cheng, A.C.; Wang, M.S.; Yin, Z.Q.; Jia, R.Y. Regulatory Role of Host MicroRNAs in Flaviviruses Infection. Front. Microbiol. 2022, 13, 869441. [Google Scholar] [CrossRef] [PubMed]
- Hayes, C.G. West Nile Virus: Uganda, 1937, to New York City, 1999. Ann. N. Y. Acad. Sci. 2001, 951, 25–37. [Google Scholar] [CrossRef]
- Andreadis, T.G.; Anderson, J.F.; Tirrell-Peck, S.J. Multiple isolations of eastern equine encephalitis and Highlands J viruses from mosquitoes (Diptera:Culicidae) during a 1996 epizootic in southeastern Connecticut. J. Med. Entomol. 1998, 35, 296–302. [Google Scholar] [CrossRef]
- Hurlbut, H.S. Effect of environmental-temperature upon transmission of St-Louis encephalitis virus by Culex-pipiens-quinquefasciatus. J. Med. Entomol. 1973, 10, 1–12. [Google Scholar] [CrossRef]
- Turell, M.J. Members of the Culex pipies complex as vectors of viruses. J. Am. Mosq. Control. Assoc. 2012, 28, 123–126. [Google Scholar] [CrossRef] [PubMed]
- Bartholomay, L.C.; Michel, K. Mosquito Immunobiology: The Intersection of Vector Health and Vector Competence. Annu. Rev. Entomol. 2018, 63, 145–167. [Google Scholar] [CrossRef]
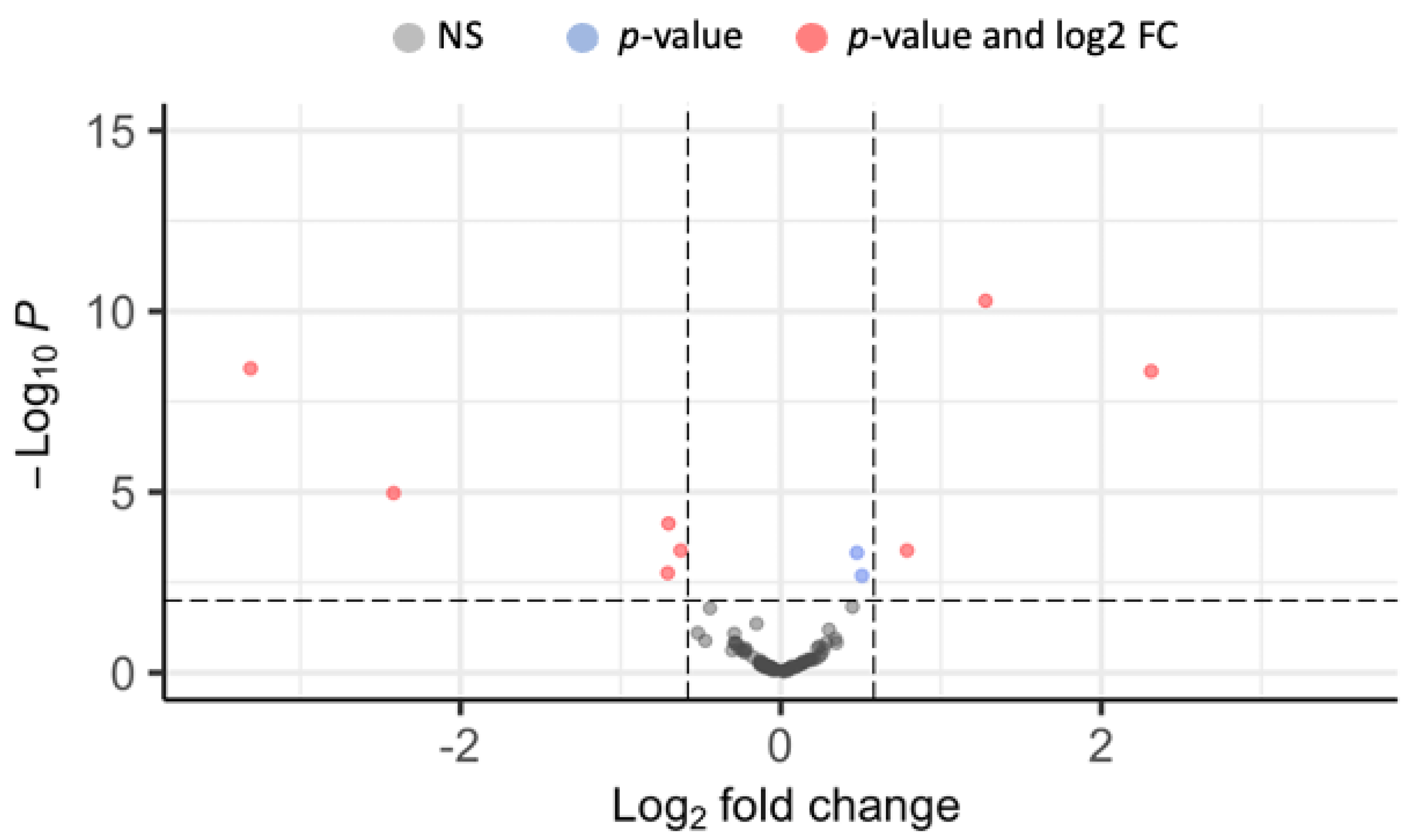
| miRNA | Fold Change | Putative Biological Function | Functional Category |
|---|---|---|---|
| miR-92a | −1.542 ** | Predicted Target: NS5 region of DENV-1 [47] | Immunity |
| miR-1891 | 1.725 ** | Validated function: Fecundity and adult longevity [48], Predicted Target: Facilitated trehalose transporter Tret1 | Reproduction Energy Utilization |
| miR-2941-3p | −5.341 ** | Predicted Target: defensin-C | Immunity |
| miR-8-5p | −1.625 ** | Predicted Target: AKH1 [49], | Energy Utilization |
| Validated Target: Drosophila Jun, dJun [50] in Drosophila | Immunity | ||
