Collembola of the Cavalum and Landeiro Caves (Madeira, Portugal) †
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
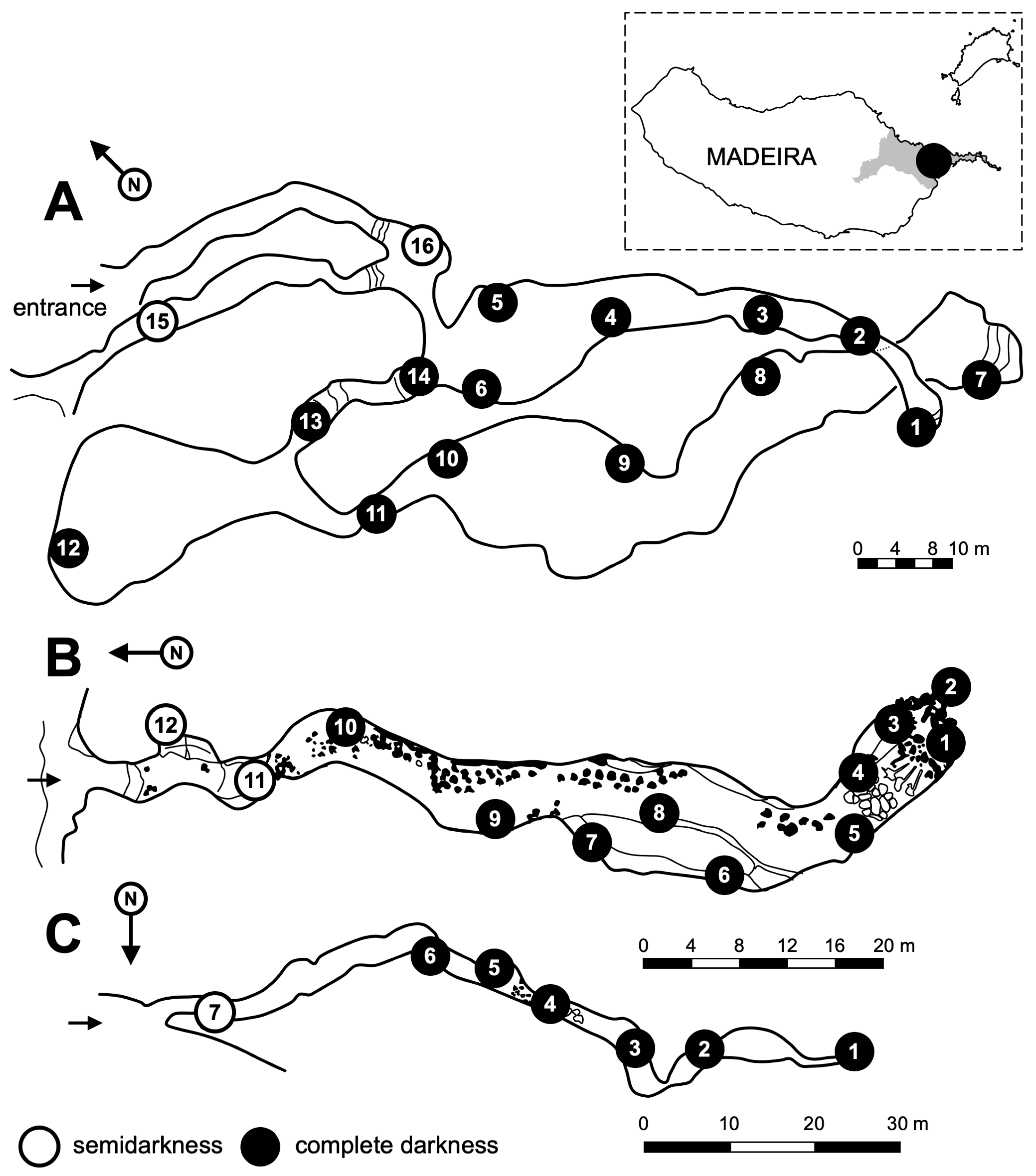
2.1. Geomorphological Characteristics of Studied Lava Tubes
2.2. Site
2.3. Environmental Parameters
2.4. Sampling
2.5. Material Procesing
2.6. Photographing
2.7. Nomenclature
3. Results
3.1. Faunistic
3.2. Taxonomy
3.2.1. Ceratophysella gibbosa (Bagnall, 1940) [59]
3.2.2. Deuteraphorura cf. ghidinii (Denis, 1938) [28]
Remarks
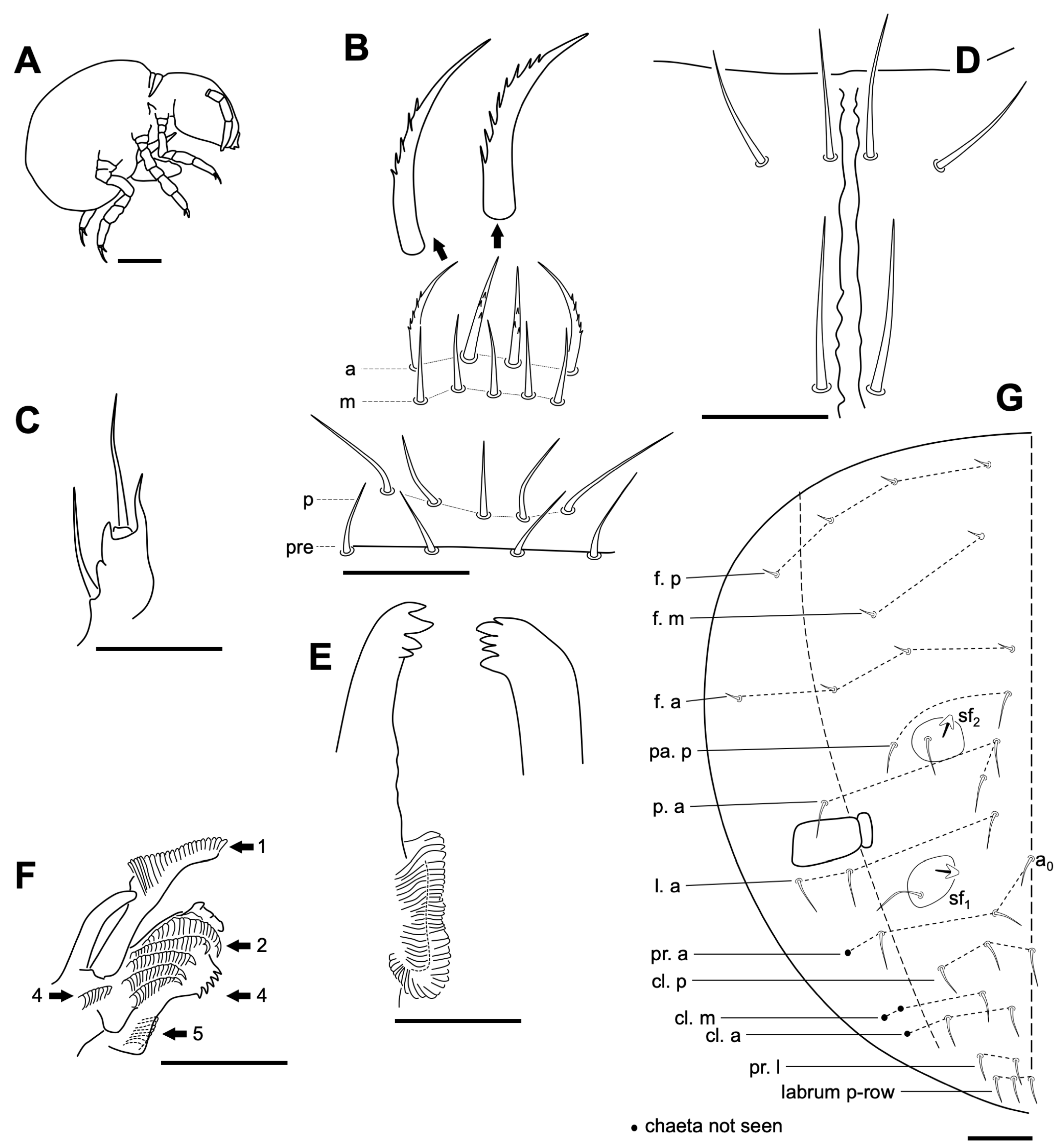
3.2.3. Onychiurus sp.
Remarks
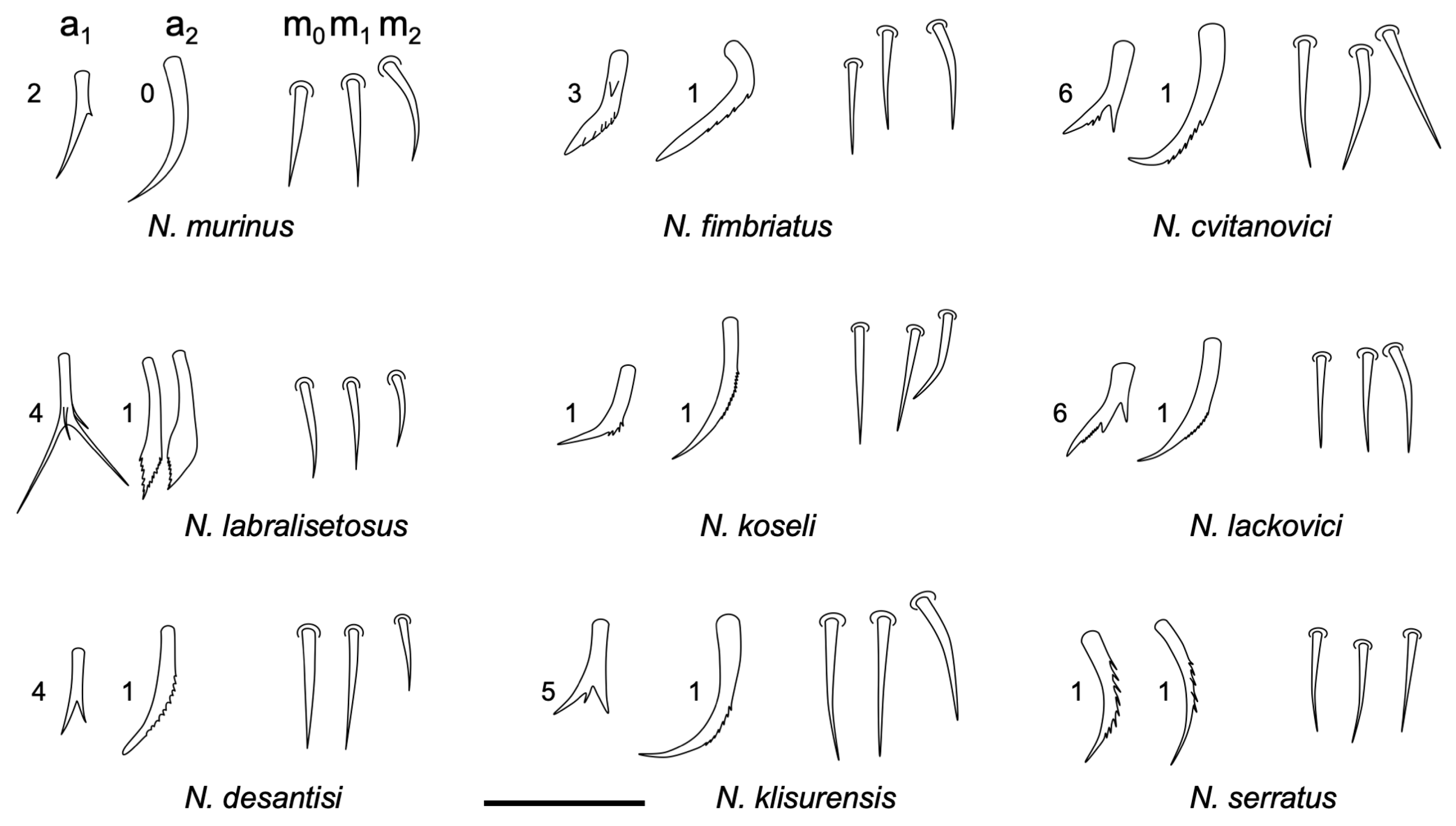
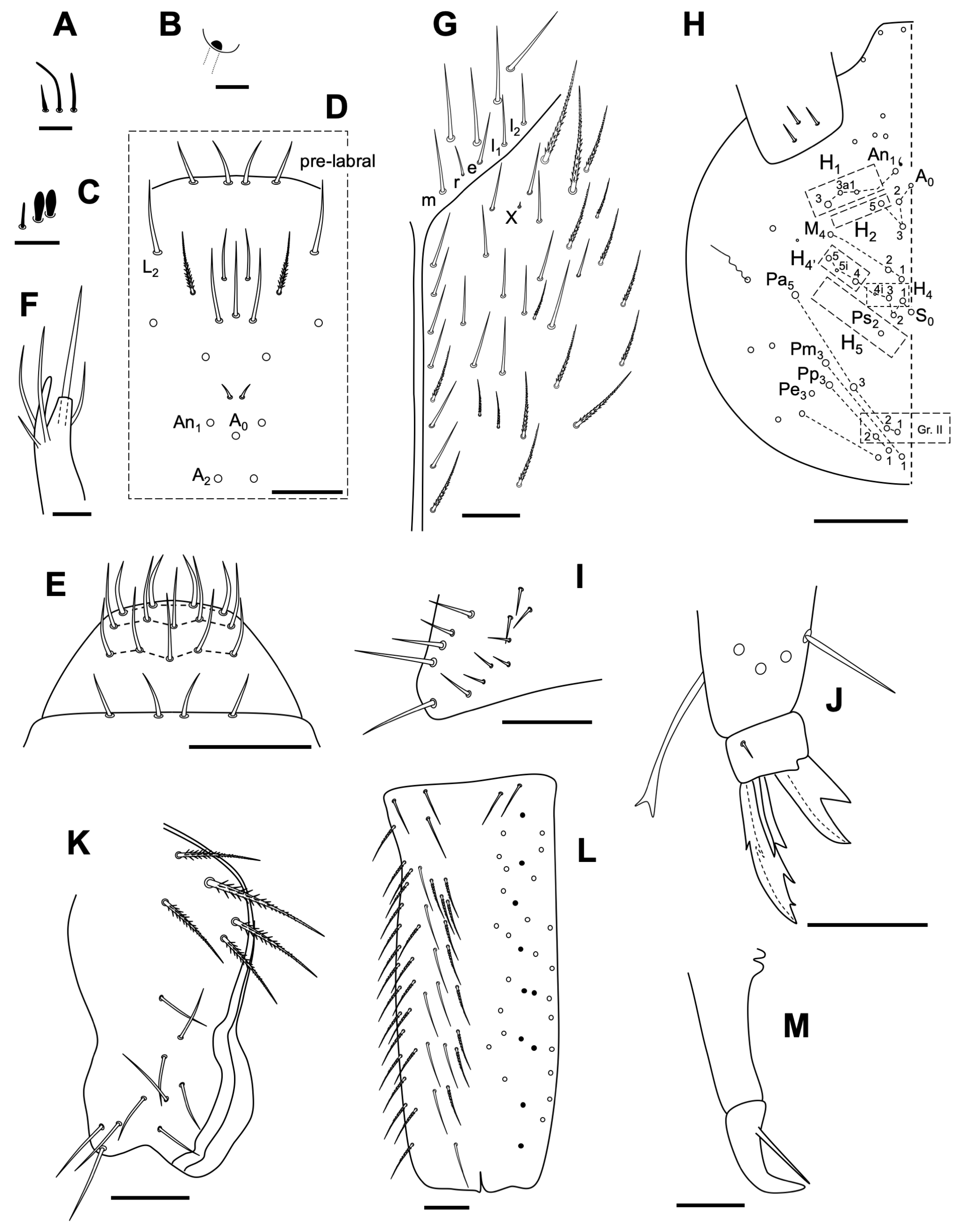
3.2.4. Neelus serratus Jordana & Baquero sp. nov.
Type Locality
Type Material
Etymology
Diagnosis
Description
Habitat
Remarks
3.2.5. Heteromurus major (Moniez, 1889) [73]
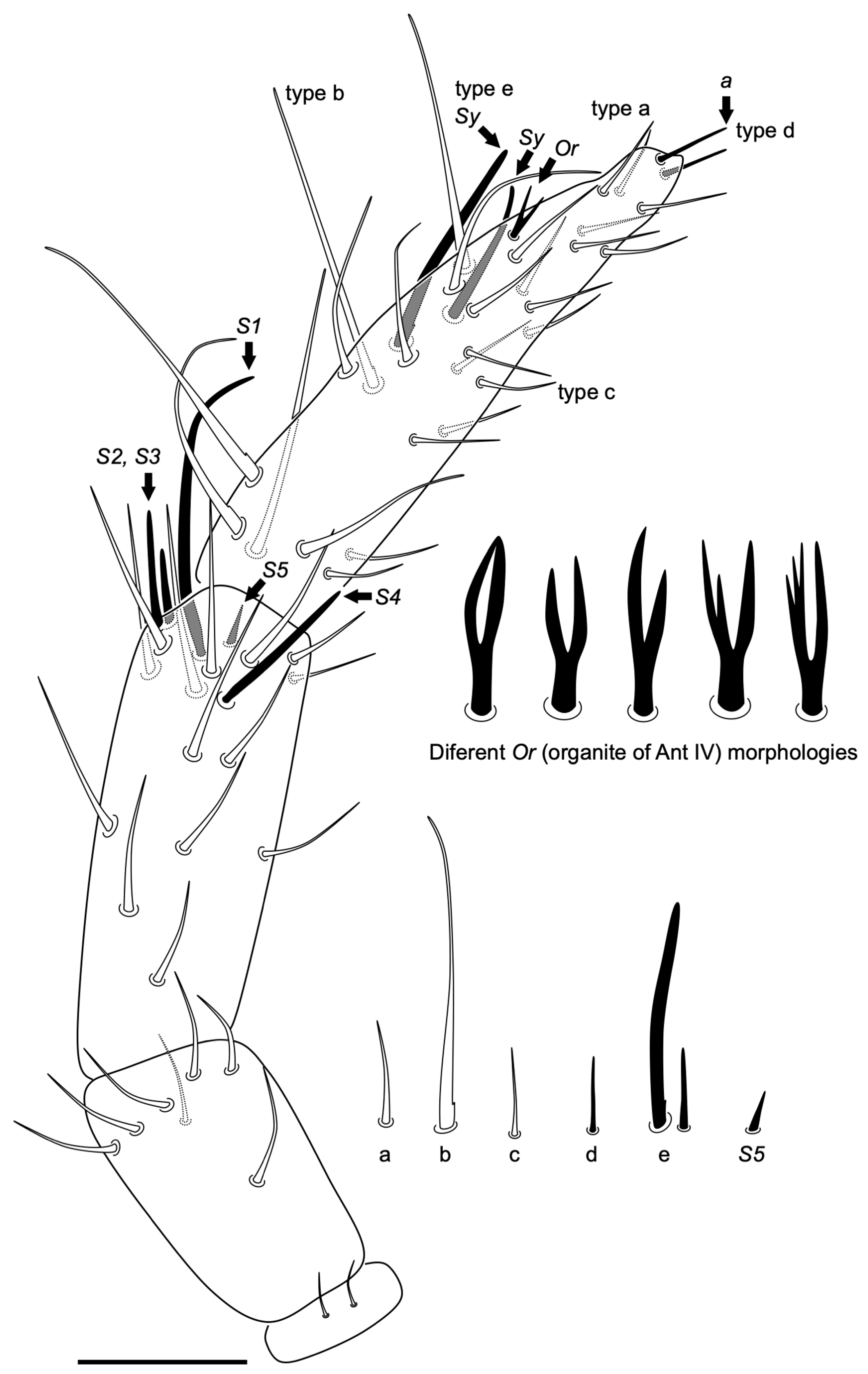
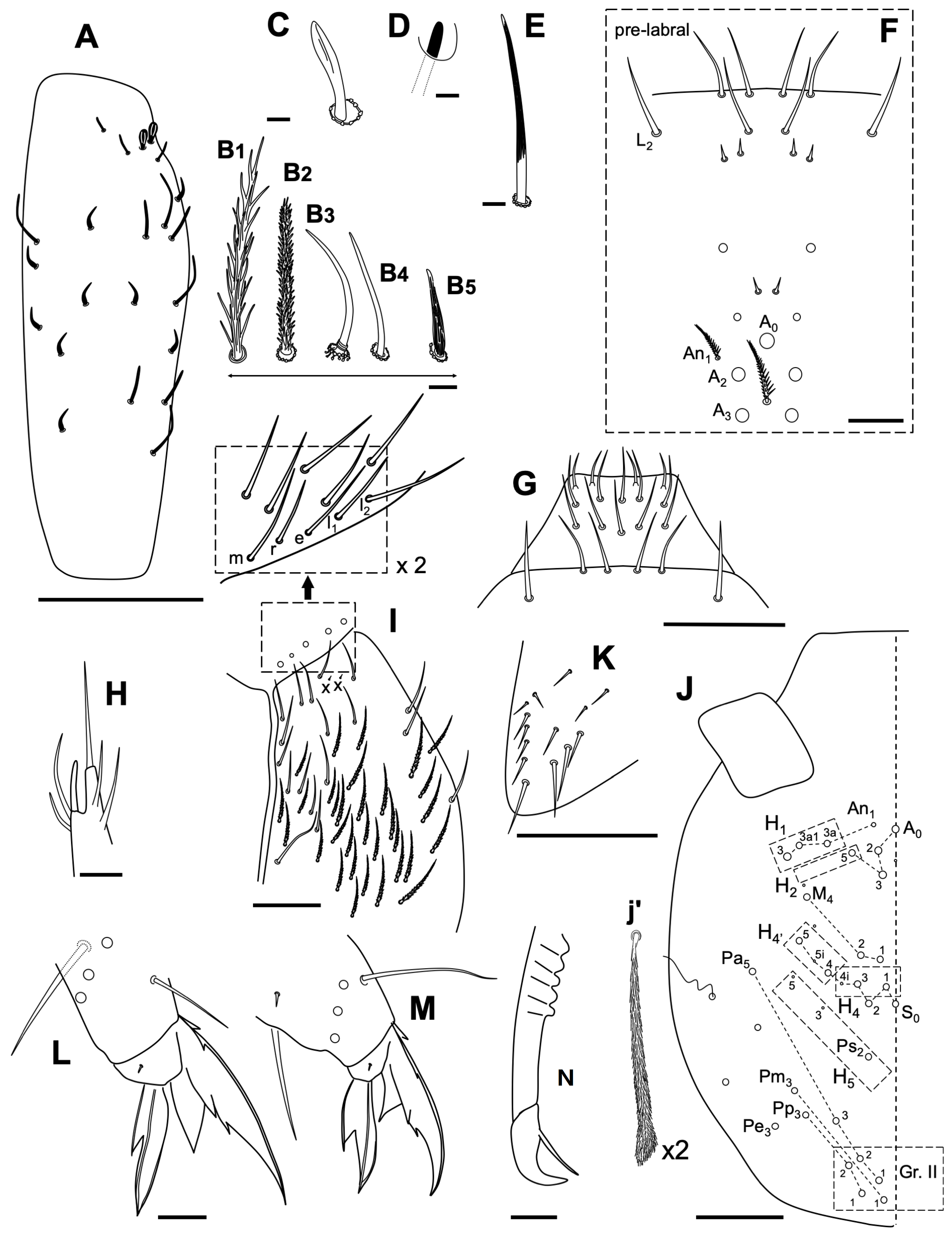
3.2.6. Coecobrya decemsetosa Jordana & Baquero sp. nov.
Type Locality
Type Material
Etymology
Diagnosis
Description
Habitat
Remarks
3.2.7. Coecobrya octoseta Jordana & Baquero sp. nov.
Type Locality
Type Material
Etymology
Diagnosis
Description
Habitat
Remarks
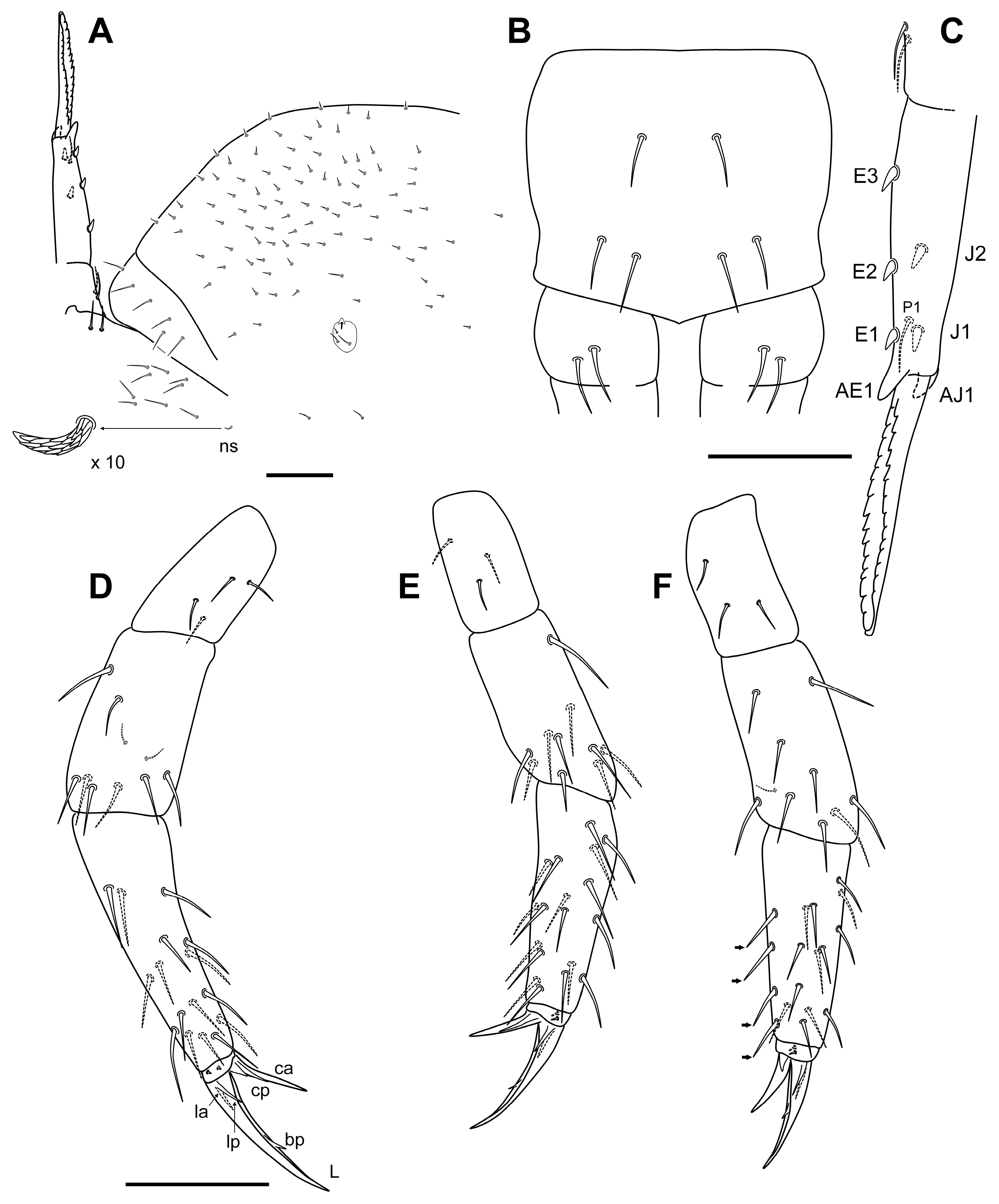
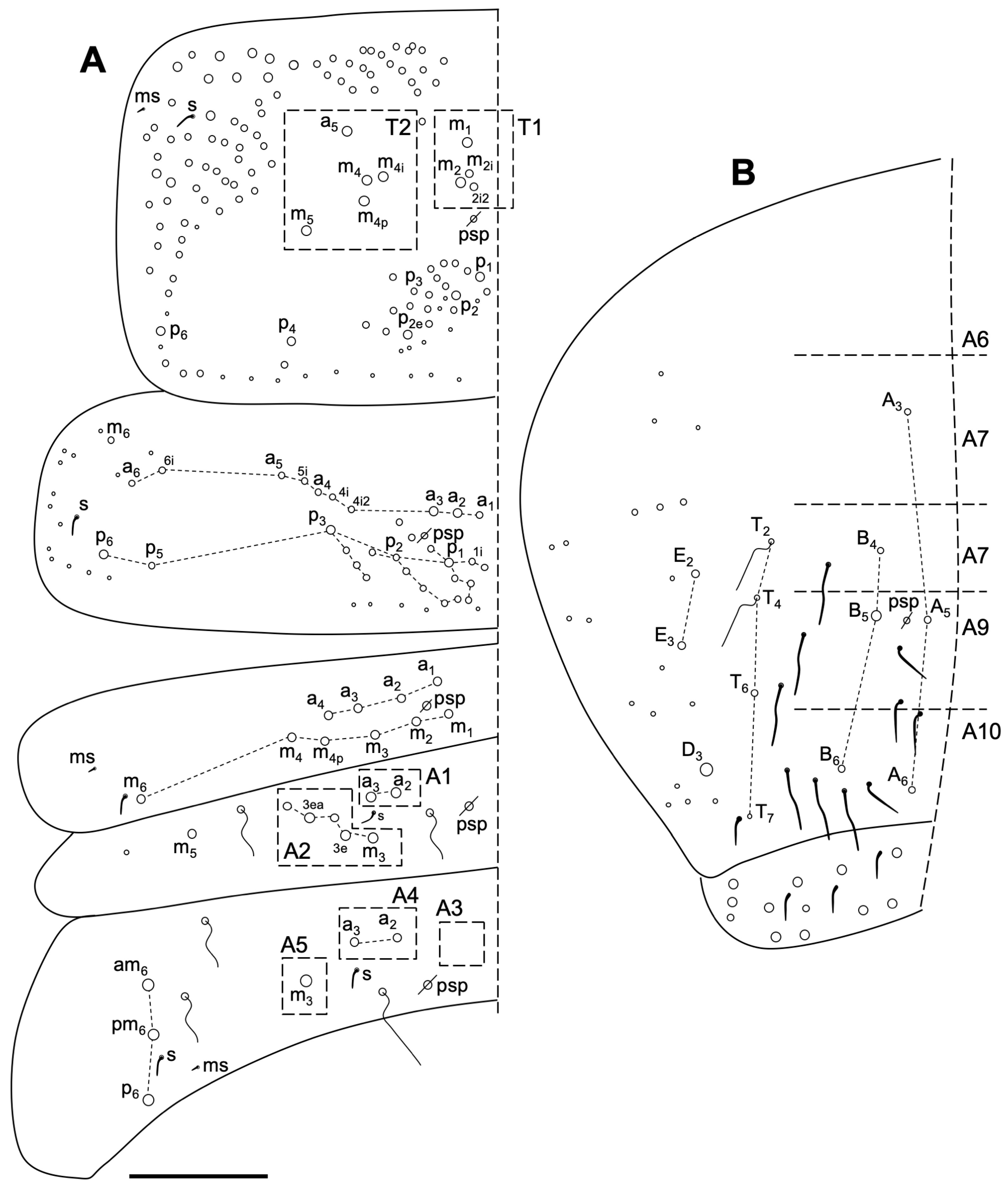
3.2.8. Sinella duodecimoculata Jordana & Baquero sp. nov.
Type Locality
Type Material
Etymology
Diagnosis
Description
Habitat
Remarks
3.2.9. Lepidocyrtus curvicollis Bourlet, 1839 [90]
Habitat and Distribution
3.2.10. Lepidocyrtus flexicollis Gisin, 1965 [93]
Habitat and Distribution
3.2.11. Entomobrya pazaristei Denis, 1933 [96]
Habitat and Distribution
3.2.12. Pygmarrhopalites elegans (Cassagnau & Delamare-Deboutteville, 1953) [27]
Habitat and Distribution
3.2.13. Pygmarrhopalites sp. 1
Remarks
3.2.14. Disparrhopalites patrizii (Cassagnau & Delamare Deboutteville, 1953) [27]
Habitat and Distribution
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Oromí, P.; Arechavaleta, M.; de la Cruz, S.; García, R.; Izquierdo, I.; López, H.; Zurita, N. Animal diversity in the volcanic subterranean environment, with special emphasis on the Canary Islands. Boletín SEDECK 2021, 16, 25–50. [Google Scholar]
- Naranjo, M.; López, H.; Martín, S.; Suárez, D.; Oromí, P. Troglobiontes de Gran Canaria. Vida Bajo el Volcán (Troglobionts of Gran Canaria. Life Under the Volcano); Cam-PDS Editores SL: Las Palmas de Gran Canaria, Spain, 2019; pp. 1–106. [Google Scholar]
- Medina, A.L.; Oromí, P. First data on the superficial underground compartment on La Gomera (Canary Islands). Mem. Biosp. 1990, 17, 87–91. [Google Scholar]
