Effect of Different Host Plants on the Diversity of Gut Bacterial Communities of Spodoptera frugiperda (J. E. Smith, 1797)
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect Rearing and Sample Collection
2.2. DNA Extraction, Library Preparation, and High-Throughput Sequencing
2.3. Statistical and Bioinformatics Analysis
3. Results
3.1. General Profile of 16S rRNA Sequencing Data
3.2. Comparison of the Bacterial Communities
3.3. Functional Prediction of Microbiota
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shin, S.C.; Kim, S.H.; You, H.; Kim, B.; Kim, A.C.; Lee, K.A.; Yoon, J.H.; Ryu, J.H.; Lee, W.J. Drosophila microbiome modulates host developmental and metabolic homeostasis via insulin signaling. Science 2011, 334, 670–674. [Google Scholar] [CrossRef]
- Douglas, A.E. Multiorganismal insects: Diversity and function of resident microorganisms. Annu. Rev. Entomol. 2015, 60, 17. [Google Scholar] [CrossRef]
- Cazemier, A.E.; Hackstein, J.; Camp, H.; Rosenberg, J.; Drift, C. Bacteria in the intestinal tract of different species of Arthropods. Microb. Ecol. 1997, 33, 189–197. [Google Scholar] [CrossRef]
- Baumann, P.; Baumann, L.; Lai, C.Y.; Rouhbakhsh, D.; Moran, N.A.; Clark, M.A. Genetics, physiology, and evolutionary relationships of the genus Buchnera: Intracellular symbionts of aphids. Annu. Rev. Microbiol. 2003, 49, 55–94. [Google Scholar] [CrossRef] [PubMed]
- Zientz, E.; Dandekar, T.; Gross, R. Metabolic interdependence of obligate intracellular bacteria and their insect hosts. Microbiol. Mol. Biol. Rev. 2004, 68, 745–770. [Google Scholar] [CrossRef]
- Hedges, L.M.; Brownlie, J.C.; Neill, S.L.O.; Johnson, K.N. Wolbachia and virus protection in insects. Science 2008, 322, 702. [Google Scholar] [CrossRef] [PubMed]
- Mahadav, A.; Gerling, D.; Gottlieb, Y.; Czosnek, H.; Ghanim, M. Parasitization by the wasp Eretmocerus mundus induces transcription of genes related to immune response and symbiotic bacteria proliferation in the whitefly Bemisia tabaci. BMC Genom. 2008, 9, 342. [Google Scholar] [CrossRef]
- Stouthamer, R.; Breeuwer, J.A.; Hurst, G.D. Wolbachia pipientis: Microbial manipulator of arthropod reproduction. Annu. Rev. Microbiol. 1999, 53, 71–102. [Google Scholar] [CrossRef]
- Kikuchi, Y.; Hayatsu, M.; Hosokawa, T.; Nagayama, A.; Tago, K.; Fukatsu, T. Symbiont-mediated insecticide resistance. Proc. Natl. Acad. Sci. USA 2012, 109, 8618–8622. [Google Scholar] [CrossRef] [PubMed]
- Duan, R.; Xu, H.; Gao, S.; Gao, Z.; Wang, N. Effects of different hosts on bacterial communities of parasitic wasp nasonia vitripennis. Front. Microbiol. 2020, 11, 1435. [Google Scholar] [CrossRef]
- Li, H.; Zhao, C.; Yang, Y.; Zhou, Z.; Qi, J.; Li, C. The influence of gut microbiota on the fecundity of Henosepilachna vigintioctopunctata (Coleoptera: Coccinellidae). J. Insect Sci. 2021, 21, 15. [Google Scholar] [CrossRef]
