Timing of Fungal Insecticide Application to Avoid Solar Ultraviolet Irradiation Enhances Field Control of Rice Planthoppers
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Fungal Formulations
2.2. Rice–Shrimp Rotation Paddy Fields
2.3. Field Trials
2.4. Sampling for RPH Population and Control Efficacy
2.5. Weather Data
2.6. Statistical Analysis
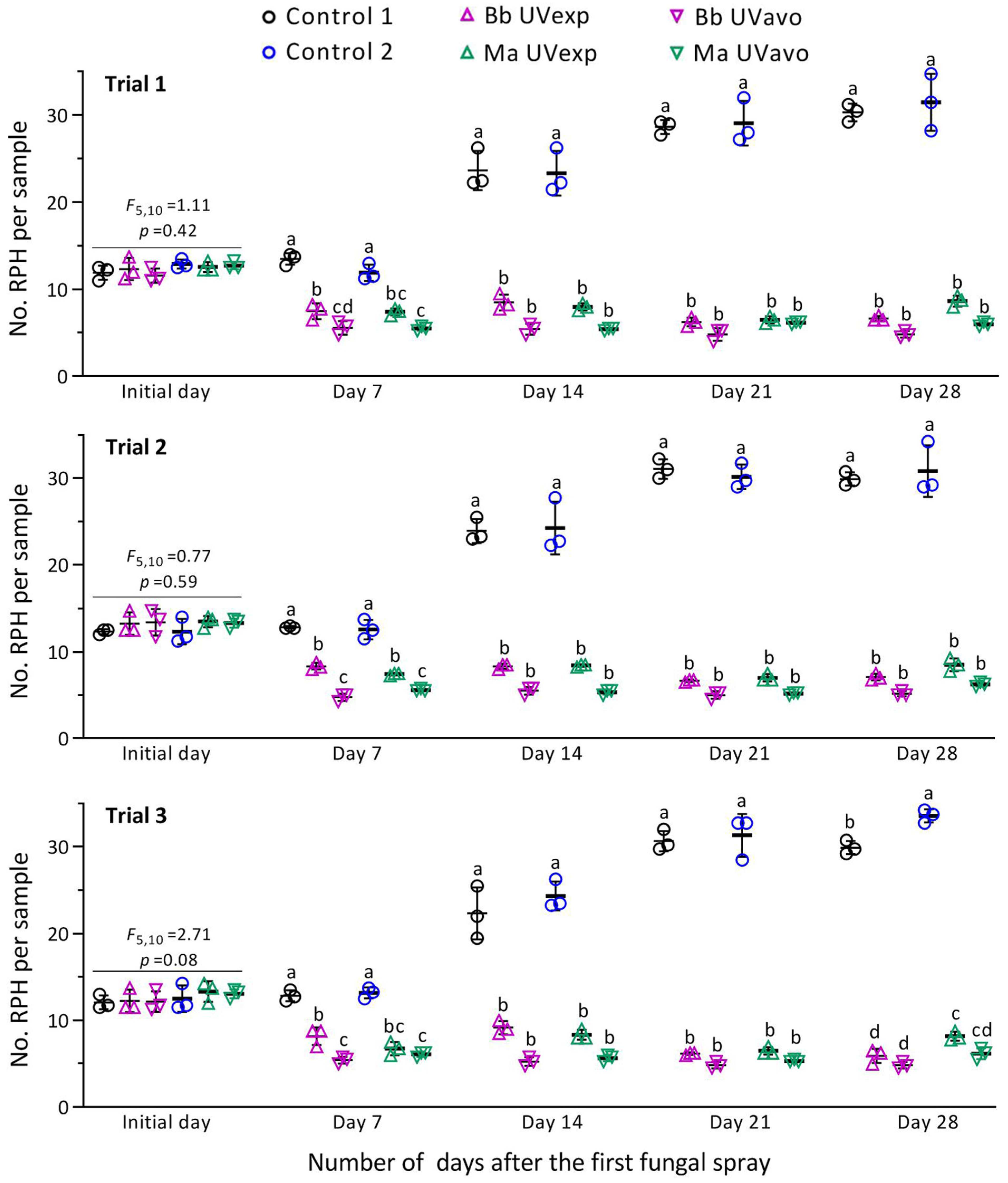
3. Results
3.1. Weather Conditions during Field Trials
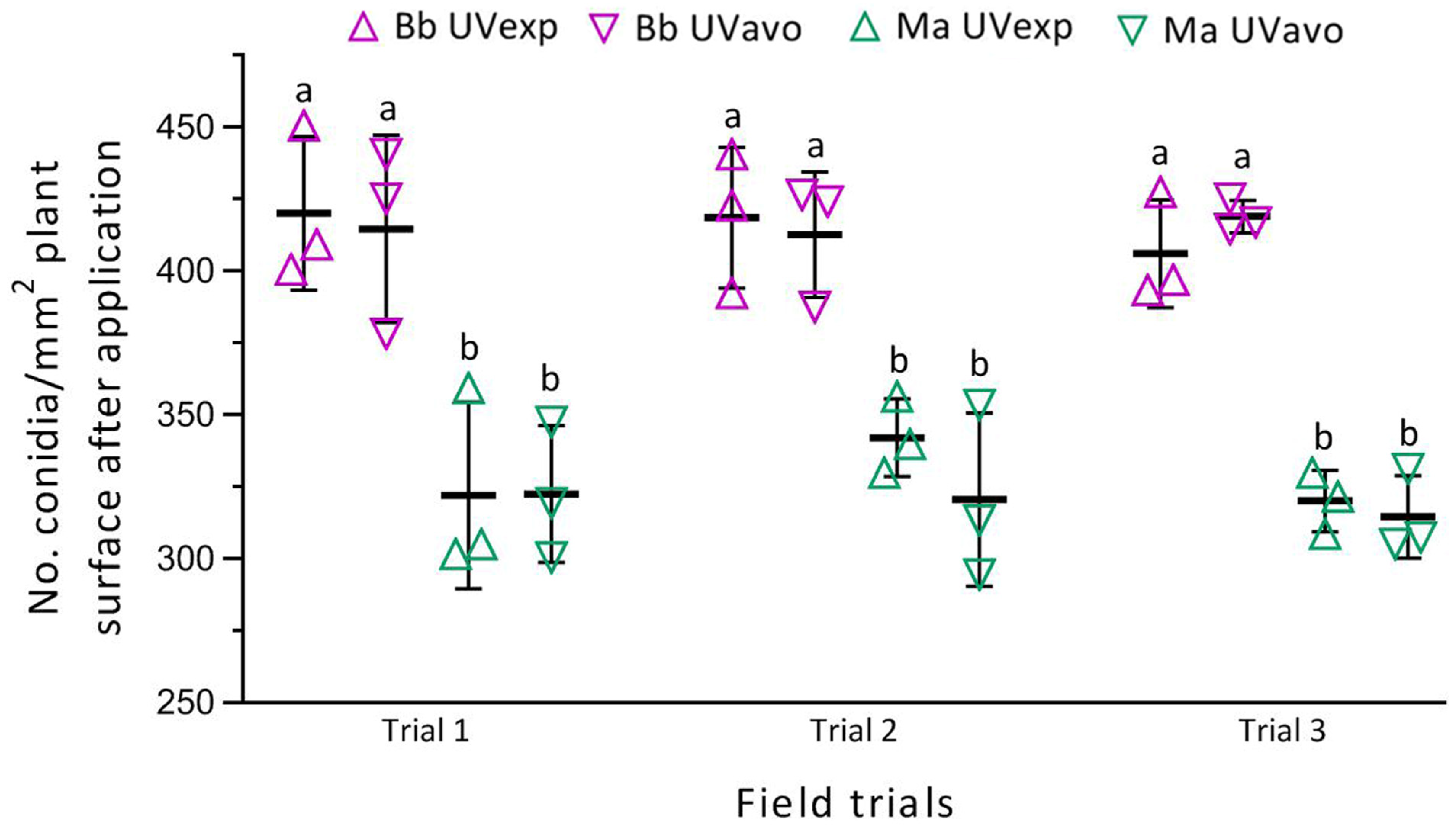
3.2. Deposition Rates of Formulated Conidia after Spray
3.3. Suppression of RPH Population by Fungal Sprays
3.4. Control Efficacies of Two Fungal Insecticides against RPH Population
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, H.; Chang, X.L.; Wang, Y.P.; Lu, M.H.; Liu, W.C.; Zhai, B.; Hu, G. The early northward migration of the white-backed planthopper (Sogatella furcifera) is often hindered by heavy precipitation in southern China during the preflood season in May and June. Insects 2019, 10, 158. [Google Scholar] [CrossRef]
- Feng, C.H.; Zhai, B.P.; Zhang, X.X.; Tang, J.Y. Climatology of low-level jet and northward migration of rice planthoppers. Acta Ecol. Sen. 2002, 22, 559–565. [Google Scholar]
- Furuno, A.; Chino, M.; Otuka, A.; Watanabe, T.; Matsumura, M.; Suzuki, Y. Development of a numerical simulation model for long-range migration of rice planthoppers. Agric. For. Meteorol. 2005, 133, 197–209. [Google Scholar] [CrossRef]
- Otuka, A.; Matsumura, M.; Watanabe, T.; Van Dinh, T. A migration analysis for rice planthoppers, Sogatella furcifera (Horvath) and Nilaparvata lugens (Stal) (Homoptera: Delphacidae), emigrating from northern Vietnam from April to May. Appl. Entomol. Zool. 2008, 43, 527–534. [Google Scholar] [CrossRef]
- Qi, H.H.; Zhang, Y.H.; Cheng, D.F.; Han, E.B.; Sun, J.R. Radar observation and trajectory analysis on the autumn return migration of the brown planthopper, Nilaparvata lugens (Homoptera: Delphacidae) in 2009 in China. Acta Entomol. Sen. 2010, 53, 1256–1264. [Google Scholar]
