Potential of Entomopathogenic Nematodes to Control the Cabbage Stem Flea Beetle Psylliodes chrysocephala
Abstract
Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Insects and Plants
2.2. Entomopathogenic Nematodes
2.3. Mortality Bioassay
2.4. Compatibility between Entomopathogenic Nematodes and Adjuvants
2.5. Statistical Analysis
3. Results
3.1. Mortality Bioassay
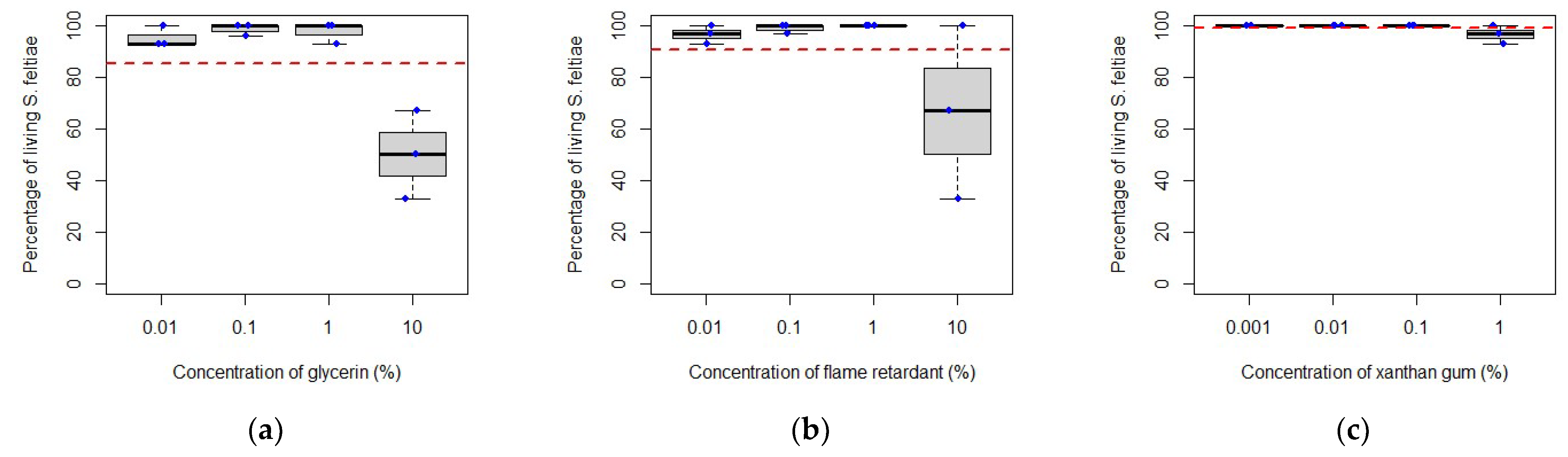
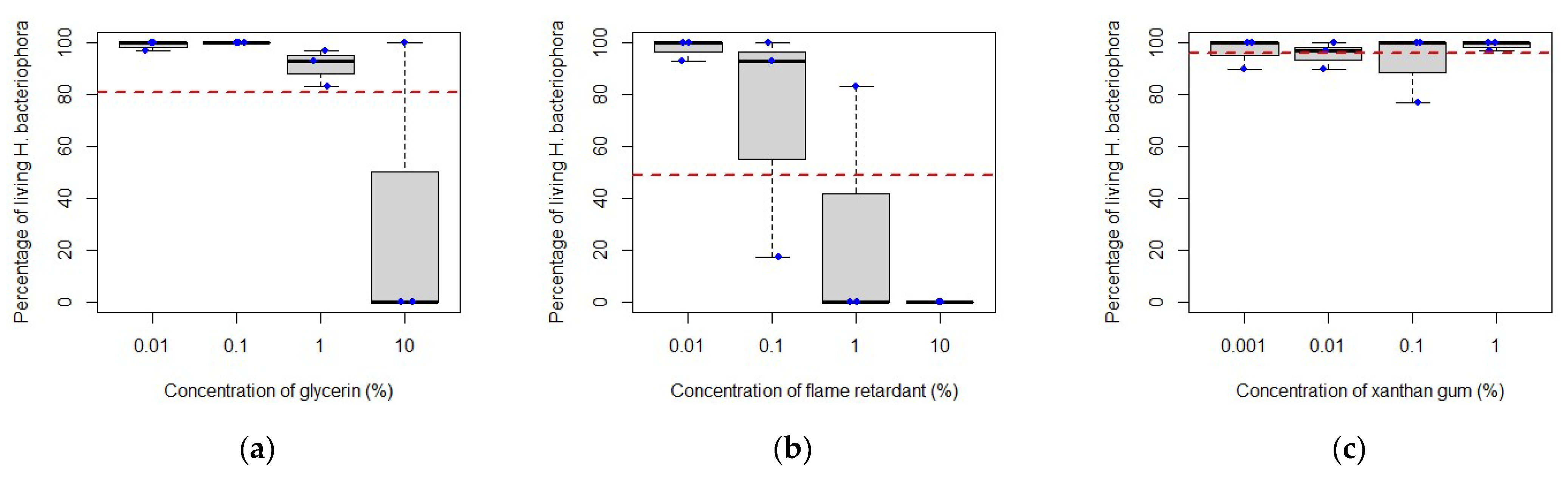
3.2. Compatibility between Entomopathogenic Nematodes and Adjuvants
4. Discussion
4.1. Mortality Bioassay
4.2. Compatibility between Entomopathogenic Nematodes and Adjuvants
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Defra Structure of the Agricultural Industry in England and the UK in June. Available online: https://www.gov.uk/government/statistical-data-sets/structure-of-the-agricultural-industry-in-england-and-the-uk-at-june (accessed on 4 January 2022).
- Scott, C.; Bilsborrow, P.E. The Impact of the EU Neonicotinoid Seed-Dressing Ban on Oilseed Rape Production in England. Pest Manag. Sci. 2019, 75, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Alford, D.V.; Gould, H.J. Surveys of Pest Incidence on Oil-Seed Rape in the UK. In Proceedings of the Eighth British Insecticide and Fungicide Conference, Hotel Metropole, Brighton, England, 17–20 November 1975; British Crop Protection Council: Hampshire, UK, 1976; Volumes 1–3, pp. 489–495. [Google Scholar]
- Winfield, A.L. Management of Oilseed Rape Pests in Europe. Agric. Zool. Rev. 1992, 5, 51–95. [Google Scholar]
- Ferguson, A.W.; Klukowski, Z.; Walczak, B.; Clark, S.J.; Mugglestone, M.A.; Perry, J.N.; Williams, I.H. Spatial Distribution of Pest Insects in Oilseed Rape: Implications for Integrated Pest Management. Agric. Ecosyst. Environ. 2003, 95, 509–521. [Google Scholar] [CrossRef]
- Nicholls, C. A Review of AHDB Impact Assessments Following the Neonicotinoid Seed Treatment Restrictions in Winter Oilseed Rape. AHDB Cereals Oilseed 2016, 31, 1–18. [Google Scholar]
- Ortega-Ramos, P.A.; Coston, D.J.; Seimandi-Corda, G.; Mauchline, A.L.; Cook, S.M. Integrated Pest Management Strategies for Cabbage Stem Flea Beetle (Psylliodes chrysocephala) in Oilseed Rape. GCB Bioenergy 2021, 14, 267–286. [Google Scholar] [CrossRef]
- Leach, J.E.; Darby, R.J.; Williams, I.H.; Fitt, B.D.; Rawlinson, C.J. Factors Affecting Growth and Yield of Winter Oilseed Rape (Brassica napus), 1985–1989. J. Agric. Sci. 1994, 122, 405–413. [Google Scholar] [CrossRef]
