Saliva-Mediated Contrasting Effects of Two Citrus Aphid Species on Asian Citrus Psyllid Feeding Behavior and Plant Jasmonic Acid Pathway
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Plants and Insects
2.2. Aphid Pre-Infestation Treatments
2.3. Saliva Collection and Infiltration
2.4. Detection of D. citri Feeding Behavior
2.5. Expression of Genes Involved in SA and JA Defense Pathways
2.6. Statistical Analysis
3. Results
3.1. Effect of Aphid Pre-Infestation on the Feeding Behavior of D. citri
3.2. Effect of A. spiraecola and A. citricidus Saliva Infiltration on the Feeding Behavior of D. citri
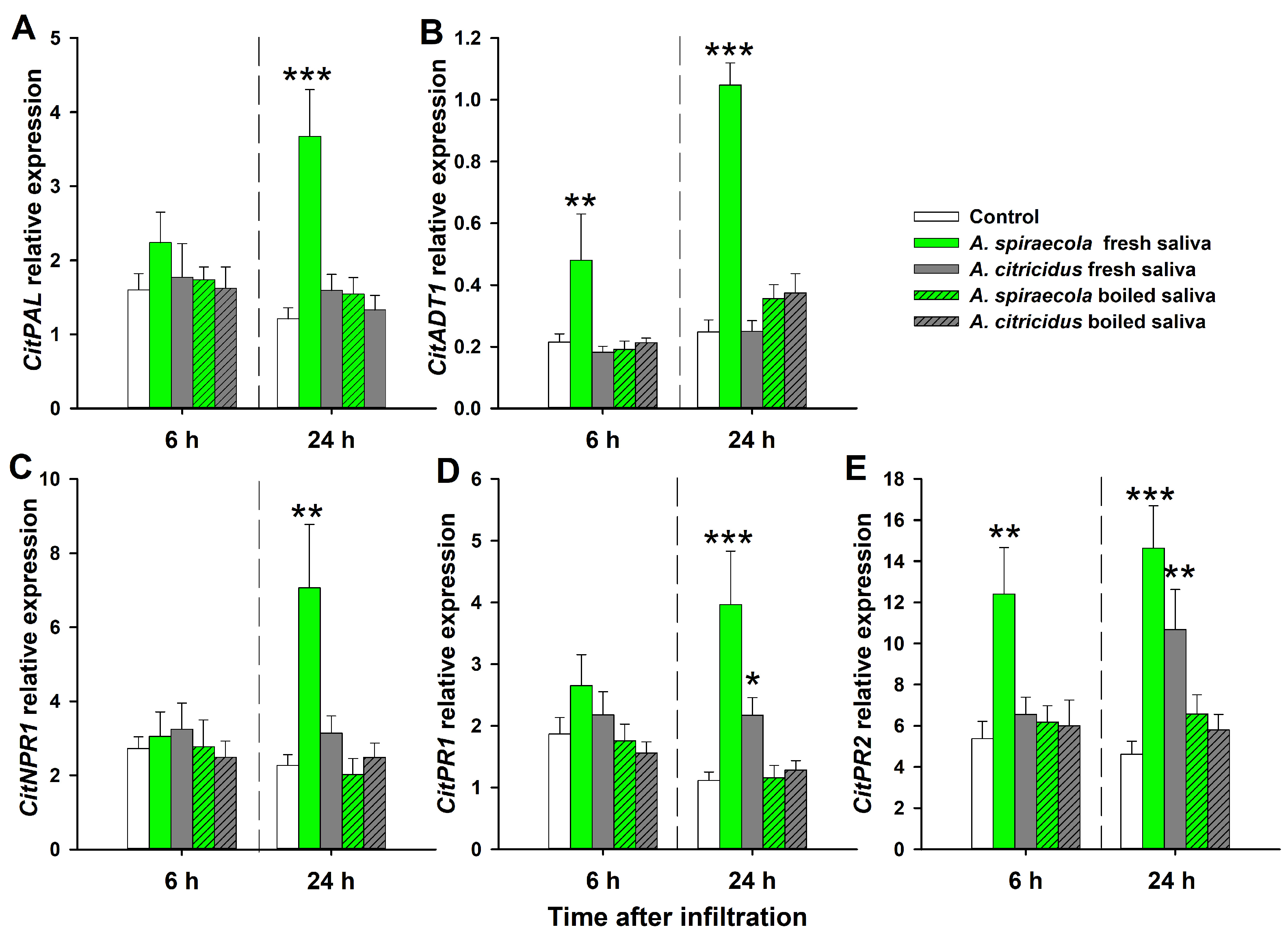
3.3. Expression of Genes Involved in Phytohormone-Dependent Defense after Citrus Aphid Saliva Infiltration
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Acevedo, F.E.; Rivera-Vega, L.J.; Chung, S.H.; Ray, S.; Felton, G.W. Cues from chewing insects—The intersection of DAMPs, HAMPs, MAMPs and effectors. Curr. Opin. Plant Biol. 2015, 26, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Furstenberg-Hagg, J.; Zagrobelny, M.; Bak, S. Plant defense against insect herbivores. Int. J. Mol. Sci. 2013, 14, 10242–10297. [Google Scholar] [CrossRef] [PubMed]
- Thaler, J.S.; Humphrey, P.T.; Whiteman, N.K. Evolution of jasmonate and salicylate signal crosstalk. Trends Plant Sci. 2012, 17, 260–270. [Google Scholar] [CrossRef] [PubMed]
- Wasternack, C.; Feussner, I. The oxylipin pathways: Biochemistry and function. Annu. Rev. Plant Biol. 2018, 69, 363–386. [Google Scholar] [CrossRef]
- Jaouannet, M.; Rodriguez, P.A.; Thorpe, P.; Lenoir, C.J.G.; MacLeod, R.; Escudero-Martinez, C.; Bos, J.I.B. Plant immunity in plant-aphid interactions. Front. Plant Sci. 2014, 5, 663. [Google Scholar] [CrossRef]
- Howe, G.A.; Jander, G. Plant immunity to insect herbivores. Annu. Rev. Plant Biol. 2008, 59, 41–66. [Google Scholar] [CrossRef]
- Zhang, P.-J.; Li, W.D.; Huang, F.; Zhang, J.M.; Xu, F.C.; Lu, Y.B. Feeding by whiteflies suppresses downstream jasmonic acid signaling by eliciting salicylic acid signaling. J. Chem. Ecol. 2013, 39, 612–619. [Google Scholar] [CrossRef]
- Erb, M.; Reymond, P. Molecular interactions between plants and insect herbivores. Annu. Rev. Plant Biol. 2019, 70, 527–557. [Google Scholar] [CrossRef]
- Huang, H.J.; Zhang, C.X.; Hong, X.Y. How does saliva function in planthopper–host interactions? Arch. Insect Biochem. Physiol. 2019, 100, e21537. [Google Scholar] [CrossRef]
