Dispersal Behavior Characters of Spodoptera frugiperda Larvae
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Plants and Insects
2.2. Larval Dispersal Observations
2.2.1. The Percentage of 1st- to 3rd-Instar Larvae Ballooning and Crawling
2.2.2. The Length of the Ballooning Silk Thread
2.2.3. The Percentage of Larvae Ballooning Twice
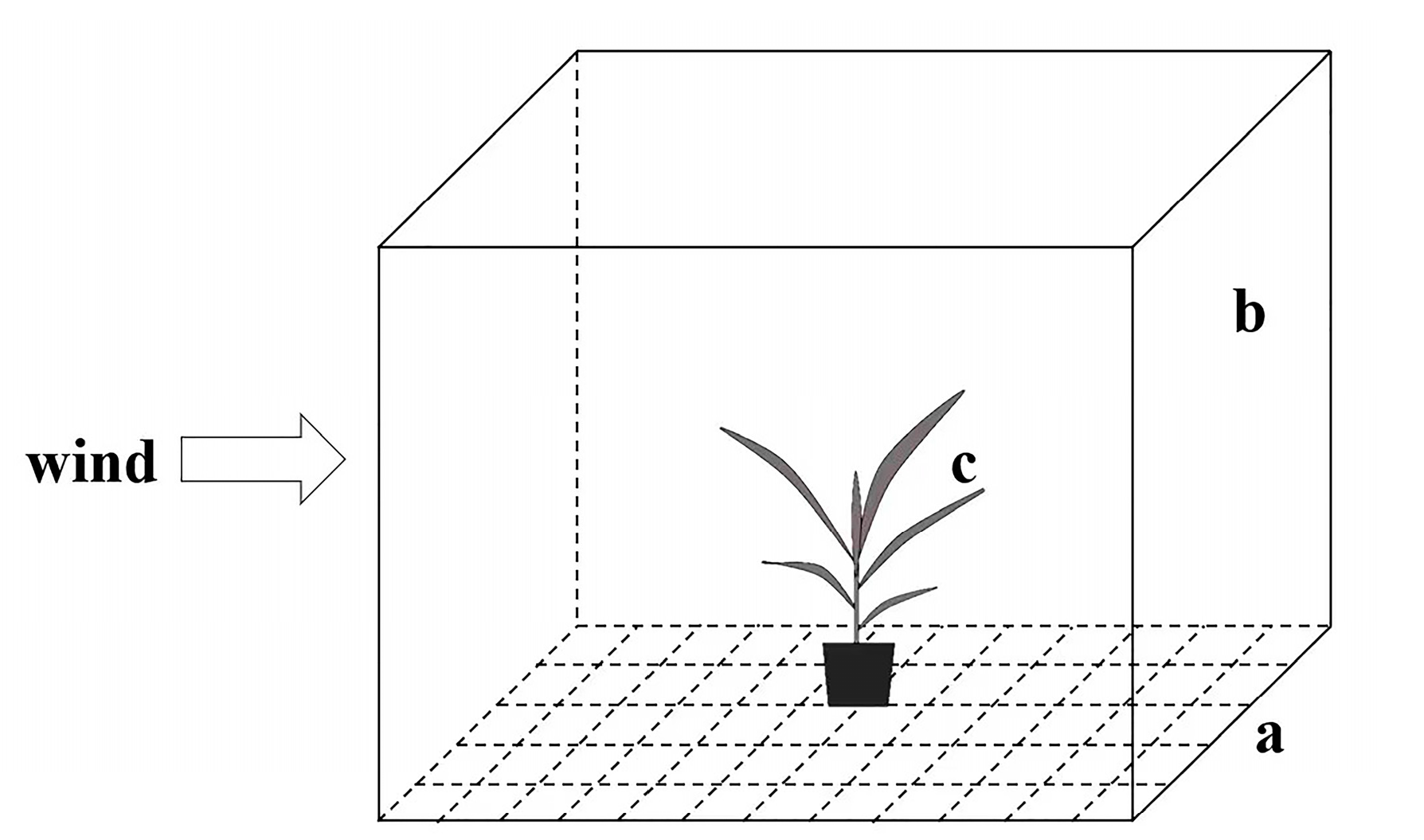
2.3. Impact of Airflow on Ballooning Behavior
2.4. Statistical Analysis
3. Results
3.1. Larval Dispersal Behavior
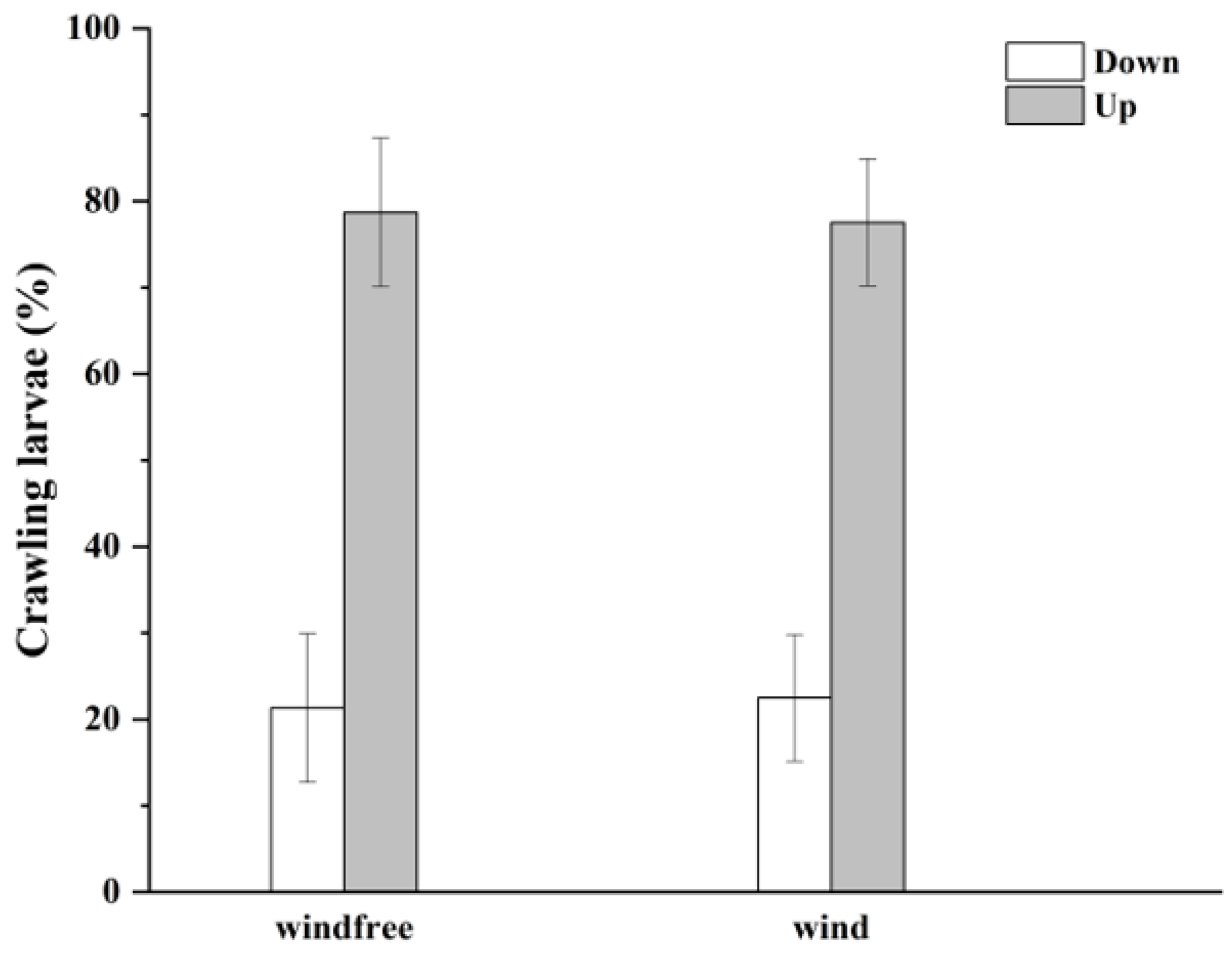
3.2. Effect of Airflow on Larvae Ballooning
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- FAO. Forecasting threats to the food chain affecting food security in countries and regions. In Food Chain Crisis Early Warning Bulletin; Food and Agriculture Organization of the United Nations: Rome, Italy, 2020; Volume 36. [Google Scholar]
- Overton, K.; Maino, J.L.; Day, R.; Umina, P.A.; Bett, B.; Carnovale, D.; Ekesi, S.; Meagher, R.; Reynolds, O.L. Global crop impacts, yield losses and action thresholds for fall armyworm (Spodoptera frugiperda): A review. Crop Prot. 2021, 145, 105641. [Google Scholar] [CrossRef]
- Goergen, G.; Kumar, P.L.; Sankung, S.B.; Togola, A.; Tamò, M. First report of outbreaks of the fall armyworm Spodoptera frugiperda (JE Smith) (Lepidoptera, Noctuidae), a new alien invasive pest in West and Central Africa. PLoS ONE 2016, 11, e0165632. [Google Scholar] [CrossRef] [PubMed]
- Early, R.; González-Moreno, P.; Murphy, S.T.; Day, R. Forecasting the global extent of invasion of the cereal pest Spodoptera frugiperda, the fall armyworm. NeoBiota 2018, 40, 25–50. [Google Scholar] [CrossRef]
- Sharanabasappa; Kalleshwaraswamy, C.M.; Asokan, R.; Swamy, M.; Maruthi, M.S.; Pavithra, H.; Hegbe, K.; Navi, S.; Prabhu, S.; Goergen, G. First report of the fall armyworm, Spodoptera frugiperda (JE Smith) (Lepidoptera: Noctuidae), an alien invasive pest on maize in India. Pest. Manag. Hortic. Ecosyst. 2018, 24, 23–29. [Google Scholar]