- Schulz, R.R.; Daebeler, F. Zum Schaden Durch Den Rapserdfloh (Psylliodes chrysocephala L.), Insbesondere Seiner Imagines. Heft 1984, 38, 113–115. [Google Scholar]
- Alford, D.V.; Cooper, D.A.; Williams, I.R. Insect Pests of Oilseed Rape; Home Grown Cereals Authority (HGCA) London: London, UK, 1991. [Google Scholar]
- Broschewitz, B.; Steinbach, P.; Goltermann, S. Einfluss Stengelbewohnender Tierischer Schaderreger Auf Den Befall von Winterraps Mit Phoma Lingam Und Botrytis Cinerea. Gesunde Pflanz. 1993, 45, 106–110. [Google Scholar]
- Bonnemaison, L.; Jourdheuil, P. L’altise d’hiver Du Colza (Psylliodes chrysocephala L.). Ann. Des Épiphyties 1954, 4, 345–524. [Google Scholar]
- Williams, J.J.W.; Carden, P.W. Cabbage Stem Flea Beetle in East Anglia. Plant Pathol. 1961, 10, 85–95. [Google Scholar] [CrossRef]
- Graham, C.W.; Alford, D.V. The Distribution and Importance of Cabbage Stem Flea Beetle (Psylliodes chrysocephala (L.)) on Winter Oilseed Rape in England. Plant Pathol. 1981, 30, 141–145. [Google Scholar] [CrossRef]
- Nilsson, C. Yield Losses in Winter Rape Caused by Cabbage Stem Flea Beetle Larvae (Psylliodes chrysocephala (L.)) [Brassica Napus Var. Oleifera]. Bull. SROP Fr. 1990, 13, 53–56. [Google Scholar]
- Nilsson, C. Strategies for the Control of Cabbage Stem Flea Beetle on Winter Rape in Sweden. IOBC WPRS Bull. 2002, 25, 133–142. [Google Scholar]
- Williams, I.H. The Major Insect Pests of Oilseed Rape in Europe and Their Management: An Overview. In Biocontrol-Based Integrated Management of Oilseed Rape Pests; Springer: Dordrecht, The Netherlands, 2010; pp. 1–43. [Google Scholar]
- European Commission Commission Implementing Regulation (EU). No 485/2013 of 24 May 2013 Amending Implementing Regulation (EU) No 540/2011, as Regards the Conditions of Approval of the Active Substances Clothianidin, Thiamethoxam and Imidacloprid, and Prohibiting the Use and Sale of Seeds Treated with Plant Protection Products Containing Those Active Substances. Off. J. Eur. Union L 2013, 139, 12–26. [Google Scholar]
- Bass, C.; Field, L.M. Neonicotinoids. Curr. Biol. 2018, 28, R772–R773. [Google Scholar] [CrossRef]
- Højland, D.H.; Nauen, R.; Foster, S.P.; Williamson, M.S.; Kristensen, M. Incidence, Spread and Mechanisms of Pyrethroid Resistance in European Populations of the Cabbage Stem Flea Beetle, Psylliodes chrysocephala L. (Coleoptera: Chrysomelidae). PLoS ONE 2015, 10, e0146045. [Google Scholar] [CrossRef]
