Effects of γ-Irradiation on Mating Behavior of Red Palm Weevil, Rhynchophorus ferrugineus (Olivier, 1790) (Coleoptera: Dryophthoridae)
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insects and Male Irradiation
2.2. Experimental Design
- No-choice: wild female + irradiated male (60 or 80 Gy). A control was set up with a wild female and a wild male.
- Choice: wild female + irradiated male (60 or 80 Gy) + wild male. A control was set up with one wild female and two wild males.
- The interval from T0 (time when the individuals were placed in the containers) and the first mating, to compare the mating competition between fertile and sterile males;
- Duration of each mating;
- The total number of mating events that occurred in 4 h.
2.3. Data Analysis
2.3.1. No-Choice Test
2.3.2. Choice Test
3. Results
3.1. No-Choice Test
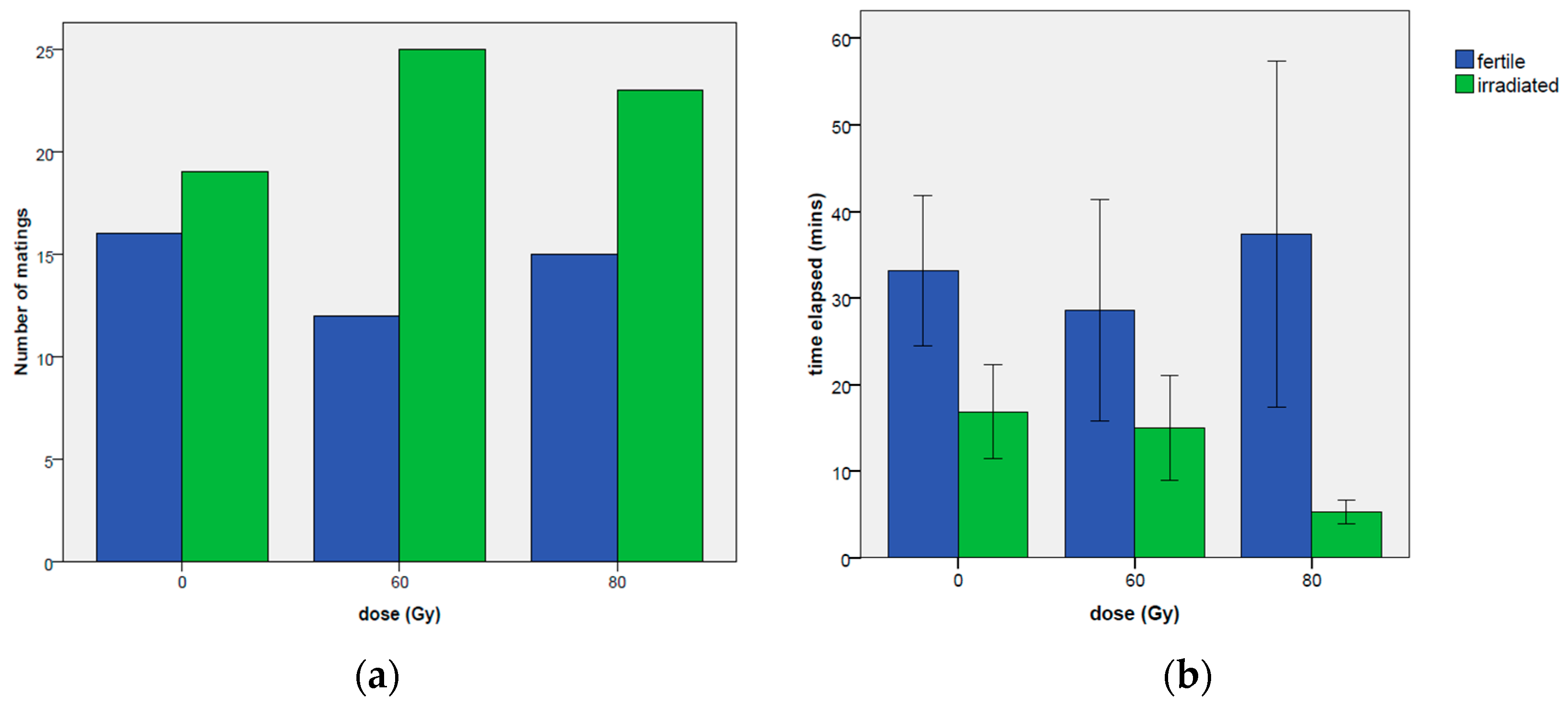
3.1.1. Mating Occurrence
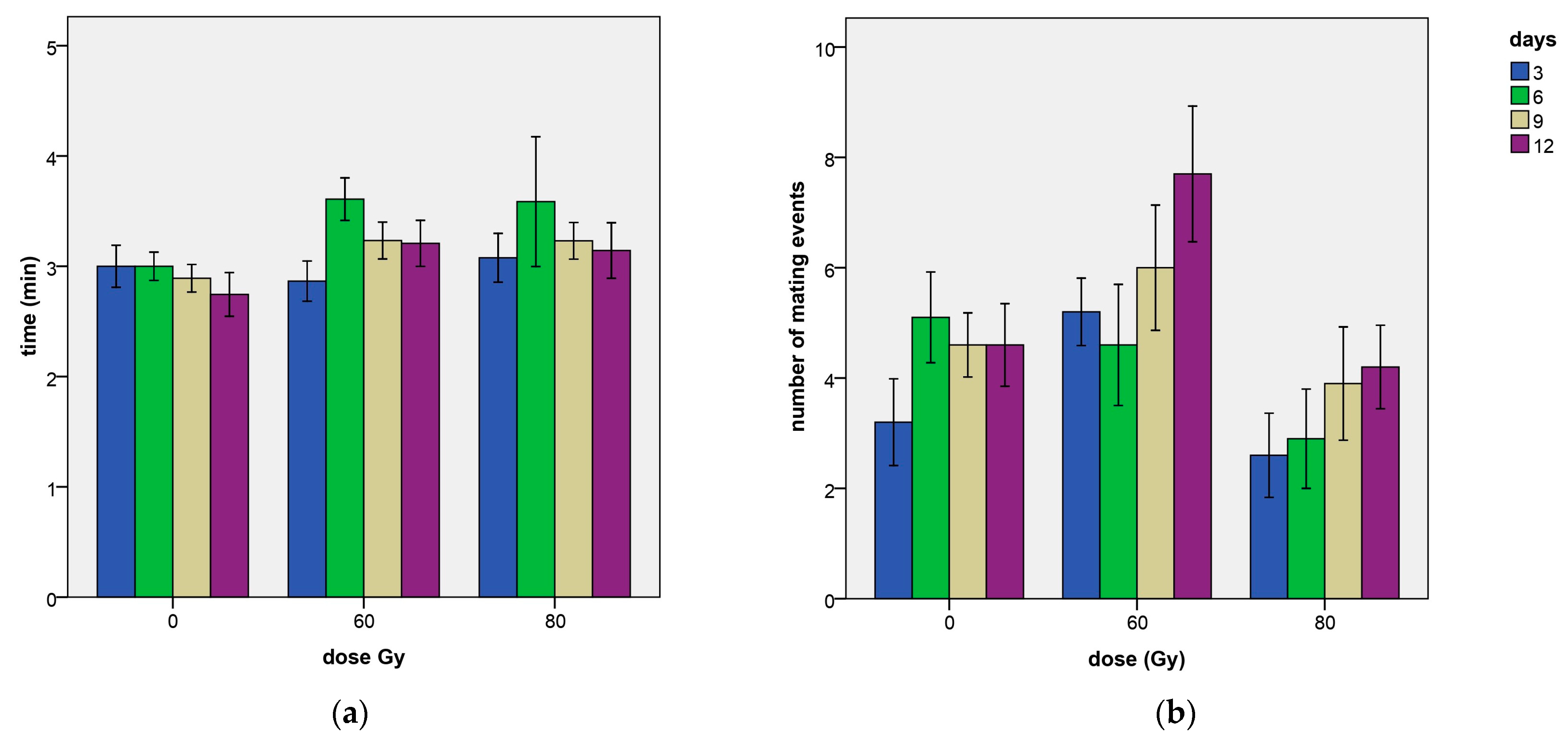
3.1.2. Mating Event Duration
3.1.3. Number of Mating Events per Day
3.1.4. Time Elapsed before the First Mating
3.2. Choice Test
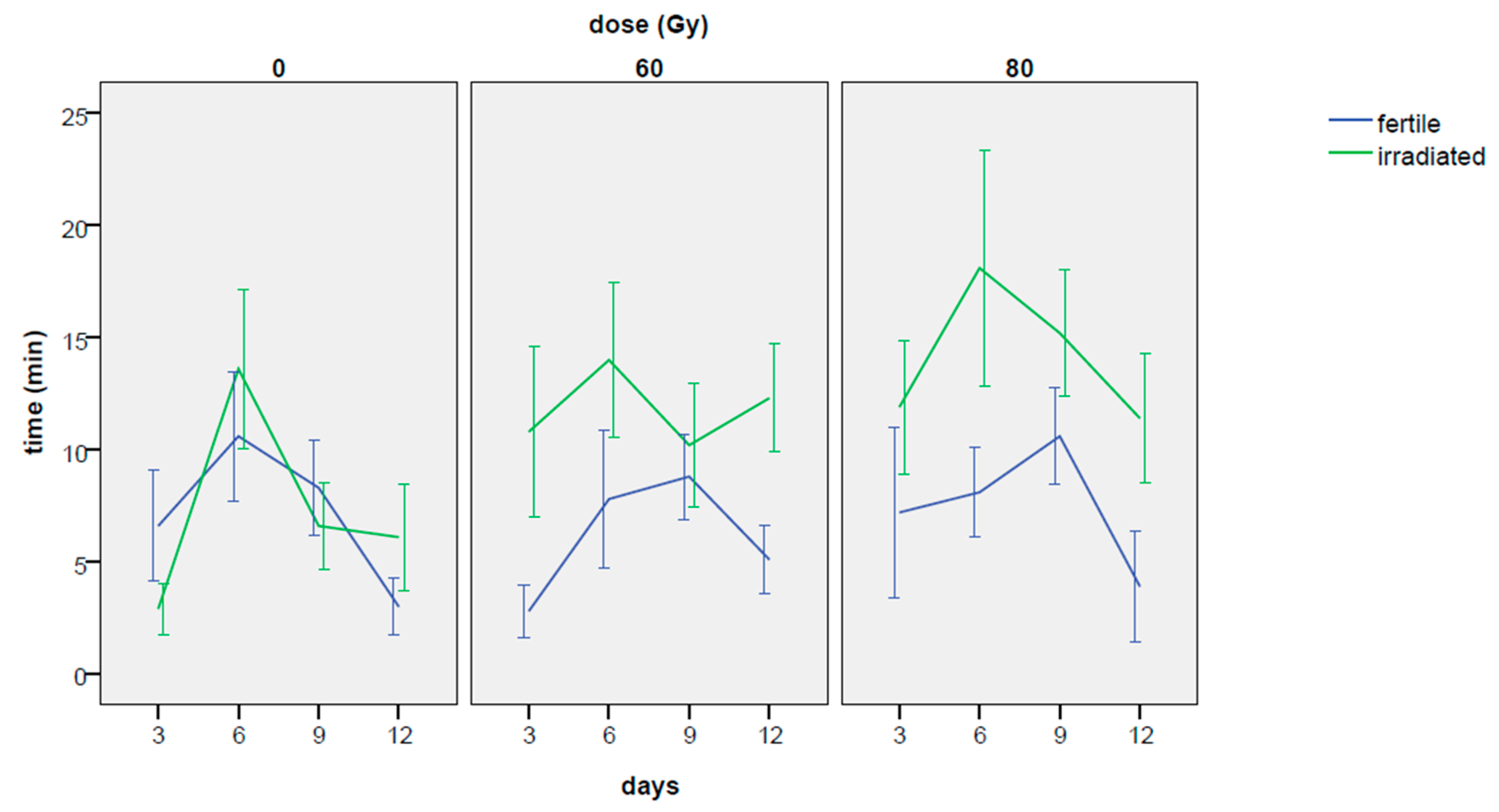
3.2.1. Total Amount of Time Spent in Mating
3.2.2. Mating Event Duration
3.2.3. Number of Mating Events Per Day
3.2.4. Occurrence of First Mating and Time Elapsed before the First Mating
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Dose Gy | Day | Proportion of Mating (0,1) | Duration of Mating (min) | Mating Events Day−1 (n.) | Time Elapsed before 1st Mating (min) |
|---|---|---|---|---|---|
| 0 | 3 | 0.040 | 3.00 | 3.20 | 58.89 |
| ±0.0064 | ±0.30 | ±0.57 | ±26.95 | ||
| 6 | 0.063 | 3.00 | 5.10 | 46.20 | |
| ±0.0095 | ±0.24 | ±0.64 | ±17.36 | ||
| 9 | 0.059 | 2.89 | 4.60 | 20.80 | |
| ±0.0089 | ±0.25 | ±0.72 | ±8.40 | ||
| 12 | 0.054 | 2.74 | 4.60 | 20.50 | |
| ±0.0082 | ±0.24 | ±0.81 | ±8.82 | ||
| Total mean | 0.054 | 2.90 | 4.38 | 30.66 | |
| ±0.0040 | ±0.13 | ±0.37 | ±6.56 | ||
| 60 | 3 | 0.062 | 2.87 | 5.20 | 21.00 |
| ±0.0091 | ±0.23 | ±0.73 | ±10.78 | ||
| 6 | 0.069 | 3.61 | 4.60 | 72.80 | |
| ±0.0100 | ±0.27 | ±0.82 | ±28.14 | ||
| 9 | 0.080 | 3.23 | 6.00 | 47.70 | |
| ±0.0115 | ±0.23 | ±0.92 | ±21.96 | ||
| 12 | 0.095 | 3.21 | 7.70 | 39.90 | |
| ±0.0133 | ±0.20 | ±1.03 | ±22.95 | ||
| Total mean | 0.077 | 3.22 | 5.87 | 29.57 | |
| ±0.005 | ±0.12 | ±0.54 | ±6.90 | ||
| 80 | 3 | 0.033 | 3.08 | 2.6 | 115.80 |
| ±0.0052 | ±0.35 | ±0.44 | ±30.17 | ||
| 6 | 0.043 | 3.59 | 2.9 | 95.30 | |