- Mondal, H.A. Aphid saliva: A powerful recipe for modulating host resistance towards aphid clonal propagation. Arthropod-Plant Interact. 2020, 14, 547–558. [Google Scholar] [CrossRef]
- Huang, H.J.; Ye, Z.X.; Lu, G.; Zhang, C.X.; Chen, J.P.; Li, J.M. Identification of salivary proteins in the whitefly Bemisia tabaci by transcriptomic and LC–MS/MS analyses. Insect Sci. 2021, 28, 1369–1381. [Google Scholar] [CrossRef]
- Su, Y.-L.; Li, J.-M.; Li, M.; Luan, J.-B.; Ye, X.-D.; Wang, X.-W.; Liu, S.-S. Transcriptomic analysis of the salivary glands of an invasive whitefly. PLoS ONE 2012, 7, e39303. [Google Scholar] [CrossRef]
- Wu, Z.Z.; Qu, M.Q.; Chen, M.S.; Lin, J.T. Proteomic and transcriptomic analyses of saliva and salivary glands from the Asian citrus psyllid, Diaphorina citri. J. Proteom. 2021, 238, 104136. [Google Scholar] [CrossRef]
- Guo, H.; Zhang, Y.; Tong, J.; Ge, P.; Wang, Q.; Zhao, Z.; Zhu-Salzman, K.; Hogenhout, S.A.; Ge, F.; Sun, Y. An aphid-secreted salivary protease activates plant defense in phloem. Curr. Biol. 2020, 30, 4826–4836.e7. [Google Scholar] [CrossRef]
- De Vos, M.; Jander, G. Myzus persicae (green peach aphid) salivary components induce defence responses in Arabidopsis thaliana. Plant Cell Environ. 2009, 32, 1548–1560. [Google Scholar] [CrossRef]
- Naessens, E.; Dubreuil, G.; Giordanengo, P.; Baron, O.L.; Minet-Kebdani, N.; Keller, H.; Coustau, C. A secreted MIF cytokine enables aphid feeding and represses plant immune responses. Curr. Biol. 2015, 25, 1898–1903. [Google Scholar] [CrossRef]
- Su, Q.; Peng, Z.; Tong, H.; Xie, W.; Wang, S.; Wu, Q.; Zhang, J.; Li, C.; Zhang, Y. A salivary ferritin in the whitefly suppresses plant defenses and facilitates host exploitation. J. Exp. Bot. 2019, 70, 3343–3355. [Google Scholar] [CrossRef]
- Elzinga, D.A.; De Vos, M.; Jander, G. Suppression of plant defenses by a Myzus persicae (Green Peach Aphid) salivary effector protein. Mol. Plant Microbe Interact. 2014, 27, 747–756. [Google Scholar] [CrossRef]
- Cui, N.; Lu, H.; Wang, T.; Zhang, W.; Kang, L.; Cui, F. Armet, an aphid effector protein, induces pathogen resistance in plants by promoting the accumulation of salicylic acid. Philos. Trans. R. Soc. B 2019, 374, 20180314. [Google Scholar] [CrossRef]
- Ye, W.; Yu, H.; Jian, Y.; Zeng, J.; Ji, R.; Chen, H.; Lou, Y. A salivary EF-hand calcium-binding protein of the brown planthopper Nilaparvata lugens functions as an effector for defense responses in rice. Sci. Rep. 2017, 7, 40498. [Google Scholar] [CrossRef]