- Navasero, M.V.; Navasero, M.M.; Burgonio, G.A.S.; Ardez, K.P.; Ebuenga, M.D.; Beltran, M.J.B.; Bato, M.B.; Gonzales, P.G.; Magsino, G.L.; Caoili, B.L. Detection of the fall armyworm, Spodoptera frugiperda (JE Smith) (Lepidoptera: Noctuidae) using larval morphological characters, and observations on its current local distribution in the Philippines. Philipp. Entomol. 2019, 33, 171–184. [Google Scholar]
- FAO. Integrated Management of the Fall Armyworm on Maize: A Guide for Farmer Field Schools in Africa; Food and Agriculture Organization of the United Nations: Rome, Italy, 2018; 130p. [Google Scholar]
- Montezano, D.G.; Specht, A.; Sosa-Gómez, D.R.; Roque-Specht, V.F.; Malaquias, J.V.; Paula-Moraes, S.V.; Peterson, J.A.; Hunt, T.E. Biotic potential and reproductive parameters of Spodoptera frugiperda (J. E. Smith, 1797) (Lepidoptera: Noctuidae). J. Agric. Sci. 2019, 11, 240–252. [Google Scholar] [CrossRef]
- Lee, G.-S.; Seo, B.Y.; Lee, J.; Kim, H.-J.; Song, J.H.; Lee, W.H. First report of the fall armyworm, Spodoptera frugiperda (Smith, 1797) (Lepidoptera, Noctuidae), a new migratory pest in Korea. Korean J. Appl. Entomol. 2020, 59, 73–78. [Google Scholar]
- Ye, H.; Li, Y.-P.; Feng, D.; Li, C.-L. Construction of prevention and control barriers for Spodoptera frugiperda in the border areas of Yunnan Province. J. Yunnan Univ. 2022, 44, 1054–1061. [Google Scholar] [CrossRef]
- Pitre, H.N.; Mulrooney, J.E.; Hogg, D.B. Fall armyworm (Lepidoptera: Noctuidae) oviposition: Crop preferences and egg distribution on plants. J. Econ. Entomol. 1983, 76, 463–466. [Google Scholar] [CrossRef]
- Sotelo-Cardona, P.; Chuang, W.-P.; Lin, M.-Y.; Chiang, M.-Y.; Ramasamy, S. Oviposition preference not necessarily predicts offspring performance in the fall armyworm, Spodoptera frugiperda (Lepidoptera: Noctuidae) on vegetable crops. Sci. Rep. 2021, 11, 15885. [Google Scholar] [CrossRef]
- McDonald, G. Oviposition and larval dispersal of the common armyworm, Mythimna convecta (Walker) (Lepidoptera: Noctuidae). Aust. J. Ecol. 1991, 16, 385–393. [Google Scholar] [CrossRef]
- He, L.-M.; Ge, S.-S.; Chen, Y.-C.; Wu, Q.-L.; Jiang, Y.-Y.; Wu, K.-M. The developmental threshold temperature, effective accumulated temperature and prediction mode of developmental duration of fall armyworm, Spodoptera frugiperda. Plant Prot. 2019, 45, 18–26. [Google Scholar]