- Oromí, P.; Pérez, A.J.; Martín, J.L.; Martín, N. La fauna Subterránea, Pobladora de un Hábitat Inhóspito. Legados del Fuego: Reservorios de una Asombrosa Biota y Refugios Ancestrales; Publicaciones del Ayuntamiento de La Orotava: Tenerife, Spain, 2018; pp. 82–131. [Google Scholar]
- Martín, J.L.; Izquierdo, I.; Oromí, P. El género Loboptera en Canarias: Descripción de cinco nuevas especies hipogeas (Blattaria, Blattellidae). Vieraea 1999, 27, 255–286. [Google Scholar]
- Oromí, P. Canary Islands: Biospeleology. In Encyclopedia of Caves and Karst Science; Gunn, J., Ed.; Taylor and Francis Group: New York, NY, USA, 2004; pp. 179–181. [Google Scholar]
- Macías-Hernández, N.; López, S.C.; Roca-Cusachs, M.; Oromí, P.; Arnedo, M.A. A geographical distribution database of the genus Dysdera in the Canary Islands (Araneae, Dysderidae). Zookeys 2016, 625, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Enghoff, H. Three new species of Dolichoiulus millipedes from the underground of Gran Canaria, with notes on the circumscription of the genus (Diplopoda, Julida, Julidae). Eur. J. Tax. 2012, 15, 1–12. [Google Scholar] [CrossRef]
- Hoch, H.; Asche, M. Evolution and speciation of cave-dwelling Fulgoroidea in the Canary Islands (Homoptera: Cixiidae and Meenoplidae). Zool. J. Linn. Soc. 1993, 109, 53–101. [Google Scholar] [CrossRef]
- Borges, P.A.V.; Serrano, A.R.M.; Amorim, I.R. New species of caved welling beetles (Coleoptera: Carabidae: Trechinae) from the Azores. J. Nat. Hist. 2004, 38, 1303–1313. [Google Scholar] [CrossRef]
- Rodrigues de Gouveia, F.A.S. Principais grutas existentes na ilha da Madeira. Bol. Da Soc. Port. Espeleol. 1963, 1, 34–42. [Google Scholar]
- Relvas, P.; Monteiro, V. Grutas do Cavalum, Machico-Madeira. Algarocho 1987, 9, 5–8. [Google Scholar]
- Nóbrega, A.; Correia, J.P. Ilha da Madeira. Espeleo Divulg. 1985, 4, 36–43. [Google Scholar]
- Vandel, A. Les Isopodes terrestres de l’archipel Madérien. Mém. Du Mus. Natl. D’hist. Nat.-Sér. A Zool. 1960, 22, 1–156. [Google Scholar]
- Zaragoza, J.A.; Aguín-Pombo, D.; Nunes, E. Paraliochthonius cavalensis, nueva espécie cavernícola de Madeira (Arachnida, Pseudoscorpiones, Chthoniidae). Rev. Ibér. Aracnol. 2004, 9, 343–351. [Google Scholar]
- Reboleira, A.S.P.S.; Enghoff, H. Insular species swarm goes underground: Two new troglobiont Cylindroiulus millipedes from Madeira (Diplopoda: Julidae). Zootaxa 2014, 3785, 481–489. [Google Scholar] [CrossRef]
- Strinati, P.; Condé, B. Grottes et Palpigrades de Madére. Mem. Biosp. 1995, 22, 161–168. [Google Scholar]
- Wunderlich, J. Die Spinnen-Fauna der Makaronesischen Inseln. Taxonomie, Ökologie, Biogeographie und Evolution. Beitr. Araneol. 1992, 1, 1–619. [Google Scholar]