| ±0.0065 | ±0.35 | ±0.50 | ±27.45 | ||
| 9 | 0.055 | 3.23 | 3.9 | 75.50 | |
| ±0.0080 | ±0.29 | ±0.56 | ±28.02 | ||
| 12 | 0.055 | 3.14 | 4.2 | 43.60 | |
| ±0.0079 | ±0.27 | ±0.63 | ±23.22 | ||
| Total mean | 0.047 | 3.25 | 3.40 | 49.15 | |
| ±0.005 | ±0.16 | ±0.43 | ±9.08 | ||
| Mean of days | 3 | 0.045 | 2.95 | 3.67 | 65.45 |
| ±0.003 | ±0.17 | ±0.45 | ±15.30 | ||
| 6 | 0.059 | 3.36 | 4.20 | 71.43 | |
| ±0.004 | ±0.17 | ±0.56 | ±14.32 | ||
| 9 | 0.065 | 3.12 | 4.83 | 48.00 | |
| ±0.004 | ±0.15 | ±0.55 | ±12.473 | ||
| 12 | 0.068 | 3.06 | 5.50 | 34.67 | |
| ±0.004 | ±0.14 | ±0.60 | ±11.04 | ||
| Total | 0.059 | 3.12 | 4.55 | 54.80 | |
| ±0.003 | ±0.10 | ±0.28 | ±6.72 |
| Duration of Mating | 0 Gy | 60 Gy | 80 Gy | Total | ||||
|---|---|---|---|---|---|---|---|---|
| Min | n | % | n | % | n | % | n | % |
| 1 | 71 | 43.3 | 76 | 32.3 | 46 | 33.8 | 193 | 36.5 |
| 2 | 70 | 39.7 | 90 | 38.3 | 52 | 38.2 | 212 | 38.7 |
| 3 | 24 | 13.6 | 44 | 18.7 | 21 | 15.4 | 89 | 15.9 |
| ≥4 | 11 | 6.5 | 25 | 10.6 | 17 | 12.5 | 53 | 9.9 |
| Total | 176 | 100 | 235 | 100 | 136 | 100 | 547 | 100 |
| Categories of Time | 0 Gy | 60 Gy | 80 Gy | Total | ||||
|---|---|---|---|---|---|---|---|---|
| min | n | % | n | % | n | % | n | % |
| <10 | 13 | 32.5 | 15 | 37.5 | 6 | 15.0 | 34 | 28.3 |
| 10–30 | 16 | 40.0 | 8 | 20.0 | 11 | 27.5 | 35 | 29.2 |
| 30–60 | 4 | 10.0 | 11 | 27.5 | 7 | 17.5 | 22 | 18.3 |
| 60–120 | 4 | 5.0 | 2 | 5.0 | 5 | 12.5 | 11 | 9.2 |
| 120–240 | 2 | 5.0 | 1 | 2.5 | 4 | 10.0 | 7 | 5.8 |
| No mating | 1 | 2.5 | 3 | 7.5 | 7 | 17.5 | 11 | 9.2 |
| Total | 40 | 33.3 | 40 | 33.3 | 40 | 33.3 | 120 | 100 |
| Day | Dose (Gy) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 60 | 80 | Total * | ||||||
| n. | Male Type | Mins | % | Mins | % | Mins | % | Mins | % |
| fertile | 66 | (69.5) | 28 | (20.6) | 72 | (37.7) | 100 | (31.6) | |
| 3 | irradiated | 29 | (30.5) | 108 | (79.4) | 119 | (62.3) | 227 | (69.4) |
| total | 95 | (100.0) | 136 | (100.0) | 191 | (100.0) | 327 | (100.0) | |
| fertile | 106 | (43.8) | 78 | (33.8) | 81 | (30.9) | 159 | (33.1) | |
| 6 | irradiated | 136 | (56.2) | 140 | (64.2) | 181 | (69.1) | 321 | (66.9) |
| total | 242 | (100.0) | 218 | (100.0) | 262 | (100.0) | 480 | (100.0) | |
| fertile | 83 | (55.7) | 88 | (46.3) | 106 | (41.1) | 194 | (43.3) | |
| 9 | irradiated | 66 | (44.3) | 102 | (53.7) | 152 | (58.9) | 254 | (56.7) |
| total | 149 | (100.0) | 190 | (100.0) | 258 | (100.0) | 413 | (100.0) | |