- Sparks, A.N. A review of the biology of the fall armyworm. Fla. Entomol. 1979, 62, 82–87. [Google Scholar] [CrossRef]
- Du Plessis, H.; Schlemmer, M.L.; van den Berg, J. The Effect of temperature on the development of Spodoptera frugiperda (Lepidoptera: Noctuidae). Insects 2020, 11, 228. [Google Scholar] [CrossRef] [PubMed]
- Kenis, M.; Benelli, G.; Biondi, A.; Calatayud, P.-A.; Day, R.; Desneux, N.; Harrison, R.D.; Kriticos, D.J.; Rwomushana, I.; van den Berg, J. Invasiveness, biology, ecology, and management of the fall armyworm, Spodoptera frugiperda. Entomol. Gen. 2022. [Google Scholar] [CrossRef]
- De Groote, H.; Kimenju, S.C.; Munyua, B.; Palmas, S.; Kassie, M.; Bruce, A. Spread and impact of fall armyworm (Spodoptera frugiperda JE Smith) in maize production areas of Kenya. Agric. Ecosyst. Environ. 2020, 292, 106804. [Google Scholar] [CrossRef]
- Ye, H.; Li, Y.-P.; Feng, D.; Xu, Q.-Y. Ecological effects of river valley topography on the migration of Spodoptera frugiperda. J. Yunnan Univ. 2022, 44, 188–194. [Google Scholar] [CrossRef]
- Levin, L.A. Recent progress in understanding larval dispersal: New directions and digressions. Integr. Comp. Biol. 2006, 46, 282–297. [Google Scholar] [CrossRef]
- Rhainds, M.G.; Gries, G.T.; Ho, C.T.; Chew, P.S. Dispersal by bagworm larvae, Metisa plana: Effects of population density, larval sex, and host plant attributes. Ecol. Entomol. 2002, 27, 204–212. [Google Scholar] [CrossRef]
- Ongaratto, S.; Baldin, E.L.; Hunt, T.E.; Luhr, N.; Heier, K.R.; Robinson, E.A. Larval movement of Anticarsia gemmatalis (Lepidoptera: Erebidae) on vegetative and reproductive stage non-Bt soybean. Res. Sq. 2022. [Google Scholar] [CrossRef]
- Zalucki, M.P.; Clarke, A.R.; Malcolm, S.B. Ecology and behavior of first instar larval Lepidoptera. Annu. Rev. Entomol. 2002, 47, 361–393. [Google Scholar] [CrossRef]
- Pepi, A.A.; Broadley, H.J.; Elkinton, J.S. Density-dependent effects of larval dispersal mediated by host plant quality on populations of an invasive insect. Oecologia 2016, 182, 499–509. [Google Scholar] [CrossRef] [PubMed]
- Moore, R.G.; Hanks, L.M. Aerial dispersal and host plant selection by neonate Thyridopteryx ephemeraeformis (Lepidoptera: Psychidae). Ecol. Entomol. 2004, 29, 327–335. [Google Scholar] [CrossRef]