- Grafton-Cardwell, E.E.; Stelinski, L.L.; Stansly, P.A. Biology and Management of Asian Citrus Psyllid, Vector of the Huanglongbing Pathogens. Annu. Rev. Entomol. 2013, 58, 413–432. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.Q.; Jiang, H.-B.; Liu, T.Y.; Yang, L.; Fan, J.Y.; Xiong, Y.; Jing, T.X.; Lou, B.H.; Dou, W.; Wang, J.J. A transcriptomic and proteomic analysis of the Diaphorina citri salivary glands reveals genes responding to Candidatus Liberibacter asiaticus. Front. Physiol. 2020, 11, 582505. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Killiny, N. The secreted salivary proteome of Asian citrus psyllid Diaphorina citri. Physiol. Entomol. 2018, 43, 324–333. [Google Scholar] [CrossRef]
- Michaud, J. Biological control of Asian citrus psyllid, Diaphorina citri (Hemiptera: Psyllidae) in Florida: A preliminary report. Entomol. News. 2002, 113, 216–222. [Google Scholar]
- Gao, J.; Xiu, B.; Sun, Z.; Arthurs, S.; Guo, H.; Gu, S.; Long, J.; Xia, C.; Hussain, M.; Mao, R. Aphis spiraecola and Aphis (Toxoptera) citricidus differently manipulate plant metabolism to gain fitness in terms of population abundance or dispersal. Entomol. Exp. Et Appl. 2022, 170, 168–181. [Google Scholar] [CrossRef]
- Gao, J.; Tao, T.; Arthurs, S.P.; Ye, F.; An, X.; Hussain, M.; Mao, R. Plant jasmonic acid mediated contrasting effects of two citrus aphid species on Diaphorina citri Kuwayama. Pest Manag. Sci. 2023, 79, 811–820. [Google Scholar] [CrossRef]
- Capinera, J.L. Melon Aphid or Cotton Aphid, Aphis gossypii Glover (Insecta: Hemiptera: Aphididae); University of Florida Cooperative Extension Service, Institute of Food and Agricultural Sciences, Extension: Gainesville, FL, USA, 2000. [Google Scholar]
- McCoy, C.W.; Samson, R.A.; Boucias, D.G.; Osborne, L.S.; Pena, J.E.; Buss, L.J. Pathogens Infecting Insects and Mites of Citrus; LLC Friends of Microbes: Winter Park, FL, USA, 2009; p. 193. [Google Scholar]
- Zhang, Y.; Fan, J.; Francis, F.; Chen, J.L. Watery saliva secreted by the grain aphid Sitobion avenae stimulates aphid resistance in wheat. J. Agric. Food Chem. 2017, 65, 8798–8805. [Google Scholar] [CrossRef]
- Francis, M.I.; Kostenyuk, I.; Orbović, V.; Loskutov, A.; Zolotukhin, M.; Graham, J.H. Automated needle-free injection method for delivery of bacterial suspensions into citrus leaf tissues. J. Phytopathol. 2011, 159, 347–351. [Google Scholar] [CrossRef]
- Ebert, T.A.; Backus, E.A.; Cid, M.; Fereres, A.; Rogers, M.E. A new SAS program for behavioral analysis of electrical penetration graph data. Comput. Electron. Agric. 2015, 116, 80–87. [Google Scholar] [CrossRef]