- Willis, C.E.; Foster, S.P.; Zimmer, C.T.; Elias, J.; Chang, X.; Field, L.M.; Williamson, M.S.; Davies, T.E. Investigating the Status of Pyrethroid Resistance in UK Populations of the Cabbage Stem Flea Beetle (Psylliodes chrysocephala). Crop Prot. 2020, 138, 105316. [Google Scholar] [CrossRef]
- IBMA Definition: Bioprotection as the Global Term for All Biocontrol Technologies. Available online: https://ibma-global.org/wp-content/uploads/2020/12/ibmadefinitionleafletweb.pdf (accessed on 5 April 2023).
- Hokkanen, H.M.; Menzler-Hokkanen, I.; Butt, T.M. Pathogens of Oilseed Rape Pests. In Biocontrol Oilseed Rape Pests; Blackwell Science Ltd.: Hoboken, NJ, USA, 2003; pp. 299–322. [Google Scholar]
- Bednarek, A.; Nowicki, T. Effect of Intrapopulation Factors in the Nematodes Steinernema Feltiae (Steinernematidae) on Intensity of Steinernema Feltiae. Zesz. Probl. Postępów Nauk Rol. 1986, 323, 199–212. [Google Scholar]
- Shapiro-Ilan, D.; Hazir, S.; Glazer, I. Basic and Applied Research: Entomopathogenic Nematodes. In Microbial Control of Insect and Mite Pests; Elsevier: Amsterdam, The Netherlands, 2017; pp. 91–105. [Google Scholar]
- Bedding, R.A.; Molyneux, A.S. Penetration of Insect Cuticle by Infective Juveniles of Heterorhabditis spp. (Heterorhabditidae: Nematoda). Nematologica 1982, 28, 354–359. [Google Scholar] [CrossRef]
- Peters, A.; Ehlers, R.-U. Susceptibility of Leatherjackets (Tipula paludosa and Tipula oleracea; Tipulidae; Nematocera) to the Entomopathogenic Nematode Steinernema feltiae. J. Invertebr. Pathol. 1994, 63, 163–171. [Google Scholar] [CrossRef]
- Akhurst, R.J.; Boemare, N.E. Biology and Taxonomy of Xenorhabdus. In Entomopathogenic Nematodes in Biological Control; CRC Press: Boca Raton, FL, USA, 1990; pp. 75–90. [Google Scholar] [CrossRef]
- Boemare, N.E.; Akhurst, R.J.; Mourant, R.G. DNA Relatedness between Xenorhabdus spp. (Enterobacteriaceae), Symbiotic Bacteria of Entomopathogenic Nematodes, and a Proposal to Transfer Xenorhabdus luminescens to a New Genus, Photorhabdus Gen. Nov. Int. J. Syst. Evol. Microbiol. 1993, 43, 249–255. [Google Scholar] [CrossRef]
- Gaugler, R. Entomopathogenic Nematology; CABI Publishing: Wallingford, UK, 2002. [Google Scholar]
- Poinar, G.O., Jr. Taxonomy and Biology of Steinernematidae and Heterorhabditidae. In Entomopathogenic Nematodes in Biological Control; Gaugler, R., Kaya, H.K., Eds.; CRC Press: Boca Raton, FL, USA, 1990; pp. 23–61. [Google Scholar]
- Hassan, A.A. Comparison between Field Research and Controlled Laboratory Research. Arch. Clin. Biomed. Res. 2017, 1, 101–104. [Google Scholar] [CrossRef]