| fertile | 30 | (33.0) | 51 | (29.3) | 39 | (25.5) | 90 | (28.5) | |
| 12 | irradiated | 61 | (67.0) | 123 | (70.7) | 114 | (74.5) | 237 | (72.5) |
| total | 91 | (100.0) | 174 | (100.0) | 153 | (100.0) | 327 | (100.0) | |
| Total | fertile | 285 | (49.4) | 245 | (34.1) | 298 | (34.6) | 543 | (34.3) |
| irradiated | 292 | (50.6) | 473 | (65.9) | 566 | (65.4) | 1039 | (65.7) | |
| Hours | Dose (Gy) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 60 | 80 | Total * | ||||||
| n. | Male Type | Mins | (%) | Mins | % | Mins | (%) | Mins | % |
| fertile | 78 | (41.7) | 96 | (37.1) | 114 | (32.4) | 210 | (34.4) | |
| 1st (0–60 min) | irradiated | 109 | (58.3) | 163 | (62.9) | 238 | (67.6) | 401 | 65.6 |
| total | 187 | (100.0) | 259 | (100.0) | 352 | (100.0) | 611 | (100.0) | |
| fertile | 94 | (63.6) | 59 | (30.1) | 54 | (30.5) | 113 | (31.3) | |
| 2nd (61–120 min) | irradiated | 75 | (44.4) | 137 | (69.9) | 123 | (69.5) | 260 | (69.7) |
| total | 169 | (100.0) | 196 | (100.0) | 177 | (100.0) | 373 | (100.0) | |
| fertile | 55 | (46.6) | 53 | 38.4 | 87 | (43.7) | 140 | (41.5) | |
| 3rd | irradiated | 63 | (53.4) | 85 | 61.6 | 112 | (56.3) | 197 | (58.5) |
| total | 118 | (100.0) | 138 | (100.0) | 199 | (100.0) | 337 | (100.0) | |
| fertile | 58 | (56.3) | 37 | 30.6 | 43 | (31.6) | 80 | (30.7) | |
| 4th | irradiated | 45 | (43.7) | 88 | 70.4 | 93 | (68.4) | 181 | (69.3) |
| total | 103 | (100.0) | 125 | (100.0) | 136 | (100.0) | 261 | (100.0) | |
| Total | fertile | 285 | (49.4) | 245 | 34.1 | 298 | (34.6) | 543 | (34.3) |
| (4 h) | irradiated | 292 | (50.6) | 473 | 65.9 | 566 | (65.4) | 1039 | (65.7) |
References
- Wattanapongsiri, A. A Revision of the Genera Rhynchophorus and Dynamis (Coleoptera: Curculionidae). Sci. Bull. Dep. Agric. Thail. 1966, 1, 328. [Google Scholar]
- Murphy, S.; Briscoe, B. The Red Palm Weevil as an Alien Invasive: Biology and the Prospects For Biological Control as a Component of IPM a Threat to Palms. Biocontrol News Inf. 1999, 20, 35–46. [Google Scholar]
- Thomas, M.C. Giant Palm Weevils of the Genus Rhynchophorus (Coleoptera: Curculionidae) and Their Threat to Florida Palms. Florida Department of Agriculture and Consumer Services, Division of Plant Industry. DACS-P-01682: 1-2. 2010. Available online: https://www.fdacs.gov/content/download/66344/file/pest_alert_-_giant_palm_weevils_of_the_genus_rhynchophorus.pdf (accessed on 15 January 2021).