- Erber, D. Thalasophilus pieperi n. sp., a new cavernicolous carabid beetle from Madeira. Bocagiana 1990, 140, 1–7. [Google Scholar]
- Serrano, A.R. Medon vicentensis n. sp., a new species of eyeless roverbeetle (Coleoptera: Staphylinidae: Paederinae) from a cave in the Island of Madeira. Bocagiana 1993, 165, 1–7. [Google Scholar]
- Mahnert, V. Les pseudoscorpions (Arachnida) des grottes des iles Canaries, avec description de deux especes nouvelles du genre Paraliochthonius Beier. Mem. Biosp. 1989, 16, 41–46. [Google Scholar]
- Machado, A. Nuevos carabidos microftalmos de la Isla de La Palma, Islas Canarias (Coleoptera, Carabidae). Nouv. Rev. Entomol. (Nouv. Sér.) 1989, 6, 369–372. [Google Scholar]
- Oromí, P.; Borges, P.A.V. New Trechodinae and Trechinae from the Azores (Coleoptera: Carabidae). Bocagiana 1991, 152, 1–10. [Google Scholar]
- Borges, P.A.V.; Oromí, P. The Azores. In Encyclopaedia Biospeleologica Tome I; Juberthie, C., Decu, V., Eds.; Société de Biospéologie: Bucharest, Romania, 1994; pp. 605–610. [Google Scholar]
- Oromí, P.; Izquierdo, I. Canary Islands. In Encyclopaedia Biospeologica. Société de Biospéologie; Juberthie, C., Decu, V., Eds.; Moulis-Bucarest (Academie Roumaine): Bucharest, Romania, 1994; pp. 631–639. [Google Scholar]
- Brum da Silveira, A.; Prada, S.; Ramalho, R.; Madeira, J.; Fonseca, P.; Canha, E.; Brilha, J. Inventariação do Património Geológico da Ilha da Madeira. Secretaria Regional do Ambiente e Recursos Naturais-Relatório Final. 2012; 414p. Available online: https://geodiversidade.madeira.gov.pt (accessed on 1 May 2022).
- Cassagnau, P.; Delamare-Deboutteville, C. Les Arrhopalites et Pararrhopalites d’Europe (Collemboles Symphypléones cavernicoles). Notes Biosp. 1953, VIII, 133–146. [Google Scholar]
- Denis, J.R. Collemboles d’Italie (principalment cavernicoles); sixieme note sur la faune italienne des Collemboles. Boll. Soc. Adr. 1938, 36, 95–165. [Google Scholar]
- Gisin, H. Notes sur les Collemboles, avec démembrement des Onychiurus armatus, ambulans at fimetarius auctorum. Bull. Soc. Roy. Ent. Egypt 1952, 25, 1–22. [Google Scholar]
- Delamare-Deboutteville, C.; Bassot, J.M. Collemboles Symphypleones de Madère et remarques biogéographiques. Vie Milieu 1957, 8, 76–85. [Google Scholar]
- Gisin, H. Collemboles récoltés par M. Bassot a Madère. Vie Milieu 1957, 8, 473–478. [Google Scholar]
- Nunes, E. Os Tubos de Lava de Machico (Ilha da Madeira, Portugal): Biodiversidade e Conservação. Bachelor’s Thesis, Departamento de Biologia, Universidade da Madeira, Funchal, Portugal, 2005; 50p. [Google Scholar]