- Fujii, T.; Sanada-Morimura, S.; Oe, T.; Ide, M.; Thanh, D.V.; Chien, H.V.; Tuong, P.V.; Loc, P.M.; Cuong, L.Q.; Liu, Z.W.; et al. Long-term field insecticide susceptibility data and laboratory experiments reveal evidence for cross resistance to other neonicotinoids in the imidacloprid-resistant brown planthopper Nilaparvata lugens. Pest Manag. Sci. 2020, 76, 480–486. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, M.; Sanada-Morimura, S.; Otuka, A.; Ohtsu, R.; Sakumoto, S.; Takeuchi, H.; Satoh, M. Insecticide susceptibilities in populations of two rice planthoppers, Nilaparvata lugens and Sogatella furcifera, immigrating into Japan in the period 2005–2012. Pest Manag. Sci. 2014, 70, 615–622. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, M.; Sanada-Morimura, S.; Otuka, A.; Sonoda, S.; Thanh, D.V.; Chien, H.V.; Tuong, P.V.; Loc, P.M.; Liu, Z.W.; Zhu, Z.R.; et al. Insecticide susceptibilities of the two rice planthoppers Nilaparvata lugens and Sogatella furcifera in East Asia, the Red River Delta, and the Mekong Delta. Pest Manag. Sci. 2018, 74, 456–464. [Google Scholar] [CrossRef]
- Nakao, T. Insecticide resistance in rice planthoppers. In Advances in Agrochemicals: Ion Channels and G Protein-Coupled Receptors (GPCRs) as Targets for Pest Control, Vol. 2: GPCRs and Ion Channels; Gross, A.D., Ozoe, Y., Coats, J.R., Eds.; American Chemical Society: Washington, DC, USA, 2017; pp. 23–39. [Google Scholar]
- Song, X.Y.; Peng, Y.X.; Wang, L.X.; Ye, W.N.; Pei, X.G.; Zhang, Y.C.; Zhang, S.; Gao, C.F.; Wu, S.F. Monitoring, cross-resistance, inheritance, and fitness costs of brown planthoppers, Nilaparvata lugens, resistance to pymetrozine in China. Pest Manag. Sci. 2022, 78, 3980–3987. [Google Scholar] [CrossRef]
- Wu, J.C. Pesticide-induced resurgence of rice planthoppers and scientific application of pesticides. Acta Phytophyl. Sin. 2017, 44, 919–924. [Google Scholar]
- Chen, Y.H.; Bernal, C.C.; Tan, J.; Horgan, F.G.; Fitzgerald, M.A. Planthopper “adaptation” to resistant rice varieties: Changes in amino acid composition over time. J. Insect Physiol. 2011, 57, 1375–1384. [Google Scholar] [CrossRef]
- Horgan, F.G.; Cruz, A.P.; Arida, A.; Ferrater, J.B.; Bernal, C.C. Adaptation by the brown planthopper to resistant rice: A test of female-derived virulence and the role of yeast-like symbionts. Insects 2021, 12, 908. [Google Scholar] [CrossRef]