- Inghilesi, A.; Mazza, G.; Cini, A.; Cervo, R. Comportamento Sociale E Riproduttivo Del Punteruolo Rosso Delle Palme: Approfondire Le Conoscenze Per Contrastare Questo Flagello. Atti Accad. Naz. Ital. Entomol. 2014, 61, 189–192. [Google Scholar]
- Sacchetti, P.; Camera, A.; Granchietti, A.; Rosi, M.; Marzialetti, P. Prima Segnalazione In Italia Del Curculionide Delle Palme, Rhynchophorus ferrugineus. Not. Del Cent. Sper. Per Il Vivaismo Di Pist. 2005, 144, 6–9. [Google Scholar]
- Inghilesi, A.F.; Mazza, G.; Cervo, R.; Cini, A. A Network of Sex and Competition: The Promiscuous Mating System of an Invasive Weevil. Curr. Zool. 2015, 61, 85–97. [Google Scholar] [CrossRef]
- AlDosary, N.; AlDobai, S.; Faleiro, J. Review on The Management of Red Palm Weevil Rhynchophorus ferrugineus Olivier in Date Palm Phoenix dactylifera L. Emir. J. Food Agric. 2016, 28, 34. [Google Scholar] [CrossRef]
- El-Sebay, Y. Ecological Studies on the Red Palm Weevil Rhynchophorus ferrugineus Oliv. (Coleoptera Curculionidae) in Egypt. Egypt. J. Agric. Res. 2003, 81, 523–529. [Google Scholar] [CrossRef]
- Faleiro, J.R. A Review of the Issues and Management of the Red Palm Weevil Rhynchophorus ferrugineus (Coleoptera: Rhynchophoridae) in Coconut and Date Palm During the Last One Hundred Years. Int. J. Trop. Insect Sci. 2006, 26, 135–154. [Google Scholar]
- Ince, S.; Porcelli, F. Egg Laying and Egg Laying Behavior of Red Palm Weevil, Rhynchophorus ferrugineus (Olivier) 1790 (Coleoptera: Curculionidae). Agric. Biol. J. N. Am. 2011, 2, 1368–1374. [Google Scholar] [CrossRef]
- Kaakeh, W. Longevity, Fecundity, and Fertility of The Red Palm Weevil, Rynchophorus ferrugineus Olivier (Coleoptera: Curculionidae) on Natural and Artificial Diets. Emir. J. Food Agric. 2005, 17, 23. [Google Scholar] [CrossRef]
- Musmeci, S.; Cristofaro, M.; Arnone, S.; Sasso, R.; Baccaro, S.; Pasquali, A.; Catarci, S. Controllo Del Punteruolo Rosso Mediante La Tecnica Dell’Insetto Sterile (SIT): Utopia o Realtà. Atti Accad. Naz. Ital. Entomol. 2013, 61, 239–246. [Google Scholar]
- Dembilio, O.; Jacas, J.A. Basic Bio-Ecological Parameters of The Invasive Red Palm Weevil, Rhynchophorus Ferrugineus (Coleoptera: Curculionidae), In Phoenix canariensis Under Mediterranean Climate. Bull. Entomol. Res. 2011, 101, 153–163. [Google Scholar] [CrossRef]
- Klassen, W. Area-wide integrated pest management and the sterile insect technique. In Sterile Insect Technique: Principles and Practice in Area-Wide Integrated Pest Management; Dyck, V.A., Hendrichs, J., Robinson, A.S., Eds.; Springer: Dordrecht, The Netherlands, 2005; pp. 39–68. ISBN 978-1-4020-4051-1. [Google Scholar]
- Llácer, E.; Santiago-Álvarez, C.; Jacas, J.A. Could Sterile Males Be Used to Vector a Microbiological Control Agent? The case of Rhynchophorus ferrugineus and Beauveria bassiana. Bull. Entomol. Res. 2013, 103, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Knipling, E.F. Possibilities of Insect Control or Eradication Through the Use of Sexually Sterile Males. J. Econ. Entomol. 1955, 48, 459–462. [Google Scholar] [CrossRef]
- Knipling, E.F. The potential role of sterility for pest control. In Principles of Insect Chemosterilization; LaBrecque, G.C., Smith, C.N., Eds.; Century-Crofts: Appleton, NY, USA, 1968; pp. 7–40. [Google Scholar]
- Robinson, A.S. Genetic Basis of the Sterile Insect Technique. In Sterile Insect Technique: Principles and Practice in Area-Wide Integrated Pest Management; CRC: Boca Raton, FL, USA, 2021. [Google Scholar]
- Snow, J.W. Radiation, insects and eradication in North America: An overview from screw worm to boll worm. In Modern Insect Control: Nuclear Techniques and Biotechnology, Proceedings of an International Symposium on Modern Insect Control: Nuclear Techniques and Biotechnology, Vienna, Austria, 16–20 November 1987; IAEA International Atomic Energy Agency (IAEA): Vienna, Austria, 1988; ISBN 978-92-0-010388-9. [Google Scholar]
- Vreysen, M.J.; Saleh, K.M.; Ali, M.Y.; Abdulla, A.M.; Zhu, Z.R.; Juma, K.G.; Dyck, V.A.; Msangi, A.R.; Mkonyi, P.A.; Feldmann, H.U. Glossina austeni (Diptera: Glossinidae) Eradicated on The Island of Unguja, Zanzibar, using the Sterile Insect Technique. J. Econ. Entomol. 2000, 93, 123–135. [Google Scholar] [CrossRef] [PubMed]
- Hendrichs, J.; Franz, G.; Rendon, P. Increased Effectiveness and Applicability of The Sterile Insect Technique Through Male-Only Releases for Control of Mediterranean Fruit Flies During Fruiting Seasons. J. Appl. Entomol. 1995, 119, 371–377. [Google Scholar] [CrossRef]