- Arbea, J.I.; Baquero, E.; Beruete, E.; Pérez Fernández, T.; Jordana, R. Catálogo de los colémbolos cavernícolas del área iberobalear e islas macaronésicas septentrionales (Collembola). Bol. Soc. Ent. Aragonesa 2021, 68, 1–80. [Google Scholar]
- Calandri, G. Le grotte laviche di Madeira (Portugal). Boll. G.S.I. CAI 1991, 21, 19–27. [Google Scholar]
- Calandri, G. L’anidrini carbonica nelle grotte lavichedi Madeira. Boll. G.S.I. CAI 1992, 22, 12–19. [Google Scholar]
- Hernández, J.J.; Oromí, P.; Lainez, A.; Ortega, G.; Pérez, A.E.; López, J.S.; Medina, A.L.; Izquierdo, I.; Sala, L.; Zurita, N.; et al. Catálogo espeleológico de Tenerife; Cabildo de Tenerife: Santa Cruz de Tenerife, España, 1995; 168p. [Google Scholar]
- Arechavaleta, M.; Zurita, N.; Camacho, A.; Oromí, P. La fauna invertebrada de tres cavidades volcánicas del Parque Nacional del Teide (Tenerife): Los Roques, Cuevas Negras y Chavao. Rev. Acad. Canar. Cienc. 1998, 10, 65–78. [Google Scholar]
- Arechavaleta, M.; Sala, L.L.; Oromi, P. La fauna invertebrada de la Cueva de Felipe Reventón (Icod de los Vinos, Tenerife, Islas Tenerife). Vieraea 1999, 27, 229–244. [Google Scholar]
- Schneider, C. Morphological review of the order Neelipleona (Collembola) through the redescription of the type species of Acanthoneelidus, Neelides and Neelus. Zootaxa 2017, 4308, 1–94. [Google Scholar] [CrossRef]
- Gisin, H. Espèces nouvelles et lignées évolutives de Pseudosinella endogés (Collembola). Mém. Est. Mus. Zool. Univ. Coimbra 1967, 301, 1–25. [Google Scholar]
- Zhang, F.; Bedos, A.; Deharveng, L. Cave-dwelling Coecobrya from southern China with a survey of clypeal chaetae in Entomobryoidea (Collembola). Eur. J. Taxon. 2016, 226, 1–21. [Google Scholar] [CrossRef]
- Fjellberg, A. The Labial Palp in Collembola. Zool. Anz. 1999, 237, 309–330. [Google Scholar]
- Chen, J.-X.; Christiansen, K.A. The genus Sinella with special reference to Sinella s. s. (Collembola: Entomobryidae) of China. Orient. Insects 1993, 27, 1–54. [Google Scholar] [CrossRef]
- Chen, J.-X.; Christiansen, K.A. Subgenus Coecobrya of the genus Sinella (Collembola: Entomobryidae) with special reference to the species of China. Ann. Entomol. Soc. Am. 1997, 90, 1–19. [Google Scholar] [CrossRef]
- Mari Mutt, J.A. A revision of the genus Dicranocentrus Schött (Insecta: Collembola: Entomobryidae). Univ. P.R. Agr. Exp. Sta. Bull. 1979, 259, 1–79. [Google Scholar]
- Soto-Adames, F.N. Postembryonic development of the dorsal chaetotaxy in Seira dowlingi (Collembola, Entomobryidae); with an analysis of the diagnostic and phylogenetic significance of primary chaetotaxy in Seira. Zootaxa 2008, 1683, 1–31. [Google Scholar] [CrossRef]