- Huang, S.H.; Lai, P.Y.; Hwang, S.Y.; Borhara, K.; Huang, W.R.; Wang, S.Y. Climate variability shifting immigrated rice planthoppers in Taiwan. Climate 2022, 10, 71. [Google Scholar] [CrossRef]
- Li, X.J.; Hu, Y.Y.; Ma, J.; Wang, Y.C.; Wang, L.; Lu, M.H.; Zhai, B.P. Influence of 2015–2016 El Nino on the occurrence of rice planthoppers in China. Acta Entomol. Sin. 2017, 60, 450–463. [Google Scholar]
- Liu, J.P.; Zhuang, J.; Huang, W.K.; Chi, H.; Wang, C.H.; Hua, H.X.; Wu, G. Different adaptability of the brown planthopper, Nilaparvata lugens (Stål), to gradual and abrupt increases in atmospheric CO2. J. Pest Sci. 2020, 93, 979–991. [Google Scholar] [CrossRef]
- Wan, G.J.; Dang, Z.H.; Wu, G.; Parajulee, M.N.; Ge, F.; Chen, F.J. Single and fused transgenic Bacillus thuringiensis rice alter the species-specific responses of non-target planthoppers to elevated carbon dioxide and temperature. Pest Manag. Sci. 2014, 70, 734–742. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.R.; Cheng, J.A.; Jiang, M.X.; Zhang, X.Y. Complex influence of rice variety, fertilization timing, and insecticide on population dynamics of Sogatella furcifera (Horvath), Nilaparvata lugens (Stål) (Homoptera: Delphacidae) and their natural enemies in rice in Hangzhou, China. J. Pest Sci. 2004, 77, 65–74. [Google Scholar] [CrossRef]
- Zhu, P.Y.; Zheng, X.S.; Xu, H.X.; Johnson, A.C.; Heong, K.L.; Gurr, G.M.; Lu, Z.X. Nitrogen fertilizer promotes the rice pest Nilaparvata lugens via impaired natural enemy, Anagrus flaveolus, performance. J. Pest Sci. 2020, 93, 757–766. [Google Scholar] [CrossRef]
- Ling, Y.; Ang, L.; Zhang, W.L. Current understanding of the molecular players involved in resistance to rice planthoppers. Pest Manag. Sci. 2019, 75, 2566–2574. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, W.L. Genetic and biochemical mechanisms of rice resistance to planthopper. Plant Cell Rep. 2016, 35, 1559–1572. [Google Scholar]
- Yao, F.L.; You, M.S.; Vasseur, L.; Yang, G.; Zheng, Y.K. Polycultural manipulation for better regulation of planthopper populations in irrigated rice-based ecosystems. Crop Prot. 2012, 34, 104–111. [Google Scholar] [CrossRef]