- Veldtman, R.; McGeoch, M.A.; Scholtz, C.H. Fine-scale abundance and distribution of wild silk moth pupae. Bull. Entomol. Res. 2007, 97, 15–27. [Google Scholar] [CrossRef]
- Yang, G.; Wiseman, B.R.; Espelie, K.E. Movement of neonate fall armyworm (Lepidoptera: Noctuidae) larvae on resistant and susceptible genotypes of corn. Environ. Entomol. 1993, 22, 547–553. [Google Scholar] [CrossRef]
- Pannuti, L.E.R.; Baldin, E.L.L.; Hunt, T.E.; Paula-Moraes, S.V. On-plant larval movement and feeding behavior of fall armyworm (Lepidoptera: Noctuidae) on reproductive corn stages. Environ. Entomol. 2016, 45, 192–200. [Google Scholar] [CrossRef]
- Navik, O.; Shylesha, A.N.; Patil, J.; Venkatesan, T.; Lalitha, Y.; Ashika, T.R. Damage, distribution and natural enemies of invasive fall armyworm Spodoptera frugiperda (JE smith) under rainfed maize in Karnataka, India. Crop Prot. 2021, 143, 105536. [Google Scholar] [CrossRef]
- Wan, J.; Huang, C.; Li, C.-Y.; Zhou, H.-X.; Ren, Y.-I.; Li, Z.-Y.; Xing, L.-S.; Zhang, B.; Qiao, X.; Liu, B.; et al. Biology, invasion and management of the agricultural invader: Fall armyworm, Spodoptera frugiperda (Lepidoptera: Noctuidae). J. Integr. Agric. 2021, 20, 646–663. [Google Scholar] [CrossRef]
- He, L.-M.; Wang, T.-l.; Chen, Y.-C.; Ge, S.-S.; Wyckhuys, K.A.G.; Wu, K.-M. Larval diet affects development and reproduction of East Asian strain of the fall armyworm, Spodoptera frugiperda. J. Integr. Agric. 2021, 20, 736–744. [Google Scholar] [CrossRef]
- Chapman, J.W.; Williams, T.; Escribano, A.; Caballero, P.; Cave, R.D.; Goulson, D. Age-related cannibalism and horizontal transmission of a nuclear polyhedrosis virus in larval Spodoptera frugiperda. Ecol. Entomol. 1999, 24, 268–275. [Google Scholar] [CrossRef]
- Morrill, W.L.; Greene, G. Distribution of fall armyworm larvae. 2. Influence of biology and behavior of larvae on selection of feeding sites. Environ. Entomol. 1973, 2, 415–418. [Google Scholar] [CrossRef]
- Chapman, J.W.; Williams, T.; Martínez, A.M.; Cisneros, J.; Caballero, P.; Cave, R.D.; Goulson, D. Does cannibalism in Spodoptera frugiperda (Lepidoptera: Noctuidae) reduce the risk of predation? Behav. Ecol. Sociobiol. 2000, 48, 321–327. [Google Scholar] [CrossRef]