- Chen, B.; Du, K.; Sun, C.; Vimalanathan, A.; Liang, X.; Li, Y.; Wang, B.; Lu, X.; Li, L.; Shao, Y. Gut bacterial and fungal communities of the domesticated silkworm (Bombyx mori) and wild mulberry-feeding relatives. ISME J. 2018, 12, 2252–2262. [Google Scholar] [CrossRef]
- Wang, Z.L.; Wang, T.Z.; Zhu, H.F.; Pan, H.B.; Yu, X.P. Diversity and dynamics of microbial communities in brown planthopper at different developmental stages revealed by high-throughput amplicon sequencing. Insect Sci. 2020, 27, 883–894. [Google Scholar] [CrossRef]
- Wang, X.; Sun, S.; Yang, X.; Cheng, J.; Liu, X. Variability of gut microbiota across the life cycle of Grapholita molesta (Lepidoptera: Tortricidae). Front. Microbiol. 2020, 11, 1366. [Google Scholar] [CrossRef]
- Liu, Y.; Shen, Z.; Yu, J.; Li, Z.; Liu, X.; Xu, H. Comparison of gut bacterial communities and their associations with host diets in four fruit borers. Pest Manag. Sci. 2019, 76, 1353–1362. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Li, W.; Luo, J.; Zhang, L.J.; Cui, J. Biodiversity of the microbiota in Spodoptera exigua (Lepidoptera: Noctuidae). J. Appl. Microbiol. 2018, 126, 1199–1208. [Google Scholar] [CrossRef]
- Jones, A.G.; Mason, C.J.; Felton, G.W.; Hoover, K. Host plant and population source drive diversity of microbial gut communities in two polyphagous insects. Sci. Rep. 2019, 9, 2792. [Google Scholar] [CrossRef]
- Zhang, B.; Leonard, S.P.; Li, Y.; Moran, N.A. Obligate bacterial endosymbionts limit thermal tolerance of insect host species. Proc. Natl. Acad. Sci. USA 2019, 116, 24712–24718. [Google Scholar] [CrossRef]
- Wang, G.; Dittmer, J.; Douglas, B.; Huang, L.; Brucker, R.M. Coadaptation between host genome and microbiome under long-term xenobiotic-induced selection. Sci. Adv. 2021, 7, abd4473. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.Y.; Saqib, H.S.A.; Chen, J.H.; Ruan, Q.Q.; You, M.S. Differential profiles of gut microbiota and metabolites associated with host shift of Plutella xylostella. Int. J. Mol. Sci. 2020, 21, 6283. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Zhang, X.; Liu, X.; Dong, Y.; Yan, Z.; Lv, D.; Wang, P.; Li, Y. Comparison of gut bacterial communities of Grapholita molesta (Lepidoptera: Tortricidae) reared on different host plants. Int. J. Mol. Sci. 2021, 22, 6843. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.M.; Choi, M.Y.; Kim, J.W.; Lee, S.A.; Ahn, J.H.; Song, J.; Kim, S.H.; Weon, H.Y. Effects of diet type, developmental stage, and gut compartment in the gut bacterial communities of two Cerambycidae species (Coleoptera). J. Microbiol. 2017, 55, 21–30. [Google Scholar] [CrossRef]
- Todd, E.L.; Poole, R.W. Keys and illustrations for the armyworm moths of the noctuid genus Spodoptera Guenée from the western hemisphere. Ann. Entomol. Soc. Am. 1980, 6, 722–738. [Google Scholar] [CrossRef]
- Goergen, G.; Kumar, P.L.; Sankung, S.B.; Togola, A.; Tamò, M.; Luthe, D.S. First report of outbreaks of the fall armyworm Spodoptera frugiperda (J E Smith) (Lepidoptera, Noctuidae), a new alien invasive pest in west and central africa. PLoS ONE 2016, 11, e0165632. [Google Scholar] [CrossRef]