- Ren, W.Z.; Hu, L.L.; Guo, L.; Zhang, J.; Tang, L.; Zhang, E.T.; Zhang, J.E.; Luo, S.M.; Tang, J.J.; Chen, X. Preservation of the genetic diversity of a local common carp in the agricultural heritage rice-fish system. Proc. Natl. Acad. Sci. USA 2018, 115, E546–E554. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Hu, L.L.; Tang, J.J.; Wu, X.; Li, N.N.; Yuan, Y.G.; Yang, H.H.; Zhang, J.E.; Luo, S.M.; Chen, X. Ecological mechanisms underlying the sustainability of the agricultural heritage rice-fish coculture system. Proc. Natl. Acad. Sci. USA 2011, 108, E1381–E1387. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.L.; Zhao, L.F.; Tang, J.J.; Guo, L.; Ding, L.L.; Zhang, J.; Ren, W.Z.; Chen, X. Extension potential of rice-fish co-culture system: A case study of 10 provinces in South China. Chin. J. Eco-Agric. 2019, 27, 981–993. [Google Scholar]
- Ye, Y.Y.; Ren, W.Z.; Zhang, S.X.; Zhao, L.F.; Tang, J.J.; Hu, L.L.; Chen, X. Genetic diversity of fish in aquaculture and of common carp (Cyprinus carpio) in traditional rice-fish coculture. Agriculture 2022, 12, 997. [Google Scholar] [CrossRef]
- Zhang, J.; Hu, L.L.; Ren, W.Z.; Guo, L.; Tang, J.J.; Shu, M.A.; Chen, X. Rice-soft shell turtle coculture effects on yield and its environment. Agric. Ecosyst. Environ. 2016, 224, 116–122. [Google Scholar] [CrossRef]
- de Faria, M.; Wraight, S.P. Mycoinsecticides and mycoacaricides: A comprehensive list with worldwide coverage and international classification of formulation types. Biol. Control 2007, 43, 237–256. [Google Scholar] [CrossRef]
- Feng, M.G.; Pu, X.Y. Time-concentration-mortality modeling of the synergistic interaction of Beauveria bassiana and imidacloprid against Nilaparvata lugens. Pest Manag. Sci. 2005, 61, 363–370. [Google Scholar] [CrossRef]
- Jin, S.F.; Feng, M.G.; Ying, S.H.; Mu, W.J.; Chen, J.Q. Evaluation of alternative rice planthopper control by the combined action of oil-formulated Metarhizium anisopliae and low-rate buprofezin. Pest Manag. Sci. 2011, 67, 36–43. [Google Scholar] [CrossRef]
- Lee, S.J.; Yu, J.S.; Nai, Y.S.; Parker, B.L.; Skinner, M.; Kim, J.S. Beauveria bassiana sensulato granules for management of brown planthopper, Nilaparvata lugens in rice. BioControl 2015, 60, 263–270. [Google Scholar] [CrossRef]