- Miranda, M.P.; Yamamoto, P.T.; Garcia, R.B.; Lopes, J.P.A.; Lopes, J.R.S. Thiamethoxam and imidacloprid drench applications on sweet orange nursery trees disrupt the feeding and settling behaviour of Diaphorina citri (Hemiptera: Liviidae). Pest Manag. Sci. 2016, 72, 1785–1793. [Google Scholar] [CrossRef]
- Carmo-Sousa, M.; Garcia, R.B.; Wulff, N.A.; Fereres, A.; Miranda, M.P. Drench application of systemic insecticides disrupts probing behavior of Diaphorina citri (Hemiptera: Liviidae) and inoculation of Candidatus Liberibacter asiaticus. Insects 2020, 11, 314. [Google Scholar] [CrossRef]
- Nehela, Y.; Hijaz, F.; Elzaawely, A.A.; El-Zahaby, H.M.; Killiny, N. Citrus phytohormonal response to Candidatus Liberibacter asiaticus and its vector Diaphorina citri. Physiol. Mol. Plant Pathol. 2018, 102, 24–35. [Google Scholar] [CrossRef]
- Ibanez, F.; Suh, J.H.; Wang, Y.; Stelinski, L.L. Long-term, sustained feeding by Asian citrus psyllid disrupts salicylic acid homeostasis in sweet orange. BMC Plant Biolog. 2019, 19, 493. [Google Scholar] [CrossRef]
- Oliveira Coqueiro, D.S.; de Souza, A.A.; Takita, M.A.; Rodrigues, C.M.; Kishi, L.T.; Machado, M.A. Transcriptional profile of sweet orange in response to chitosan and salicylic acid. BMC Genom. 2015, 16, 1–14. [Google Scholar]
- Zhao, W.; Baldwin, E.A.; Bai, J.; Plotto, A.; Irey, M. Comparative analysis of the transcriptomes of the calyx abscission zone of sweet orange insights into the huanglongbing-associated fruit abscission. Hortic. Res. 2019, 6, 71. [Google Scholar] [CrossRef]
- Ohgushi, T. Indirect interaction webs: Herbivore-induced effects through trait change in plants. Annu. Rev. Ecol. Evol. Syst. 2005, 36, 81–105. [Google Scholar] [CrossRef]
- Hu, X.S.; Liu, X.F.; Zhao, H.Y. Development and application of electrical penetration graph (EPG) technique. Plant Prot. 2006, 32, 1–4. [Google Scholar]
- Liu, B.M.; Yan, F.M.; Chu, D.; Pan, H.P.; Jiao, X.G.; Xie, W.; Wu, Q.J.; Wang, S.L.; Xu, B.Y.; Zhou, X.G.; et al. Difference in feeding behaviors of two invasive whiteflies on host plants with different suitability: Implication for competitive displacement. Int. J. Biol. Sci. 2012, 8, 697–706. [Google Scholar] [CrossRef]
- Nowak, H.; Komor, E. How aphids decide what is good for them: Experiments to test aphid feeding behaviour on Tanacetum vulgare (L. ) using different nitrogen regimes. Oecologia 2010, 163, 973–984. [Google Scholar]