| miR-283 | −1.631 * | Predicted Target: ATP synthase lipid-binding protein | Energy Utilization |
| miR-2952-3p | −9.911 *** | Predicted Target: Hydroxyacylglutathione hydrolase | Energy Utilization |
| novel-miR1 | 2.425 *** | Predicted Target: ankyrin repeat and MYND domain-containing protein 2 | - |
| novel-miR4 | 4.962 *** | Predicted Target: CPIJ003731, uncharacterized protein | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marzec, S.; Siperstein, A.; Zhou, A.; Holzapfel, C.M.; Bradshaw, W.E.; Meuti, M.E.; Armbruster, P.A. MicroRNA Expression Prior to Biting in a Vector Mosquito Anticipates Physiological Processes Related to Energy Utilization, Reproduction and Immunity. Insects 2023, 14, 700. https://doi.org/10.3390/insects14080700
Marzec S, Siperstein A, Zhou A, Holzapfel CM, Bradshaw WE, Meuti ME, Armbruster PA. MicroRNA Expression Prior to Biting in a Vector Mosquito Anticipates Physiological Processes Related to Energy Utilization, Reproduction and Immunity. Insects. 2023; 14(8):700. https://doi.org/10.3390/insects14080700
Chicago/Turabian StyleMarzec, Sarah, Alden Siperstein, Angela Zhou, Christina M. Holzapfel, William E. Bradshaw, Megan E. Meuti, and Peter A. Armbruster. 2023. "MicroRNA Expression Prior to Biting in a Vector Mosquito Anticipates Physiological Processes Related to Energy Utilization, Reproduction and Immunity" Insects 14, no. 8: 700. https://doi.org/10.3390/insects14080700
APA StyleMarzec, S., Siperstein, A., Zhou, A., Holzapfel, C. M., Bradshaw, W. E., Meuti, M. E., & Armbruster, P. A. (2023). MicroRNA Expression Prior to Biting in a Vector Mosquito Anticipates Physiological Processes Related to Energy Utilization, Reproduction and Immunity. Insects, 14(8), 700. https://doi.org/10.3390/insects14080700