- Benedict, M.Q.; Knols, B.G.; Bossin, H.C.; Howell, P.I.; Mialhe, E.; Caceres, C.; Robinson, A.S. Colonisation and Mass Rearing: Learning from Others. Malar. J. 2009, 8, S4. [Google Scholar] [CrossRef]
- Rahalkar, G.W.; Harwalkar, M.R.; Rananavare, H.D.; Shantaram, K.; Ayengar, A.R.G. Laboratory studies on radiation sterilization of the Red Palm Weevil (Rhynchophorus ferrugineus Oliv.) males. J. Plant. Crops 1973, 1, 141–145. [Google Scholar]
- Ramachandran, C.P. Effects of Gamma Radiation on Various Stages of Red Palm Weevil, Rhynchophorus ferrugineus F. J. Nucl. Agric. Biol. 1991, 20, 218–221. [Google Scholar]
- Lance, D.R.; McInnis, D.O. Biological basis of the sterile insect technique. In Sterile Insect Technique: Principles and Practice in Area-Wide Integrated Pest Management; Dyck, V.A., Hendrichs, J., Robinson, A.S., Eds.; CRC Press: Boca Raton, FL, USA, 2021; p. 30. ISBN 9781003035572. [Google Scholar]
- Gothi, K.K.; Hire, R.S.; Vijayalakshimi, N.; Dongre, T.K. Studies on Mating Behaviour of Radio-Sterilized Males of Red Palm Weevil, Rhynchophorus ferrugineus (Oliv). J. Nucl. Agric. Biol. 2007, 36, 65–72. [Google Scholar]
- Al-Ayedh, H.Y.; Rasool, K.G. Sex Ratio and The Role of Mild Relative Humidity in Mating Behaviour of Red Date Palm Weevil Rhynchophorus ferrugineus Oliv. (Coleoptera: Curculionidae) Gamma-Irradiated Adults. J. Appl. Entomol. 2010, 134, 157–162. [Google Scholar] [CrossRef]
- Prabhu, S.T.; Dongre, T.K.; Patil, R.S. Effect of Irradiation on The Biological Activities of Red Palm Weevil, Rhynchophorus ferrugineus Olivier. Karnataka J. Agric. Sci. 2010, 23, 186–188. [Google Scholar]
- Krishnakumar, R.; Maheswari, P. Assessment of the Sterile Insect Technique to manage Red Palm Weevil Rhynchophorus ferrugineus in coconut. In Area-Wide Control of Insect Pests; Vreysen, M.J.B., Robinson, A.S., Hendrichs, J., Eds.; Springer: Dordrecht, The Netherlands, 2007; pp. 475–485. ISBN 978-1-4020-6058-8. [Google Scholar]
- Whitten, M.; Mahon, R. Misconceptions and constraints. In Sterile Insect Technique: Principles and Practice in Area-Wide Integrated Pest Management; Dyck, V.A., Hendrichs, J., Robinson, A.S., Eds.; Springer: Dordrecht, The Netherlands, 2005; pp. 601–626. ISBN 978-1-4020-4051-1. [Google Scholar]
- Musmeci, S.; Belvedere, S.; Sasso, R.; Arnone, S.; Cristofaro, M.; Nobili, P.; La Marca, A.; De Biase, A. Last-male Sperm Precedence in Rhynchophorus ferrugineus (Olivier): Observations in Laboratory Mating Experiments with Irradiated Males. Bull. Entomol. Res. 2018, 108, 93–100. [Google Scholar] [CrossRef]
- Parker, G.A. Sperm Competition and Its Evolutionary Consequences in the Insects. Biol. Rev. 1970, 45, 525–567. [Google Scholar] [CrossRef]
- Gwynne, D.T. Male mating effort, confidence of paternity, and insect sperm competition. In Sperm Competition and the Evolution of Animal Mating Systems; Smith, R.L., Ed.; Academic Press: Orlando, FL, USA, 1984; pp. 117–149. ISBN 978-0-12-652570-0. [Google Scholar]
- Calkins, C.O.; Parker, A.G. Sterile Insect Quality. In Sterile Insect Technique: Principles and Practice in Area-Wide Integrated Pest Management; Dyck, V.A., Hendrichs, J., Robinson, A.S., Eds.; Springer: Dordrecht, The Netherlands, 2005; pp. 269–296. ISBN 978-1-4020-4051-1. [Google Scholar]
- Bakri, A.; Mehta, K.; Lance, D.R. Sterilizing insects with ionizing radiation. In Sterile Insect Technique: Principles and Practice in Area-Wide Integrated Pest Management; Dyck, V.A., Hendrichs, J., Robinson, A.S., Eds.; Springer: Dordrecht, The Netherlands, 2005; pp. 233–268. ISBN 978-1-4020-4051-1. [Google Scholar]
- Mazza, G.; Inghilesi, A.F.; Stasolla, G.; Cini, A.; Cervo, R.; Benvenuti, C.; Francardi, V.; Cristofaro, M.; Arnone, S.; Roversi, P.F. Sterile Rhynchophorus ferrugineus Males Efficiently Impair Reproduction While Maintaining Their Sexual Competitiveness in a Social Context. J. Pest Sci. 2016, 89, 459–468. [Google Scholar] [CrossRef]
- Baccaro, S.; Cemmi, A.; Di Sarcina, I.; Ferrara, G. Gamma Irradiation Calliope Facility at ENEA–Casaccia Research Centre (Rome, Italy); Fusion and Technology for Nuclear Safety and Security Department Casaccia Research Centre: Rome, Italy, 2019; p. 49. Available online: https://iris.enea.it/retrieve/dd11e37c-d730-5d97-e053-d805fe0a6f04/RT-2019-04-ENEA.pdf (accessed on 6 June 2023).
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using Lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- R Core Team. A Language and Environment for Statistical Computing; R-Foundation for Computer Statistics: Vienna, Austria, 2019; Available online: https://www.r-project.org/ (accessed on 10 January 2023).