- Zhang, Y.; Fu, Y.; Francis, F.; Liu, X.; Chen, J. Insight into watery saliva proteomes of the grain aphid, Sitobion avenae. Arch. Insect Biochem. Physiol. 2021, 106, e21752. [Google Scholar] [CrossRef]
- Acevedo, F.E.; Smith, P.; Peiffer, M.; Helms, A.; Tooker, J.; Felton, G.W. Phytohormones in fall armyworm saliva modulate defense responses in plants. J. Chem. Ecol. 2019, 45, 598–609. [Google Scholar] [CrossRef] [PubMed]
- Alborn, H.T.; Hansen, T.V.; Jones, T.H.; Bennett, D.C.; Tumlinson, J.H.; Schmelz, E.A.; Teal, P.E. Disulfooxy fatty acids from the American bird grasshopper Schistocerca americana, elicitors of plant volatiles. Proc. Natl. Acad. Sci. USA 2007, 104, 12976–12981. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Singh, A.; Kaithakottil, G.G.; Mathers, T.C.; Gravino, M.; Mugford, S.T.; van Oosterhout, C.; Swarbreck, D.; Hogenhout, S.A. An aphid RNA transcript migrates systemically within plants and is a virulence factor. Proc. Natl. Acad. Sci. USA 2020, 117, 12763–12771. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Zhang, H.; Yang, Y.; Zhang, Y.; Guo, J.; Liu, W.; Wan, F. Plant defense responses induced by Bemisia tabaci Middle East—Asia Minor 1 salivary components. Entomol. Exp. Et Appl. 2016, 159, 287–297. [Google Scholar] [CrossRef]
- Ali, J.G.; Agrawal, A.A. Specialist versus generalist insect herbivores and plant defense. Trends Plant Sci. 2012, 17, 293–302. [Google Scholar] [CrossRef]
- Mostefaoui, H.; Allal-Benfekih, L.; Djazouli, Z.E.; Petit, D.; Saladin, G. Why the aphid Aphis spiraecola is more abundant on clementine tree than Aphis gossypii? Comptes Rendus Biol. 2014, 337, 123–133. [Google Scholar] [CrossRef]
- Brlansky, R.; Roy, A.; Damsteegt, V. Stem-pitting Citrus tristeza virus predominantly transmitted by the brown citrus aphid from mixed infections containing non-stem-pitting and stem-pitting isolates. Plant Dis. 2011, 95, 913–920. [Google Scholar] [CrossRef]
- Wang, L.; Wu, J. The essential role of jasmonic acid in plant-herbivore interactions—Using the wild tobacco Nicotiana attenuata as a model. J. Genet. Genom. 2013, 40, 597–606. [Google Scholar] [CrossRef]
- Cao, H.H.; Wang, S.H.; Liu, T.X. Jasmonate-and salicylate-induced defenses in wheat affect host preference and probing behavior but not performance of the grain aphid, Sitobion avenae. Insect Sci. 2014, 21, 47–55. [Google Scholar] [CrossRef]
- Zarate, S.I.; Kempema, L.A.; Walling, L.L. Silverleaf whitefly induces salicylic acid defenses and suppresses effectual jasmonic acid defenses. Plant Physiol. 2007, 143, 866–875. [Google Scholar] [CrossRef]