- Li, X.F.; Wang, G.H. Preliminary Study on the Control of Phyllotreta vittata Larvae by Entomopathogenic Nematodes. Acta Phytophylacica Sin. 1990, 17, 229–231. [Google Scholar]
- Wei, H.Y.; Wang, G.H. The control effect of a Steinernema nematode against striped flea beetle. Acta Phytophylacica Sin. 1993, 20, 61–64. [Google Scholar]
- Hou, Y.; Pang, X.; Liang, G. On the Partial Application of Steinernema Carpocapsae Strain A24 against Striped Flea Beetle Phyllotreta striolata. Acta Phytophylacica Sin. 2001, 28, 151–156. [Google Scholar]
- Yan, X.; Han, R.; Moens, M.; Chen, S.; De Clercq, P. Field Evaluation of Entomopathogenic Nematodes for Biological Control of Striped Flea Beetle, Phyllotreta striolata (Coleoptera: Chrysomelidae). BioControl 2013, 58, 247–256. [Google Scholar] [CrossRef]
- Yan, X.; Lin, Y.; Huang, Z.; Han, R. Characterisation of Biological and Biocontrol Traits of Entomopathogenic Nematodes Promising for Control of Striped Flea Beetle (Phyllotreta striolata). Nematology 2018, 20, 503–518. [Google Scholar] [CrossRef]
- Noosidum, A.; Mangtab, S.; Lewis, E.E. Biological Control Potential of Entomopathogenic Nematodes against the Striped Flea Beetle, Phyllotreta sinuata Stephens (Coleoptera: Chrysomelidae). Crop Prot. 2021, 141, 105448. [Google Scholar] [CrossRef]
- Morris, O.N. Evaluation of the Nematode, Steinernema Feltiae Filipjev, for the Control of the Crucifer Flea Beetle, Phyllotreta cruciferae (Goeze) (Coleoptera: Chrysomelidae). Can. Entomol. 1987, 119, 95–101. [Google Scholar] [CrossRef]
- Reddy, G.V.P.; Tangtrakulwanich, K.; Wu, S.; Miller, J.H.; Ophus, V.L.; Prewett, J. Sustainable Management Tactics for Control of Phyllotreta cruciferae (Coleoptera: Chrysomelidae) on Canola in Montana. J. Econ. Entomol. 2014, 107, 661–666. [Google Scholar] [CrossRef]
- Hokkanen, H.M.; Zec-Vojinovic, M.; Büchs, W.; Husberg, G.B.; Klukowski, Z.; Luik, A. Effectiveness of Entomopathogenic Nematodes in the Control of OSR Pests. In Proceedings of the International Symposium on Integrated Pest Management in Oilseed Rape, Gottingen, Germany, 3–5 April 2006. [Google Scholar]
- Hokkanen, H.M. Biological Control Methods of Pest Insects in Oilseed Rape. EPPO Bull. 2008, 38, 104–109. [Google Scholar] [CrossRef]
- Ignoffo, C.M.; Garcia, C. Influence of Conidial Color on Inactivation of Several Entomogenous Fungi (Hyphomycetes) by Simulated Sunlight. Environ. Entomol. 1992, 21, 913–917. [Google Scholar] [CrossRef]