- Midega, C.; Pittchar, J.O.; Pickett, J.A.; Hailu, G.W.; Khan, Z.R. A climate-adapted push-pull system effectively controls fall armyworm, Spodoptera frugiperda (J E Smith), in maize in East Africa. Crop Prot. 2018, 105, 10–15. [Google Scholar] [CrossRef]
- Sun, X.X.; Chao-Xing, H.U.; Jia, H.R.; Qiu-Lin, W.U.; Shen, X.J.; Zhao, S.Y.; Jiang, Y.Y.; Kong-Ming, W.U. Case study on the first immigration of fall armyworm, Spodoptera frugiperda invading into China. J. Integr. Agric. 2021, 20, 664–672. [Google Scholar] [CrossRef]
- Montezano, D.G.; Specht, A.; Sosa-Gómez, D.R.; Roque-Specht, V.F.; Hunt, T. Host Plants of Spodoptera frugiperda (Lepidoptera: Noctuidae) in the Americas. Afr. Entomol. 2018, 26, 286–300. [Google Scholar] [CrossRef]
- Gopalakrishnan, R.; Kalia, V.K. Biology and biometric characteristics of Spodoptera frugiperda (Lepidoptera: Noctuidae) reared on different host plants with regard to diet. Pest Manag. Sci. 2022, 78, 2043–2051. [Google Scholar] [CrossRef] [PubMed]
- Li-Mei, H.E.; Qiu-Lin, W.U.; Gao, X.W.; Kong-Ming, W.U. Population life tables for the invasive fall armyworm, Spodoptera frugiperda fed on major oil crops planted in China. J. Integr. Agric. 2021, 20, 745–754. [Google Scholar]
- Han, S.P.; Zhou, Y.Y.; Wang, D.; Qin, Q.J.; Song, P.; He, Y.Z. Impact of host plants on biological characteristics and Vg/VgR expression of Spodoptera frugiperda. J. Pest Sci. 2022. [Google Scholar] [CrossRef]
- Xia, X.; Lan, B.; Tao, X.; Lin, J.; You, M. Characterization of Spodoptera litura gut bacteria and their role in feeding and growth of the host. Front. Microbiol. 2020, 11, 1492. [Google Scholar] [CrossRef] [PubMed]
- Mason, C.J.; Clair, A.S.; Peiffer, M.; Gomez, E.; Hoover, K. Diet influences proliferation and stability of gut bacterial populations in herbivorous lepidopteran larvae. PLoS ONE 2020, 15, e0229848. [Google Scholar] [CrossRef] [PubMed]
- Lv, D.; Liu, X.; Dong, Y.; Yan, Z.; Zhang, X.; Wang, P.; Yuan, X.; Li, Y. Comparison of Gut Bacterial Communities of Fall Armyworm (Spodoptera frugiperda) Reared on Different Host Plants. Int. J. Mol. Sci. 2021, 22, 11266. [Google Scholar] [CrossRef] [PubMed]
- Mago, T.; Salzberg, S.L. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, 884–890. [Google Scholar] [CrossRef]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996. [Google Scholar] [CrossRef]
- Wang, Q. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef]
- Schloss, P.D.; Westcott, S.L.; Ryabin, T.; Hall, J.R.; Hartmann, M.; Hollister, E.B.; Lesniewski, R.A.; Oakley, B.B.; Parks, D.H.; Robinson, C.J. Introducing mothur: Open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl. Environ. Microbiol. 2009, 75, 7537. [Google Scholar] [CrossRef]
- Zhang, X.; Li, T.P.; Zhou, C.Y.; Zhao, D.S.; Zhu, Y.X.; Bing, X.L.; Huang, H.J.; Hong, X.Y. Antibiotic exposure perturbs the bacterial community in the small brown planthopper Laodelphax striatellus. Insect Sci. 2020, 27, 895–907. [Google Scholar] [CrossRef]