- Szeptycki, A. Chaetotaxy of the Entomobryidae and Its Phylogenetical Significance. Morpho-Systematic Studies on Collembola. IV; Państwowe Wydawnictwo Naukowe (Polska Akademia Nauk): Kraków, Poland, 1979; pp. 1–219. [Google Scholar]
- Jordana, R.; Baquero, E. A proposal of characters for taxonomic identification of Entomobrya species (Collembola, Entomobryomorpha), with description of a new species. Abh. Und Ber. Naturk. Mus Görlitz 2005, 76, 117–134. [Google Scholar]
- Zhang, F.; Yu, D.-Y.; Xu, G.-L. Transformational homology of the tergal setae during postembryonic development in the Sinella-Coecobrya group (Collembola: Entomobryidae). Contrib. Zool. 2011, 80, 213–230. [Google Scholar] [CrossRef]
- Jordana, R. Synopses on Palaearctic Collembola–CAPBRYINAE & ENTOMOBRYINI. Soil Org. 2012, 84, 1–390. [Google Scholar]
- Zhang, F.; Deharveng, L. Systematic revision of Entomobryidae (Collembola) by integrating molecular and new morphological evidence. Zool. Scr. 2015, 44, 298–311. [Google Scholar] [CrossRef]
- Lubbock, J. Notes on the Thysanura, Part IV. Trans. Linn. Soc. Lond. 1870, 27, 277–297. [Google Scholar] [CrossRef]
- Börner, C. Die Familien der Collembolen. Zool. Anz. 1913, 41, 315–322. [Google Scholar]
- D’Haese, C.A. Were the first springtails semi-aquatic? A phylogenetic approach by means of 28S rDNA and optimization alignment. Proc. R. Entomol. Soc. Lond. 2002, 269, 1143–1151. [Google Scholar] [CrossRef] [PubMed]
- Salmon, J.T. An Index to the Collembola, Volume 1. R. Soc. N. Z. Bull. 1964, 7, 1–144. [Google Scholar]
- Deharveng, L. Recent advances in Collembola systematics. Pedobiologia 2004, 48, 415–433. [Google Scholar] [CrossRef]
- Börner, C. Collembola Symphypleona, Fam. Neelidae. Gen. Insec. 1906, I, 1–5. [Google Scholar]
- Brohmer, P. Fauna von Deutschland: Ein Bestimmungsbuch Unserer Heimischen Tierwelt, 4th ed.; Quelle & Meyer: Leipzig, Germany, 1932. [Google Scholar]
- Bagnall, R.S. Notes on British Collembola. Entomol. Mon. Mag. 1940, lXXVI, 163–174. [Google Scholar]
- D’Haese, C.A. Morphological appraisal of Collembola phylogeny with special emphasis on Poduromorpha and a test of the aquatic origin hypothesis. Zool. Scr. 2003, 32, 563–586. [Google Scholar] [CrossRef]
- Absolon, K. Weitere Nachricht über europäische Höhlencollembolen und über die Gattung Aphorura A.D. MacG. (Schluß). Zool. Anz. 1901, XXIV, 385–389. [Google Scholar]
- Gervais, P. Designation of Type and Description of Genus Onychiurus. Echo Monde Savant 1841, 8, 372. [Google Scholar]
- Arbea, J.I. Review of the genus Onychiurus Gervais, 1841 (Collembola: Onychiuridae) with description of a new cave species from Southern Spain. Zootaxa 2012, 3564, 33–46. [Google Scholar] [CrossRef]