- Hothorn, T.; Bretz, F.; Westfall, P. Simultaneous Inference in General Parametric Models. Biom. J. Biom. Z. 2008, 50, 346–363. [Google Scholar] [CrossRef]
- De Beer, C.J.; Moyaba, P.; Boikanyo, S.N.B.; Majatladi, D.; Venter, G.J.; Vreysen, M.J.B. Gamma Irradiation and Male Glossina austeni Mating Performance. Insects 2020, 11, 522. [Google Scholar] [CrossRef]
- Ilboudo, K.; Camara, K.; Salou, E.W.; Gimonneau, G. Quality Control and Mating Performance of Irradiated Glossina palpalis gambiensis Males. Insects 2022, 13, 476. [Google Scholar] [CrossRef]
- Cristofaro, M.; Sforza, R.F.H.; Roselli, G.; Paolini, A.; Cemmi, A.; Musmeci, S.; Anfora, G.; Mazzoni, V.; Grodowitz, M. Effects of Gamma Irradiation on the Fecundity, Fertility, and Longevity of the Invasive Stink Bug Pest Bagrada hilaris (Burmeister) (Hemiptera: Pentatomidae). Insects 2022, 13, 787. [Google Scholar] [CrossRef]
- Bouyer, J.; Vreysen, M.J.B. Yes, Irradiated Sterile Male Mosquitoes Can Be Sexually Competitive! Trends Parasitol. 2020, 36, 877–880. [Google Scholar] [CrossRef] [PubMed]
- Roselli, G.; Anfora, G.; Suckling, D.M.; Mazzoni, V.; Vanoni, V.; Menegotti, L.; Fellin, L.; Rossi Stacconi, M.V.; Ioriatti, C.; Cristofaro, M. Effects of Irradiation on Biology and Mating Behaviour of Wild Males of Brown Marmorated Stink Bug Using a 6 MV Medical Linear Accelerator. Insects 2023, 14, 460. [Google Scholar] [CrossRef] [PubMed]
- Himuro, C.; Kohama, T.; Matsuyama, T.; Sadoyama, Y.; Kawamura, F.; Honma, A.; Ikegawa, Y.; Haraguchi, D. First Case of Successful Eradication of the Sweet Potato Weevil, Cylas formicarius (Fabricius), Using the Sterile Insect Technique. PLoS ONE 2022, 17, e0267728. [Google Scholar] [CrossRef] [PubMed]
- Suckling, D.M.; Cristofaro, M.; Roselli, G.; Levy, M.C.; Cemmi, A.; Mazzoni, V.; Stringer, L.D.; Zeni, V.; Ioriatti, C.; Anfora, G. The Competitive Mating of Irradiated Brown Marmorated Stink Bugs, Halyomorpha halys, for the Sterile Insect Technique. Insects 2019, 10, 411. [Google Scholar] [CrossRef]
- Aldawood, A.S.; Rasool, K.G.; Sukirno, S.; Husain, M.; Sutanto, K.D.; Alduailij, M.A. Semi-Artificial Diet Developed For The Successful Rearing Of Red Palm Weevil: Rhynchophorus ferrugineus (Coleoptera: Dryphthoridae) in the Laboratory. J. King Saud Univ. Sci. 2022, 34, 102272. [Google Scholar] [CrossRef]





| Time | Mating | 0 Gy | 60 Gy | 80 Gy | Total | ||||
|---|---|---|---|---|---|---|---|---|---|
| Days | Yes/No | Min | % | Min | % | Min | % | Min | % |
| 3rd | no | 2314 | (96.0) | 2261 | (93.8) | 2330 | (96.7) | 6905 | (95.5) |
| yes | 96 | (4.0) | 149 | (6.2) | 80 | (3.3) | 325 | (4.5) | |
| 6th | no | 2257 | (93.7) | 2244 | (93.1) | 2306 | (95.7) | 6807 | (94.1) |
| yes | 153 | (6.3) | 166 | (6.9) | 104 | (4.3) | 423 | (5.9) | |
| 9th | no | 2268 | (93.7) | 2216 | (91.9) | 2277 | (94.5) | 6761 | (93.8) |
| yes | 142 | (5.9) | 194 | (8.1) | 133 | (5.5) | 469 | (6.2) | |
| 12th | no | 2281 | (94.6) | 2218 | (90.5) | 2278 | (94.5) | 6739 | (93.5) |
| yes | 129 | (5.4) | 230 | 9.5 | 132 | (5.5) | 491 | (6.5) | |
| Total | no | 9120 | (94.6) | 8.939 | 92.4 | 9191 | (95.3) | 27,212 | (94.1) |
| yes | 520 | (5.4) | 739 | 7.6 | 449 | (4.7) | 1708 | (5.9) | |
| Fixed Effects: | Estimate | ±SE | z Value | p-Value |
|---|---|---|---|---|
| (Intercept) | −2.8855 | 0.1990 | −14.502 | <2 × 10−16 |
| Dose 60 Gy | 0.4658 | 0.2583 | 1.803 | 0.0714 |
| Dose 80 Gy | −0.2703 | 0.2704 | −1.000 | 0.3174 |
| 6th day | 0.4994 | 0.1336 | 3.739 | 0.0002 |
| 9th day | 0.4187 | 0.1354 | 3.093 | 0.0019 |
| 12th day | 0.3144 | 0.1380 | 2.279 | 0.0227 |
| hour2 | −0.3635 | 0.1148 | −3.166 | 0.0015 |
| hour3 | −0.6167 | 0.1181 | −5.220 | 1.8 × 10−7 |
| hour4 | −0.8324 | 0.1212 | −6.871 | 6.4 × 10−12 |
| Dose 60 Gy: 6th day | −0.3810 | 0.1777 | −2.144 | 0.0320 |
| Dose 80 Gy: 6th day | −0.2217 | 0.2019 | −1.098 | 0.2722 |
| Dose 60 Gy: 9th day | 0.1282 | 0.1766 | −0.726 | 0.4681 |
| Dose 80 Gy: 9th day | 0.1226 | 0.1980 | 0.619 | 0.5359 |
| Dose 60 Gy: 12th day | 0.1682 | 0.1765 | 0.953 | 0.3403 |
| Dose 80 Gy: 12th day | 0.2190 | 0.1999 | 1.095 | 0.2734 |
| Fixed Effects | Estimate | ±SE | z Value | p-Value |
|---|---|---|---|---|
| (Intercept) | 0.999 | 0.067 | 14.807 | <2 × 10−16 |
| Dose 60 Gy | 0.114 | 0.057 | 1.988 | 0.0468 |
| Dose 80 Gy | 0.121 | 0.065 | 1.862 | 0.0626 |
| 6th day | 0.141 | 0.074 | 1.903 | 0.0570 |
| 9th day | 0.058 | 0.073 | 0.805 | 0.4210 |
| 12th day | 0.034 | 0.071 | 0.483 | 0.6291 |