- Sokame, B.M.; Subramanian, S.; Kilalo, D.C.; Juma, G.; Calatayud, P.-A. Larval dispersal of the invasive fall armyworm, Spodoptera frugiperda, the exotic stemborer Chilo partellus, and indigenous maize stemborers in Africa. Entomol. Exp. Appl. 2020, 168, 322–331. [Google Scholar] [CrossRef]
- Bell, J.R.; Bohan, D.A.; Shaw, E.M.; Weyman, G.S. Ballooning dispersal using silk: World fauna, phylogenies, genetics and models. Bull. Entomol. Res. 2005, 95, 69–114. [Google Scholar] [CrossRef] [PubMed]
- Pannuti, L.E.R.; Paula-Moraes, S.V.; Hunt, T.E.; Baldin, E.L.L.; Dana, L.; Malaquias, J.V. Plant-to-plant movement of Striacosta albicosta (Lepidoptera: Noctuidae) and Spodoptera frugiperda (Lepidoptera: Noctuidae) in maize (Zea mays). J. Econ. Entomol. 2016, 109, 1125–1131. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Hu, S.; Chen, P.; Lyv, J.; Wu, X.; Peng, M.; Ye, H. Occurrence regularity, main influencing factors and control strategies of Spodoptera frugiperda in Yunnan. J. Yunnan Univ. 2021, 43, 190–197. [Google Scholar] [CrossRef]
- Li, Z.Y.; Dai, Q.X.; Kuang, Z.L.; Liang, M.R.; Wang, L.; Lu, Y.Y.; Chen, K.W. Effects of three artificial diets on development and reproduction of the fall armyworm Spodoptera frugiperda (J. E. Smith). Environ. Entomol. 2019, 41, 1147–1154. [Google Scholar]
- Rojas, J.C.; Kolomiets, M.V.; Bernal, J.S. Nonsensical choices? Fall armyworm moths choose seemingly best or worst hosts for their larvae, but neonate larvae make their own choices. PLoS ONE 2018, 13, e0197628. [Google Scholar] [CrossRef]
- Ali, A.; Luttrell, R.G.; Pitre, H.N. Feeding sites and distribution of fall armyworm (Lepidoptera: Noctuidae) larvae on cotton. Environ. Entomol. 1990, 19, 1060–1067. [Google Scholar] [CrossRef]
- Zhao, W.; Zheng, Q.; Qin, D.; Luo, P.; Ye, C.; Shen, S.; Cheng, D.; Huang, S.; Liu, L.; Xu, H. Azadirachtin inhibits the development and metabolism of the silk glands of Spodoptera frugiperda and affects spinning behavior. Pest Manag. Sci. 2022, 78, 5293–5301. [Google Scholar] [CrossRef]