- Massoud, Z. Contribution à la connaissance morphologique et systématique des Collemboles Neelidae. Rev. Ecol. Biol. Sol 1971, 8, 195–198. [Google Scholar]
- Folsom, J.W. Neelus murinus, representing a new thysanuran family. Psyche 1896, 7, 391–392. [Google Scholar] [CrossRef]
- Massoud, Z.; Vannier, G. Revision du genre Neelus Folsom 1896 (Collembola) et description de Neelus labralisetosus n. sp. des Iles Salomon. Rev. Ecol. Biol. Sol 1967, 4, 625–637. [Google Scholar]
- Bretfeld, G.; Trinklein, D. New Collembola Symphypleona from the tropical cloud forest of Ecuador (Insecta). Abh. Und Ber. Naturk. Mus Görlitz 2000, 72, 177–194. [Google Scholar]
- Soto-Adames, F.N.; Barra, J.-A.; Christiansen, K.; Jordana, R. Suprageneric Classification of Collembola Entomobryomorpha. Ann. Entomol. Soc. Am. 2008, 101, 501–513. [Google Scholar] [CrossRef]
- Womersley, H. On Some Collembola-Arthropleona from South Africa and Southern Rhodesia. Ann. S. Afr. Mus. 1934, 3, 441–475. [Google Scholar]
- Zhang, F.; Bellini, B.C.; Soto-Adames, F.N. New insights into the systematics of Entomobryoidea (Collembola: Entomobryomorpha): First instar chaetotaxy, homology and classification. Zool. Syst. 2019, 44, 249–278. [Google Scholar] [CrossRef]
- Absolon, K.; Kseneman, M. Trogopedetini. Vergleichende Studie über eine altertümliche höhlenbewohnende Kollembolengrupe aus den dinarischen karstgebieten. Ber. Uber Eine Nat. Wiss. Forsch. Und Geogr. Erforsch. Der Hiselm Brae Dalmatian Stud. Dem Gebeite Der Allg Kantforschung Brunn 1942, 16, 1–57. [Google Scholar]
- Wankel, H. Beiträge zur Fauna der Mährischen Höhlen. Lotos. Zeitschr. F Nat. 1860, 10, 201–206. [Google Scholar]
- Moniez, R. Faune des souterrains du départment du Nord. Rev. Biol. Nord. Fr. 1889, II, 241–262. [Google Scholar]
- Schäffer, C. Die Collembola der Umgebung von Hamburg und benachbarter Gebiete. Mitt. Naturhist. Mus. Hambg. 1896, 13, 147–216. [Google Scholar]
- Yosii, R. Höhlencollembolen Japans II. Jpn. J. Zool. 1956, 11, 608–627. [Google Scholar]
- Imms, A.D. On some Collembola from India, Burma, and Ceylon; with a Catalogue of the Oriental Species of the Order. J. Zool. Proc. Zool. Soc. Lond. 1912, 4, 80–125. [Google Scholar] [CrossRef]
- Zhang, F.; Deharveng, L.; Chen, J.X. New species and rediagnosis of Coecobrya (Collembola: Entomobryidae), with a key to the species of the genus. J. Nat. Hist. 2009, 43, 41–42. [Google Scholar] [CrossRef]
- Stach, J. Materialien zur kenntnis der Collembolen Fauna Afghanistans. Acta Zool. Crac. 1960, 5, 507–581. [Google Scholar]