- Zhu-Salzman, K.; Bi, J.L.; Liu, T.X. Molecular strategies of plant defense and insect counter-defense. Insect Sci. 2005, 12, 3–15. [Google Scholar] [CrossRef]



| Gene Name | Gene Function | Primer Sequence (5′-3′) |
|---|---|---|
| CitGAPC1α | Reference gene | F: ACTCCAGAGGGATGATGTGG |
| R: ATGGGATCTCCTCTGGGTTC | ||
| CitPAL | SA biosynthesis | F: GGGGATCTGGTTCCTCTTTC |
| R: CATAGAAGCCAGGCCAGAAC | ||
| CitADT1 | SA biosynthesis | F: CACTCCTGTTGAGGACGACA |
| R: CACCTGGTAAGCCCTGGTAA | ||
| CitNPR1 | works downstream of SA | F: TGATAAGACCTTGCCACAACAC |
| R: ACCGCAGGATTCAGATCTATGT | ||
| CitPR1 | induced by SA | F: ACTGCAATCTTGTGCATTCG |
| R: TTCACCCACAGTTTCACAGC | ||
| CitPR2 | induced by SA | F: CCTTGTTCCCGCCATGAG |
| R: GCCAAGAGCTCCAGTTTCGA | ||
| CitAOS | JA biosynthesis | F: GTTTCAGCTCGCTCCGTTAC |
| R: GAGGTTGTGACACGCTTCCT | ||
| CitAOC | JA biosynthesis | F: GCGAGTGGGAATTACAGCAG |
| R: TTAACCTGCCCACTCACTCC | ||
| CitOPR3 | JA biosynthesis | F: ATGGTGCTGATTTGGTAGCC |
| R: ACCCACTCAAAGGCGTGATA | ||
| CitPI | works downstream of JA | F: AATCTTCTCATCGCTTTATC |
| R: TGCTTCGCACTTACAACT | ||
| CitJMT | methylation of jasmonate | F: GCCTGGACAAATACGCAAGAG |
| into methyljasmonate | R: CGATTCTCCCACTGCCCTTA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, J.; Tao, T.; Arthurs, S.P.; Hussain, M.; Ye, F.; Mao, R. Saliva-Mediated Contrasting Effects of Two Citrus Aphid Species on Asian Citrus Psyllid Feeding Behavior and Plant Jasmonic Acid Pathway. Insects 2023, 14, 672. https://doi.org/10.3390/insects14080672
Gao J, Tao T, Arthurs SP, Hussain M, Ye F, Mao R. Saliva-Mediated Contrasting Effects of Two Citrus Aphid Species on Asian Citrus Psyllid Feeding Behavior and Plant Jasmonic Acid Pathway. Insects. 2023; 14(8):672. https://doi.org/10.3390/insects14080672
Chicago/Turabian StyleGao, Jing, Tonglai Tao, Steven P. Arthurs, Mubasher Hussain, Fengxian Ye, and Runqian Mao. 2023. "Saliva-Mediated Contrasting Effects of Two Citrus Aphid Species on Asian Citrus Psyllid Feeding Behavior and Plant Jasmonic Acid Pathway" Insects 14, no. 8: 672. https://doi.org/10.3390/insects14080672
APA StyleGao, J., Tao, T., Arthurs, S. P., Hussain, M., Ye, F., & Mao, R. (2023). Saliva-Mediated Contrasting Effects of Two Citrus Aphid Species on Asian Citrus Psyllid Feeding Behavior and Plant Jasmonic Acid Pathway. Insects, 14(8), 672. https://doi.org/10.3390/insects14080672