- Peng, Y.F.; Tang, J.F.; Hong, M.S.; Xie, J.Q. Suppression of rice planthopper populations by the entomopathogenic fungus Metarhizium anisopliae without affecting the rice microbiota. Appl. Environ. Microbiol. 2020, 86, e01337-20. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.F.; Liu, X.Y.; Ding, Y.C.; Jiang, W.J.; Xie, J.Q. Evaluation of Metarhizium anisopliae for rice planthopper control and its synergy with selected insecticides. Crop Prot. 2019, 121, 132–138. [Google Scholar] [CrossRef]
- Peng, G.X.; Xie, J.Q.; Guo, R.; Keyhani, N.O.; Zeng, D.Y.; Yang, P.Y.; Xia, Y.X. Long-term field evaluation and large-scale application of a Metarhizium anisopliae strain for controlling major rice pests. J. Pest Sci. 2021, 94, 969–980. [Google Scholar] [CrossRef]
- Fernandes, E.K.K.; Rangel, D.E.N.; Braga, G.U.L.; Roberts, D.W. Tolerance of entomopathogenic fungi to ultraviolet radiation: A review on screening of strains and their formulation. Curr. Genet. 2015, 61, 427–440. [Google Scholar] [CrossRef] [PubMed]
- Jaronski, S.T. Ecological factors in the inundative use of fungal entomopathogens. BioControl 2010, 55, 159–185. [Google Scholar] [CrossRef]
- Tong, S.M.; Feng, M.G. Phenotypic and molecular insights into heat tolerance of formulated cells as active ingredients of fungal insecticides. Appl. Microbiol. Biotechnol. 2020, 104, 5711–5724. [Google Scholar] [CrossRef]
- Tong, S.M.; Feng, M.G. Molecular basis and regulatory mechanisms underlying fungal insecticides’ resistance to solar ultraviolet irradiation. Pest Manag. Sci. 2022, 78, 30–42. [Google Scholar] [CrossRef]
- Huang, B.F.; Feng, M.G. Comparative tolerances of various Beauveria bassiana isolates to UV-B irradiation with a description of a modeling method to assess lethal dose. Mycopathologia 2009, 168, 145–152. [Google Scholar] [CrossRef]
- Yu, L.; Xu, S.Y.; Tong, S.M.; Ying, S.H.; Feng, M.G. Optional strategies for low-risk and non-risk applications of fungal pesticides to avoid solar ultraviolet damage. Pest Manag. Sci. 2022, 78, 4660–4667. [Google Scholar] [CrossRef]
- Bateman, R. Methods of application of microbial pesticide formulations for the control of grasshoppers and locusts. Mem. Entomol. Soc. Can. 1997, 171, 69–81. [Google Scholar] [CrossRef]