- Douglas, G.M.; Maffei, V.J.; Zaneveld, J.R.; Yurgel, S.N.; Brown, J.R.; Taylor, C.M.; Huttenhower, C.; Langille, M. PICRUSt2 for prediction of metagenome functions. Nat. Biotechnol. 2020, 38, 685–688. [Google Scholar] [CrossRef] [PubMed]
- Langille, M.; Zaneveld, J.; Caporaso, J.G.; Mcdonald, D.; Knights, D.; Reyes, J.A.; Clemente, J.C.; Burkepile, D.E.; Thurber, R.V.; Knight, R. Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nat. Biotechnol. 2013, 31, 814–821. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.; Zheng, D.; Zhong, H.; Qin, B.; Gurr, G.M.; Vasseur, L.; Lin, H.; Bai, J.; He, W.; You, M. DNA sequencing reveals the midgut microbiota of diamondback moth, Plutella xylostella (L.) and a possible relationship with insecticide resistance. PLoS ONE 2013, 8, e68852. [Google Scholar] [CrossRef]
- Chen, B.; Teh, B.S.; Sun, C.; Hu, S.; Lu, X.; Boland, W.; Shao, Y. Biodiversity and activity of the gut microbiota across the life history of the insect herbivore Spodoptera littoralis. Sci. Rep. 2016, 6, 29505. [Google Scholar] [CrossRef]
- Gao, X.; Li, W.; Luo, J.; Zhang, L.; Ji, J.; Zhu, X.; Wang, L.; Zhang, K.; Zhang, S.; Cui, J. DNA sequencing reveals bacterial communities in midgut and other parts of the larvae of Spodoptera exigua Hubner (Lepidoptera: Noctuidae). FEMS Microbiol. Lett. 2020, 367, fnaa002. [Google Scholar] [CrossRef]
- Ley, R.E. Evolution of mammals and their gut microbes. Science 2008, 320, 1647–1651. [Google Scholar] [CrossRef] [PubMed]
- Warnecke, F.; Luginbühl, P.; Ivanova, N.; Ghassemian, M.; Richardson, T.H.; Stege, J.T.; Cayouette, M.; Mchardy, A.C.; Djordjevic, G.; Aboushadi, N. Metagenomic and functional analysis of hindgut microbiota of a wood-feeding higher termite. Nature 2007, 450, 560–565. [Google Scholar] [CrossRef] [PubMed]
- Philipp, E.; Moran, N.A. The gut microbiota of insects—Diversity in structure and function. FEMS Microbiol. Rev. 2013, 37, 699–735. [Google Scholar]
- Xu, J.; Mahowald, M.A.; Ley, R.E.; Lozupone, C.A.; Micah, H.; Martens, E.C.; Bernard, H.; Coutinho, P.M.; Patrick, M.; Philippe, L. Evolution of symbiotic bacteria in the distal human intestine. PLoS Biol. 2007, 5, e156. [Google Scholar] [CrossRef]
- Ptaszyńska, A.A.; Latoch, P.; Hurd, P.J.; Polaszek, A.; Michalska-Madej, J.; Grochowalski, Ł.; Strapagiel, D.; Gnat, S.; Załuski, D.; Gancarz, M.; et al. Amplicon Sequencing of Variable 16S rRNA from Bacteria and ITS2 Regions from Fungi and Plants, Reveals Honeybee Susceptibility to Diseases Results from Their Forage Availability under Anthropogenic Landscapes. Pathogens 2021, 10, 381. [Google Scholar] [CrossRef]
- Hadapad, A.B.; Shettigar, S.K.G.; Hire, R.S. Bacterial communities in the gut of wild and mass-reared Zeugodacus cucurbitae and Bactrocera dorsalis revealed by metagenomic sequencing. BMC Microbiol. 2019, 19, 282. [Google Scholar] [CrossRef]