- Chen, J.-X.; Ma, Y.-T. A new Entomobrya species (Collembola: Entomobryidae). Entomotaxonomia 1998, 20, 235–238. [Google Scholar]
- Zhang, F.; Jantarit, S.; Nilsai, A.; Stevens, M.; Ding, Y.; Satasook, C. Species delimitation in the morphologically conserved Coecobrya (Collembola: Entomobryidae): A case study integrating morphology and molecular traits to advance current taxonomy. Zool. Scr. 2018, 47, 342–356. [Google Scholar] [CrossRef]
- Xu, G.-L.; Zhang, F. Two new species of Coecobrya (Collembola, Entomobryidae) from China, with an updated key to the Chinese species of the genus. ZooKeys 2015, 498, 17–28. [Google Scholar]
- Zhang, F.; Dong, R.-R. Three new species of Coecobrya (Collembola: Entomobryidae) from southern and northwest China. Zootaxa 2014, 3760, 260–274. [Google Scholar] [CrossRef] [PubMed]
- Christiansen, K.; Bellinger, P.F. Insects of Hawaii: Vol. 15: Collembola; University of Hawaii Press: Honolulu, HI, USA, 1992; 445p. [Google Scholar]
- Folsom, J.W. Collembola of the Grave. Psyche 1902, 9, 363–367. [Google Scholar] [CrossRef]
- Bernard, E.C.; Soto-Adames, F.N.; Wynne, J.J. Collembola of Rapa Nui (Easter Island) with descriptions of five endemic cave-restricted species. Zootaxa 2015, 3949, 239–267. [Google Scholar] [CrossRef]
- Brook, G. On a new genus of Collembola (Sinella) allied to Degeeria Nicolet. Zool. J. Linn. Soc. 1882, 16, 541–545. [Google Scholar] [CrossRef]
- Mandal, G.P.; Suman, K.K.; Bhattacharya, K.K. Two new species of collembola Sinella jaldaparaensis (Entomobryidae) and Cyphoderopsis gorumaraensis (Paronellidae) from India. J. Entomol. Zool. Stud. 2019, 7, 145–150. [Google Scholar]
- Loksa, I.; Bogojevic, J. Einige interessante Collembolen arten aus der Sandwuste von Deliblat, Jugoslawien. Opus. Zool. 1970, 10, 125–142. [Google Scholar]
- Agrell, I. Die Arthropodenfauna von Madeira nach den Ergebnissen der Reissen von Prof. Dr. O. Lundblad, Juli-August 1935. XVIII. Collembola. Ark. Zool. 1939, 31B, 1–7. [Google Scholar]
- Bourlet, A. Mémoire sur les Podures. Mem. Soc. Sci. Agric. 1839, 1, 377–417. [Google Scholar]
- Thibaud, J.M. Catalogue des collemboles de France. Zoosystema 2017, 39, 297–436. [Google Scholar] [CrossRef]
- Arbea, J.I. Checklist de Fauna Ibérica. Clase Collembola Lubbock, 1870 (Hexapoda) en la península ibérica, islas Baleares y Macaronesia. In Documentos Fauna Ibérica, 16; Ramos, M.A., Sánchez Ruiz, M., Eds.; Museo Nacional de Ciencias Naturales, CSIC: Madrid, Spain, 2021; Available online: https://www.fauna-iberica.mncn.csic.es/publicaciones/dfi/dfi-0016.pdf (accessed on 1 May 2022).
- Gisin, H. Nouvelles notes taxonomiques sur les Lepidocyrtus. Rev. Ecol. Biol. Sol 1965, 2, 519–524. [Google Scholar]