- Torres-Vila, L.M.; Stockel, J.; Roehrich, R.; Rodríguez-Molina, M.C. The relation between dispersal and survival of Lobesia botrana larvae and their density in vine inflorescences. Entomol. Exp. Appl. 1997, 84, 109–114. [Google Scholar] [CrossRef]
- Van Griethuijsen, L.I.; Trimmer, B.A. Locomotion in caterpillars. Biol. Rev. 2014, 89, 656–670. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Luo, Q.; Sun, X.; Yang, X.; Jiang, Y.; Wu, K. Comparison of morphological and biological characteristics between Spodoptera frugiperda and Spodoptera litura. China Plant Prot. 2019, 39, 26–35. [Google Scholar]
- Volp, T.M.; Zalucki, M.P.; Furlong, M.J. What defines a host? Oviposition behavior and larval performance of Spodoptera frugiperda (Lepidoptera: Noctuidae) on five putative host plants. J. Econ. Entomol. 2022, 115, 1744–1751. [Google Scholar] [CrossRef]
- Chang, N.R.; Wiseman, B.R.; Lynch, R.E.; Habeck, D.H. Fall armyworm (Lepidoptera: Noctuidae) orientation and preference for selected grasses. Fla. Entomol. 1985, 68, 296–303. [Google Scholar] [CrossRef]
- De La Rosa-Cancino, W.; Rojas, J.C.; Cruz-Lopez, L.; Castillo, A.; Malo, E.A. Attraction, feeding preference, and performance of Spodoptera frugiperda larvae (Lepidoptera: Noctuidae) reared on two varieties of maize. Environ. Entomol. 2016, 45, 384–389. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.-P.; Yao, S.-Y.; Feng, D.; Haack, R.A.; Yang, Y.; Hou, J.-L.; Ye, H. Dispersal Behavior Characters of Spodoptera frugiperda Larvae. Insects 2023, 14, 488. https://doi.org/10.3390/insects14060488
Li Y-P, Yao S-Y, Feng D, Haack RA, Yang Y, Hou J-L, Ye H. Dispersal Behavior Characters of Spodoptera frugiperda Larvae. Insects. 2023; 14(6):488. https://doi.org/10.3390/insects14060488
Chicago/Turabian StyleLi, Yong-Ping, Su-Yi Yao, Dan Feng, Robert A. Haack, Yang Yang, Jia-Lan Hou, and Hui Ye. 2023. "Dispersal Behavior Characters of Spodoptera frugiperda Larvae" Insects 14, no. 6: 488. https://doi.org/10.3390/insects14060488
APA StyleLi, Y.-P., Yao, S.-Y., Feng, D., Haack, R. A., Yang, Y., Hou, J.-L., & Ye, H. (2023). Dispersal Behavior Characters of Spodoptera frugiperda Larvae. Insects, 14(6), 488. https://doi.org/10.3390/insects14060488