- Jaronski, S.T. Ecological Factors in the Inundative Use of Fungal Entomopathogens. BioControl 2010, 55, 159–185. [Google Scholar] [CrossRef]
- Antwi, F.B.; Reddy, G.V. Efficacy of Entomopathogenic Nematodes and Sprayable Polymer Gel against Crucifer Flea Beetle (Coleoptera: Chrysomelidae) on Canola. J. Econ. Entomol. 2016, 109, 1706–1712. [Google Scholar] [CrossRef]
- Briar, S.S.; Antwi, F.; Shrestha, G.; Sharma, A.; Reddy, G.V. Potential Biopesticides for Crucifer Flea Beetle, Phyllotreta cruciferae (Coleoptera: Chrysomelidae) Management under Dryland Canola Production in Montana. Phytoparasitica 2018, 46, 247–254. [Google Scholar] [CrossRef]
- Prabhuraj; Girish, A.K.S.; Shivaleela. Persistence of Heterorhabditis indica on Chickpea Foliage. Indian J. Nematol. 2005, 35, 24–27. [Google Scholar]
- Beck, B.; Brusselman, E.; Nuyttens, D.; Moens, M.; Pollet, S.; Temmerman, F.; Spanoghe, P. Improving Foliar Applications of Entomopathogenic Nematodes by Selecting Adjuvants and Spray Nozzles. Biocontrol Sci. Technol. 2013, 23, 507–520. [Google Scholar] [CrossRef]
- Shapiro-Ilan, D.I.; Cottrell, T.E.; Mizell III, R.F.; Horton, D.L.; Behle, R.W.; Dunlap, C.A. Efficacy of Steinernema Carpocapsae for Control of the Lesser Peachtree Borer, Synanthedon Pictipes: Improved Aboveground Suppression with a Novel Gel Application. Biol. Control 2010, 54, 23–28. [Google Scholar] [CrossRef]
- Shapiro-Ilan, D.I.; Cottrell, T.E.; Mizell III, R.F.; Horton, D.L. Efficacy of Steinernema carpocapsae plus Fire Gel Applied as a Single Spray for Control of the Lesser Peachtree Borer, Synanthedon pictipes. Biol. Control 2016, 94, 33–36. [Google Scholar] [CrossRef]
- Grewal, P.S.; Selvan, S.; Gaugler, R. Thermal Adaptation of Entomopathogenic Nematodes: Niche Breadth for Infection, Establishment, and Reproduction. J. Therm. Biol. 1994, 19, 245–253. [Google Scholar] [CrossRef]
- Long, S.J.; Richardson, P.N.; Fenlon, J.S. Influence of Temperature on the Infectivity of Entomopathogenic Nematodes (Steinernema and Heterorhabditis Spp.) to Larvae and Pupae of the Vine Weevil Otiorhynchus Sulcatus (Coleoptera: Curculionidae). Nematology 2000, 2, 309–317. [Google Scholar] [CrossRef]
- AHDB, (Agriculture and Horticulture Development Board). Oilseed Rape Growth Guide. Available online: https://projectblue.blob.core.windows.net/media/Default/Imported%20Publication%20Docs/AHDB%20Cereals%20&%20Oilseeds/General/Oilseed%20rape%20growth%20guide.pdf (accessed on 29 July 2020).