- Mateos, E. The European Lepidocyrtus Bourlet, 1839 (Collembola: Entomobryidae). Zootaxa 2008, 1769, 35–59. [Google Scholar] [CrossRef]
- Rondani, C. Entomobrya pro Degeeria Nic. Dipterol. Ital. Prodromus 1861, 4, 1–40. [Google Scholar]
- Denis, J.R. Collemboles récoltés par M.P. Remy en Yougoslavie et en Macédoine grecque (Note préliminaire). Bull. Soc. Ent. Fr. 1933, 38, 211–213. [Google Scholar] [CrossRef]
- Börner, C. Neue Collembolenformen and zur Nomenclatur der Collembola Lubb. Zool. Anz. 1901, 24–23, 696–712. [Google Scholar]
- Bretfeld, G. The chaetotaxy of the small abdomen of the Symphypleona (Insecta, Collembola) an its phylogenetic interpretation. Acta Zoo. Fenn. 1994, 195, 13–17. [Google Scholar]
- Stach, J. The Apterygotan Fauna of Poland in Relation to the World-Fauna of This Group of Insects—Family: Sminthuridae; Polska Akademia Nauk: Krakowie, Poland, 1956; pp. 1–287. [Google Scholar]
- Bretfeld, G. Synopses on Palaearctic Collembola, Volume 2: Symphypleona. Abh. Ber. Naturk. Mus Görlitz 1999, 71, 1–318. [Google Scholar]
- Vargovitsh, R.S. New cave Arrhopalitidae (Collembola: Symphypleona) from the Crimea (Ukraine). Zootaxa 2009, 2047, 1–47. [Google Scholar] [CrossRef]








| Cave | CVI | CVII | CVIII | LN |
|---|---|---|---|---|
| full extension (in meters) | 300 | 110 | 92 | 85 |
| maximum entrance height (in meters) | 2.0 | 3.0 | 1.5 | 0.6 |
| maximum entrance width (in meters) | 3.5 | 3.0 | 2.0 | 0.6 |
| maximum height of the tube (in meters) | 10 | 8.0 | 5.0 | 12 |
| minimum tube height (in meters) | 0.5 | 0.6 | 0.5 | 0.5 |
| relative humidity (%) * | 80–95 | 80–95 | 80–95 | 80–95 |
| temperature (°C) | 15–18 | 15–17 | 15–95 | 16–17 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baquero, E.; Arbea, J.I.; Nunes, É.; Aguin-Pombo, D.; Mateos, E.; Jordana, R. Collembola of the Cavalum and Landeiro Caves (Madeira, Portugal). Insects 2023, 14, 525. https://doi.org/10.3390/insects14060525
Baquero E, Arbea JI, Nunes É, Aguin-Pombo D, Mateos E, Jordana R. Collembola of the Cavalum and Landeiro Caves (Madeira, Portugal). Insects. 2023; 14(6):525. https://doi.org/10.3390/insects14060525
Chicago/Turabian StyleBaquero, Enrique, Javier I. Arbea, Élvio Nunes, Dora Aguin-Pombo, Eduardo Mateos, and Rafael Jordana. 2023. "Collembola of the Cavalum and Landeiro Caves (Madeira, Portugal)" Insects 14, no. 6: 525. https://doi.org/10.3390/insects14060525
APA StyleBaquero, E., Arbea, J. I., Nunes, É., Aguin-Pombo, D., Mateos, E., & Jordana, R. (2023). Collembola of the Cavalum and Landeiro Caves (Madeira, Portugal). Insects, 14(6), 525. https://doi.org/10.3390/insects14060525