| Fixed Effects | Estimate | ±SE | z Value | p-Value |
|---|---|---|---|---|
| (Intercept) | 1.210 | 0.169 | 7.166 | 7.72 × 10−13 |
| dose60 | 0.269 | 0.204 | 1.316 | 0.188298 |
| dose80 | −0.300 | 0.213 | −1.409 | 0.158757 |
| 6th day | 0.136 | 0.130 | 1.046 | 0.295532 |
| 9th day | 0.276 | 0.126 | 2.198 | 0.027981 |
| 12th day | 0.405 | 0.122 | 3.315 | 0.000916 |
| Fixed Effects | Estimate | ±SE | z Value | p-Value |
|---|---|---|---|---|
| (Intercept) | 3.517 | 0.389 | 9.038 | <2 × 10−16 |
| Dose 60 Gy | −0.966 | 0.535 | −1.806 | 0.0709 |
| Dose 80 Gy | 0.903 | 0.548 | 1.646 | 0.0997 |
| 6th day | 0.121 | 0.460 | 0.262 | 0.7930 |
| 9th day | −0.556 | 0.476 | −1.167 | 0.2431 |
| 12th day | −0.881 | 0.458 | −1.923 | 0.0545 |
| Dose 60 Gy: 6th day | 0.836 | 0.660 | 1.266 | 0.2055 |
| Dose 80 Gy: 6th day | −0.554 | 0.668 | −0.829 | 0.4072 |
| Dose 60 Gy: 9th day | 1.290 | 0.658 | 1.961 | 0.0499 |
| Dose 80 Gy: 9th day | −0.330 | 0.667 | −0.495 | 0.6207 |
| Dose 60 Gy: 12th day | 1.323 | 0.622 | 2.126 | 0.0335 |
| Dose 80 Gy: 12th day | −0.698 | 0.655 | −1.067 | 0.2862 |
| Minutes Elapsed | ||||
|---|---|---|---|---|
| Treatment | Dose (Gy) | Total Mean | ||
| 0 | 60 | 80 | ||
| No mating | 902.3 | 888.2 | 873.6 | 877.9 |
| “fertile” | 28.5 | 24.5 | 29.8 | 27.2 |
| “irradiated” | 29.2 | 47.3 | 56.6 | 51.9 |
| Total | 960.0 | 960.0 | 960.0 | 960.0 |
| Fixed Effects | Estimate | ±SE | z Value | p-Value |
|---|---|---|---|---|
| (Intercept) | 1.2842 | 0.2032 | 6.319 | 2.64 × 10−10 |
| 6th day | 0.7007 | 0.1956 | 3.583 | 0.00034 |
| 9th day | 0.5744 | 0.1966 | 2.922 | 0.00348 |
| 12th day | 0.0329 | 0.1993 | 0.165 | 0.86885 |
| treatm: dose 0 Gy | 0.1895 | 0.2706 | 0.700 | 0.48380 |
| treatm: dose 60 Gy | 0.7599 | 0.2697 | 2.818 | 0.00484 |
| treatm: dose 80 Gy | 0.8177 | 0.2678 | 3.054 | 0.00226 |
| Dose | Days | Male(1) | Male(2) | Hours | Male(1) | Male(2) | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| (Mins) | ±SE | (Mins) | ±SE | (Mins) | ±SE | (Mins) | ±SE | |||
| 0 | 3 | 6.60 | 2.47 | 2.90 | 1.13 | 1 | 7.80 | 2.14 | 10.90 | 2.05 |
| 6 | 10.60 | 2.87 | 13.60 | 3.56 | 2 | 9.40 | 2.95 | 7.50 | 1.81 | |
| 9 | 8.30 | 2.09 | 6.60 | 1.92 | 3 | 5.50 | 1.55 | 6.30 | 1.37 | |
| 12 | 3.00 | 1.27 | 6.10 | 2.36 | 4 | 5.80 | 2.02 | 4.50 | 1.20 | |
| mean | 7.13 | 1.17 | 7.30 | 1.32 | mean | 7.13 | 1.10 | 7.30 | 0.87 | |
| 60 | 3 | 2.80 | 1.19 | 10.80 | 3.82 | 1 | 9.60 | 3.63 | 16.30 | 4.58 |
| 6 | 7.80 | 3.06 | 14.00 | 3.43 | 2 | 5.90 | 1.87 | 13.70 | 2.50 | |
| 9 | 8.80 | 1.90 | 10.20 | 2.76 | 3 | 5.30 | 1.22 | 8.50 | 1.93 | |
| 12 | 5.10 | 1.52 | 12.30 | 2.41 | 4 | 3.70 | 1.11 | 8.80 | 2.27 | |
| mean | 6.13 | 1.05 | 11.83 | 1.53 | mean | 6.13 | 1.11 | 11.83 | 1.54 | |
| 80 | 3 | 7.20 | 3.78 | 11.90 | 2.98 | 1 | 11.40 | 2.85 | 23.80 | 5.68 |
| 6 | 8.10 | 2.01 | 18.10 | 5.26 | 2 | 5.40 | 2.24 | 12.30 | 2.83 | |
| 9 | 10.60 | 2.15 | 15.20 | 2.81 | 3 | 8.70 | 2.63 | 11.20 | 2.79 | |
| 12 | 3.90 | 2.47 | 11.40 | 2.90 | 4 | 4.30 | 1.30 | 9.30 | 1.90 | |
| mean | 7.45 | 1.35 | 14.15 | 1.80 | mean | 7.45 | 1.21 | 14.15 | 1.95 | |
| Total means * | 3 | 5.53 | 1.55 | 11.35 | 2.36 | 1 | 9.60 | 1.66 | 20.05 | 3.65 |
| 6 | 8.83 | 1.52 | 16.05 | 3.09 | 2 | 6.90 | 1.38 | 13.00 | 1.84 | |
| 9 | 9.23 | 1.16 | 12.70 | 2.00 | 3 | 6.50 | 1.10 | 9.85 | 1.68 | |
| 12 | 4.00 | 1.03 | 11.85 | 1.84 | 4 | 4.60 | 0.87 | 9.05 | 1.44 | |
| mean | 6.90 | 0.69 | 12.99 | 1.18 | Total | 6.90 | 0.66 | 12.99 | 1.18 |
| Fixed Effects: | Estimate | ±SE | z Value | p-Value |
|---|---|---|---|---|
| (Intercept) | 2.003 | 0.166 | 12.075 | <2 × 10−16 |
| h2 | −0.386 | 0.126 | −3.104 | 0.001910 |
| h3 | −0.487 | 0.127 | −3.834 | 0.000126 |
| h4 | −0.756 | 0.129 | −5.846 | 5.05 × 10−9 |
| treatm: dose 0 Gy | 0.202 | 0.268 | 0.752 | 0.451903 |
| treatm: dose 60 Gy | 0.712 | 0.267 | 2.667 | 0.007645 |
| treatm: dose 80 Gy | 0.753 | 0.266 | 2.832 | 0.004619 |
| Fixed Effects | Estimate | SE | z Value | p-Value |
|---|---|---|---|---|
| (Intercept) | 1.1123 | 0.0354 | 31.465 | <2 × 10−16 |
| treatm: dose 0 Gy | 0.0130 | 0.0676 | 0.193 | 0.8472 |
| treatm: dose 60 Gy | 0.1115 | 0.0581 | 1.919 | 0.0549 |
| treatm: dose 80 Gy | 0.0291 | 0.0559 | 0.521 | 0.6025 |
| Duration of Mating (Min Day−1) | Mating Events (n. Day−1) | Time Elapsed before the 1st Mating (Min) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dose | Days | Male(1) | ±SE | Male(2) | ±SE | Male(1) | ±SE | Male(2) | ±SE | Male(1) | ±SE | Male(2) | ±SE |