- Trdan, S.; Vidrih, M.; Valič, N.; Laznik, Ž. Impact of Entomopathogenic Nematodes on Adults of Phyllotreta spp. (Coleoptera: Chrysomelidae) under Laboratory Conditions. Acta Agric. Scand. Sect. B-Soil Plant Sci. 2008, 58, 169–175. [Google Scholar]
- Svendsen, T.S.; Steenberg, T. The Potential Use of Entomopathogenic Nematodes against Typhaea stercorea. BioControl 2000, 45, 97–111. [Google Scholar] [CrossRef]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using Lme4. arXiv 2014, arXiv:14065823. [Google Scholar]
- Hothorn, T.; Bretz, F.; Westfall, P. Package Multcomp: Simultaneous Inference in General Parametric Models. Publ. Online CRAN Repos. 2015. [Google Scholar]
- Lenth, R.V. Least-Squares Means: The R Package Lsmeans. J. Stat. Softw. 2016, 69, 1–33. [Google Scholar] [CrossRef]
- De Mendiburu, F.; Simon, R. Agricolae-Ten Years of an Open Source Statistical Tool for Experiments in Breeding, Agriculture and Biology. PeerJ PrePrints 2015, 3, e1404v1. [Google Scholar]
- Wickham, H. Elegant Graphics for Data Analysis (Ggplot2). In Applied Spatial Data Analysis with R; Springer: New York, NY, USA, 2009. [Google Scholar]
- Wickham, H.; Averick, M.; Bryan, J.; Chang, W.; McGowan, L.D.; François, R.; Grolemund, G.; Hayes, A.; Henry, L.; Hester, J.; et al. Welcome to the Tidyverse. J. Open Source Softw. 2019, 4, 1686. [Google Scholar] [CrossRef]
- Murrell, P. R Graphics. Wiley Interdiscip. Rev. Comput. Stat. 2009, 1, 216–220. [Google Scholar] [CrossRef]
- Godina, G.; Vandenbossche, B.; Schmidt, M.; Sender, A.; Tambe, A.H.; Touceda-González, M.; Ehlers, R.-U. Entomopathogenic Nematodes for Biological Control of Psylliodes chrysocephala (Coleoptera: Chrysomelidae) in Oilseed Rape. J. Invertebr. Pathol. 2023, 197, 107894. [Google Scholar] [CrossRef]
- Xu, C.; De Clercq, P.; Moens, M.; Chen, S.; Han, R. Efficacy of Entomopathogenic Nematodes (Rhabditida: Steinernematidae and Heterorhabditidae) against the Striped Flea Beetle, Phyllotreta striolata. BioControl 2010, 55, 789–797. [Google Scholar] [CrossRef]

| Product Name | Manufacturer | Nematode Species | Temperature Range |
|---|---|---|---|
| Nemasys® | BASF Agricultural Solutions, Littlehampton, UK | Steinernema feltiae (90%) | 10–30 °C |
| Nemasys® C | Steinernema carpocapsae (87%) | 12–30 °C | |
| Nemasys® L | Steinernema kraussei (88%) | 5–30 °C | |
| Nemasys® H | Heterorhabditis bacteriophora (82%) | 12–30 °C |
| Product Name | Manufacturer | Active Ingredients | Concentrations | Replicates |
|---|---|---|---|---|
| Flametect Nitro D | Eco-Sol Ltd., Barry, UK | Nitrogen-based solution (34% minimum), polymer-binder system (30%) | 0.01, 0.1, 1 and 10% | 3 per concentrations |
| Xanthan gum | Sigma-Aldrich, St Louis, MO, USA | Xanthan gum from Xanthomonas campestris | 0.001, 0.01, 0.1 and 1% | 3 per concentration |
| Glycerin | Fisher Scientific, Loughborough, UK | Glycerol ≥ 99% | 0.01, 0.1, 1 and 10% | 3 per concentration |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Price, C.; Campbell, H.; Pope, T. Potential of Entomopathogenic Nematodes to Control the Cabbage Stem Flea Beetle Psylliodes chrysocephala. Insects 2023, 14, 665. https://doi.org/10.3390/insects14070665
Price C, Campbell H, Pope T. Potential of Entomopathogenic Nematodes to Control the Cabbage Stem Flea Beetle Psylliodes chrysocephala. Insects. 2023; 14(7):665. https://doi.org/10.3390/insects14070665
Chicago/Turabian StylePrice, Claire, Heather Campbell, and Tom Pope. 2023. "Potential of Entomopathogenic Nematodes to Control the Cabbage Stem Flea Beetle Psylliodes chrysocephala" Insects 14, no. 7: 665. https://doi.org/10.3390/insects14070665
APA StylePrice, C., Campbell, H., & Pope, T. (2023). Potential of Entomopathogenic Nematodes to Control the Cabbage Stem Flea Beetle Psylliodes chrysocephala. Insects, 14(7), 665. https://doi.org/10.3390/insects14070665