- Malsam, O.; Kilian, M.; Oerke, E.C.; Dehne, H.W. Oils for increased efficacy of Metarhizium anisopliae to control whiteflies. Biocontrol Sci. Technol. 2002, 12, 337–348. [Google Scholar] [CrossRef]
- Poprawski, T.J.; Parker, P.E.; Tsai, J.H. Laboratory and field evaluation of hyphomycete insect pathogenic fungi for control of brown citrus aphid (Homoptera: Aphididae). Environ. Entomol. 1999, 28, 315–321. [Google Scholar] [CrossRef]
- Vandenberg, J.D.; Sandvol, L.E.; Jaronski, S.T.; Jackson, M.A.; Souza, E.J.; Halbert, S.E. Efficacy of fungi for control of Russian wheat aphid (Homoptera: Aphididae) in irrigated wheat. Southwest. Entomol. 2001, 26, 73–85. [Google Scholar]
- Wraight, S.P.; Carruthers, R.I.; Jaronski, S.T.; Bradley, C.A.; Garza, C.J.; Galaini-Wraight, S. Evaluation of the entomopathogenic fungi Beauveria bassiana and Paecilomyces fumosoroseus for microbial control of the silverleaf whitefly, Bemisia argentifolii. Biol. Control 2000, 17, 203–217. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, W.-Y.; Wen, Z.-X.; Li, X.-J.; Hu, E.-Z.; Qi, D.-Y.; Feng, M.-G.; Tong, S.-M. Timing of Fungal Insecticide Application to Avoid Solar Ultraviolet Irradiation Enhances Field Control of Rice Planthoppers. Insects 2023, 14, 307. https://doi.org/10.3390/insects14040307
Xu W-Y, Wen Z-X, Li X-J, Hu E-Z, Qi D-Y, Feng M-G, Tong S-M. Timing of Fungal Insecticide Application to Avoid Solar Ultraviolet Irradiation Enhances Field Control of Rice Planthoppers. Insects. 2023; 14(4):307. https://doi.org/10.3390/insects14040307
Chicago/Turabian StyleXu, Wan-Ying, Zhen-Xin Wen, Xin-Jie Li, En-Ze Hu, Dan-Yi Qi, Ming-Guang Feng, and Sen-Miao Tong. 2023. "Timing of Fungal Insecticide Application to Avoid Solar Ultraviolet Irradiation Enhances Field Control of Rice Planthoppers" Insects 14, no. 4: 307. https://doi.org/10.3390/insects14040307
APA StyleXu, W.-Y., Wen, Z.-X., Li, X.-J., Hu, E.-Z., Qi, D.-Y., Feng, M.-G., & Tong, S.-M. (2023). Timing of Fungal Insecticide Application to Avoid Solar Ultraviolet Irradiation Enhances Field Control of Rice Planthoppers. Insects, 14(4), 307. https://doi.org/10.3390/insects14040307