| 0 | 3 | 3.47 | 0.39 | 2.90 | 0.38 | 1.90 | 0.71 | 1.00 | 0.39 | 108.0 | - | 1.50 | 0.50 |
| 6 | 3.03 | 0.18 | 3.02 | 0.22 | 3.50 | 0.82 | 4.50 | 1.19 | 35.00 | 10.51 | 28.50 | 16.6 | |
| 9 | 2.96 | 0.22 | 3.24 | 0.42 | 2.80 | 0.63 | 2.10 | 0.59 | 19.75 | 7.90 | 16.00 | 6.52 | |
| 12 | 3.00 | 0.30 | 3.15 | 0.28 | 1.00 | 0.42 | 2.00 | 0.63 | 18.00 | 6.00 | 13.00 | 4.36 | |
| mean | 3.10 | 0.13 | 3.08 | 0.15 | 2.30 | 0.35 | 2.40 | 0.42 | 33.17 | 8.64 | 16.86 | 5.42 | |
| 60 | 3 | 2.55 | 0.31 | 3.45 | 0.35 | 1.10 | 0.43 | 3.10 | 1.07 | - | - | 16.50 | 2.50 |
| 6 | 3.39 | 0.45 | 3.50 | 0.27 | 2.30 | 0.80 | 4.00 | 0.98 | 18.50 | 17.50 | 3.00 | 2.00 | |
| 9 | 3.14 | 0.30 | 3.81 | 0.39 | 2.80 | 0.51 | 2.70 | 0.56 | 32.00 | 16.59 | 3.50 | 0.50 | |
| 12 | 3.00 | 0.19 | 3.00 | 0.19 | 1.70 | 0.50 | 4.10 | 0.74 | - | - | 21.29 | 10.8 | |
| mean | 3.10 | 0.18 | 3.40 | 0.15 | 1.97 | 0.30 | 3.48 | 0.42 | 28.63 | 12.78 | 15.00 | 6.09 | |
| 80 | 3 | 3.00 | 0.17 | 3.16 | 0.32 | 2.40 | 1.29 | 3.70 | 0.94 | 58.67 | 56.67 | - | - |
| 6 | 3.52 | 0.42 | 3.55 | 0.18 | 2.30 | 0.58 | 5.10 | 1.35 | 47.80 | 37.47 | 3.00 | 0.58 | |
| 9 | 2.79 | 0.17 | 2.92 | 0.15 | 3.80 | 0.80 | 5.20 | 0.87 | 10.00 | 3.00 | 7.80 | 2.65 | |
| 12 | 2.44 | 0.13 | 2.82 | 0.16 | 1.60 | 0.98 | 4.00 | 0.97 | 4.00 | - | 3.33 | 0.33 | |
| mean | 2.95 | 0.13 | 3.13 | 0.10 | 2.53 | 0.47 | 4.50 | 0.52 | 37.42 | 19.98 | 5.27 | 1.36 | |
| Total mean * | 3 | 3.07 | 0.17 | 3.35 | 0.50 | 1.80 | 0.50 | 3.40 | 0.70 | 71.00 | 41.93 | 16.50 | 2.50 |
| 6 | 3.27 | 0.19 | 3.69 | 0.37 | 2.70 | 0.43 | 4.55 | 0.82 | 37.58 | 15.62 | 3.00 | 0.71 | |
| 9 | 2.95 | 0.13 | 3.14 | 0.19 | 3.13 | 0.38 | 3.95 | 0.58 | 23.15 | 8.04 | 6.57 | 2.00 | |
| 12 | 2.79 | 0.12 | 3.17 | 0.24 | 1.43 | 0.38 | 4.05 | 0.59 | 13.33 | 5.81 | 15.90 | 7.90 | |
| mean | 3.04 | 0.08 | 3.33 | 0.16 | 2.27 | 0.22 | 3.46 | 0.34 | 33.62 | 8.52 | 10.54 | 3.45 | |
| Fixed Effects: | Estimate | SE | z Value | p-Value |
|---|---|---|---|---|
| (Intercept) | −0.0366 | 0.2291 | −0.160 | 0.87303 |
| 6th day | 0.5306 | 0.1585 | 3.348 | 0.00081 |
| 9th day | 0.4429 | 0.1598 | 2.772 | 0.00557 |
| 12th day | 0.0566 | 0.1654 | 0.342 | 0.73210 |
| treatm: dose 0 Gy | 0.2704 | 0.1420 | 1.905 | 0.05683 |
| treatm: dose 60 Gy | 0.4835 | 0.1382 | 3.497 | 0.00047 |
| treatm: dose 80 Gy | 0.5722 | 0.1346 | 4.252 | 2.11 × 10−5 |
| Fixed Effects | Estimate | SE | z Value | p-Value |
|---|---|---|---|---|
| Dose 0 Gy | 0.2089 | 0.5431 | 0.385 | 0.7005 |
| Dose 60 Gy | 0.9740 | 0.5598 | 1.740 | 0.0819 |
| Dose 80 Gy | 0.5459 | 0.5339 | 1.022 | 0.3066 |
| Fixed Effects | Estimate | SE | Z Value | p-Value |
|---|---|---|---|---|
| (Intercept) | 3.2517 | 0.2305 | 14.110 | <2 × 10−16 |
| treatm:dose 0 Gy | −0.5975 | 0.3738 | −1.599 | 0.109918 |
| treatm:dose 60 Gy | −0.6204 | 0.3965 | −1.564 | 0.117722 |
| treatm:dose 80 Gy | −1.5798 | 0.4351 | −3.631 | 0.000282 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cristofaro, M.; Fornari, C.; Mariani, F.; Cemmi, A.; Guedj, M.; Ben Jamaa, M.L.; Msaad Guerfali, M.; Tabone, E.; Castellana, R.; Sasso, R.; et al. Effects of γ-Irradiation on Mating Behavior of Red Palm Weevil, Rhynchophorus ferrugineus (Olivier, 1790) (Coleoptera: Dryophthoridae). Insects 2023, 14, 661. https://doi.org/10.3390/insects14070661
Cristofaro M, Fornari C, Mariani F, Cemmi A, Guedj M, Ben Jamaa ML, Msaad Guerfali M, Tabone E, Castellana R, Sasso R, et al. Effects of γ-Irradiation on Mating Behavior of Red Palm Weevil, Rhynchophorus ferrugineus (Olivier, 1790) (Coleoptera: Dryophthoridae). Insects. 2023; 14(7):661. https://doi.org/10.3390/insects14070661
Chicago/Turabian StyleCristofaro, Massimo, Chiara Fornari, Flaminia Mariani, Alessia Cemmi, Michèle Guedj, Mohamed Lahbib Ben Jamaa, Meriem Msaad Guerfali, Elisabeth Tabone, Robert Castellana, Raffaele Sasso, and et al. 2023. "Effects of γ-Irradiation on Mating Behavior of Red Palm Weevil, Rhynchophorus ferrugineus (Olivier, 1790) (Coleoptera: Dryophthoridae)" Insects 14, no. 7: 661. https://doi.org/10.3390/insects14070661
APA StyleCristofaro, M., Fornari, C., Mariani, F., Cemmi, A., Guedj, M., Ben Jamaa, M. L., Msaad Guerfali, M., Tabone, E., Castellana, R., Sasso, R., & Musmeci, S. (2023). Effects of γ-Irradiation on Mating Behavior of Red Palm Weevil, Rhynchophorus ferrugineus (Olivier, 1790) (Coleoptera: Dryophthoridae). Insects, 14(7), 661. https://doi.org/10.3390/insects14070661