- Hui, X.; Wei, G.F.; Jia, S.H.; Huang, J.H.; Miao, X.X.; Zhou, Z.H.; Zhao, L.P.; Huang, Y.P. Microbial communities in the larval midgut of laboratory and field populations of cotton bollworm (Helicoverpa armigera). Can. J. Microbiol. 2006, 52, 1085–1092. [Google Scholar]
- Brinkmann, N.; Martens, R.; Tebbe, C.C. Origin and diversity of metabolically active gut bacteria from laboratory-bred larvae of Manduca sexta (Sphingidae, Lepidoptera, Insecta). Appl. Environ. Microb. 2008, 74, 7189–7196. [Google Scholar] [CrossRef]
- Shao, Y.; Chen, B.; Sun, C.; Ishida, K.; Hertweck, C.; Boland, W. Symbiont-derived antimicrobials contribute to the control of the Lepidopteran gut microbiota. Cell Chem. Biol. 2017, 24, 66–75. [Google Scholar] [CrossRef]
- Shao, Y.; Spiteller, D.; Tang, X.; Ping, L.; Colesie, C. Crystallization of alpha- and beta-carotene in the foregut of Spodoptera larvae feeding on a toxic food plant. Insect Biochem. Mol. Biol. 2011, 41, 273–281. [Google Scholar] [CrossRef]
- Cristina, V.; Joaquín, B.; Amparo, L.; Manuel, P. The generalist inside the specialist: Gut bacterial communities of two insect species feeding on toxic plants are dominated by Enterococcus sp. Front. Microbiol. 2016, 7, 1005. [Google Scholar]
- Paniagua, V.L.R.; Enric, F.; Martin, K.; Monika, H.; Fatouros, N.E. Bacterial symbionts in Lepidoptera: Their diversity, transmission, and impact on the host. Front. Microbiol. 2018, 9, 556. [Google Scholar] [CrossRef] [PubMed]
- Fatani, S.; Saito, Y.; Alarawi, M.; Gojobori, T.; Mineta, K. Genome sequencing and identification of cellulase genes in Bacillus paralicheniformis strains from the Red Sea. BMC Microbiol. 2021, 21, 254. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, F.; Xing, D.; Wagner, R.; Regan, J.M.; Richard, T.L.; Logan, B.E. Simultaneous cellulose degradation and electricity production by Enterobacter cloacae in a microbial fuel cell. Appl. Environ. Microb. 2009, 75, 3673–3678. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.C.; Spencer, J.L.; Curzi, M.J.; Zavala, J.A.; Seufferheld, M.J. Gut bacteria facilitate adaptation to crop rotation in the western corn rootworm. Proc. Natl. Acad. Sci. USA 2013, 110, 11917–11922. [Google Scholar] [CrossRef]
- Suen, G.; Scott, J.J.; Aylward, F.O.; Adams, S.M.; Tringe, S.G.; Pinto-Tomas, A.A.; Foster, C.E.; Pauly, M.; Weimer, P.J.; Barry, K.W.; et al. An insect herbivore microbiome with high plant biomass-degrading capacity. PLoS Genet. 2010, 6, e1001129. [Google Scholar] [CrossRef] [PubMed]
- Acevedo, F.E.; Peiffer, M.; Tan, C.W.; Stanley, B.A.; Stanley, A.; Wang, J.; Jones, A.G.; Hoover, K.; Rosa, C.; Luthe, D. Fall armyworm-associated gut bacteria modulate plant defense responses. Mol. Plant Microbe. Interact. 2017, 30, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Nuri, N.; Lauzon, C.R.; Shelly, T.E. Effect of probiotic adult diets on fitness components of sterile male mediterranean fruit flies (Diptera: Tephritidae) under laboratory and field cage conditions. J. Econ. Entomol. 2004, 97, 1570–1580. [Google Scholar]

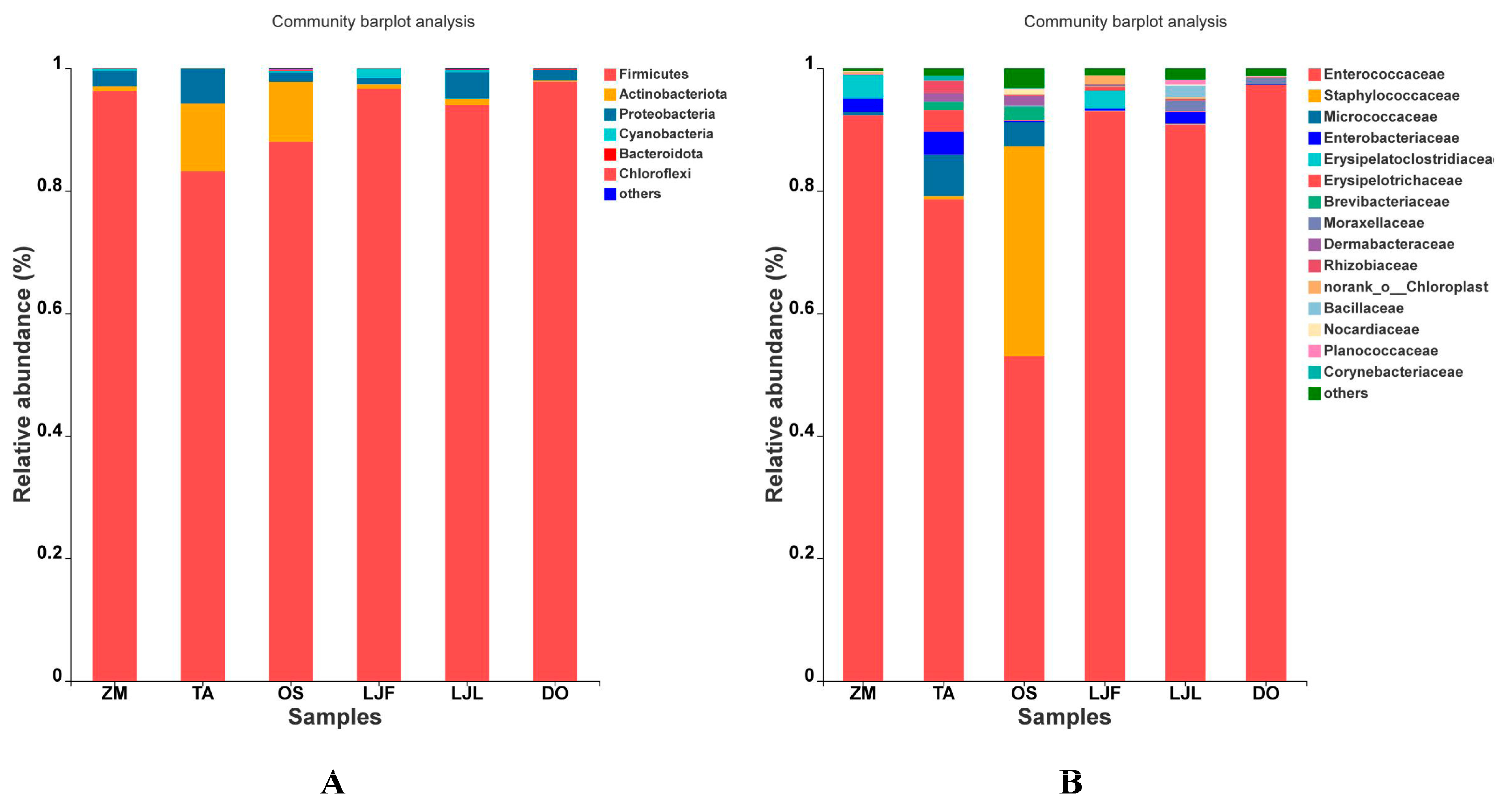
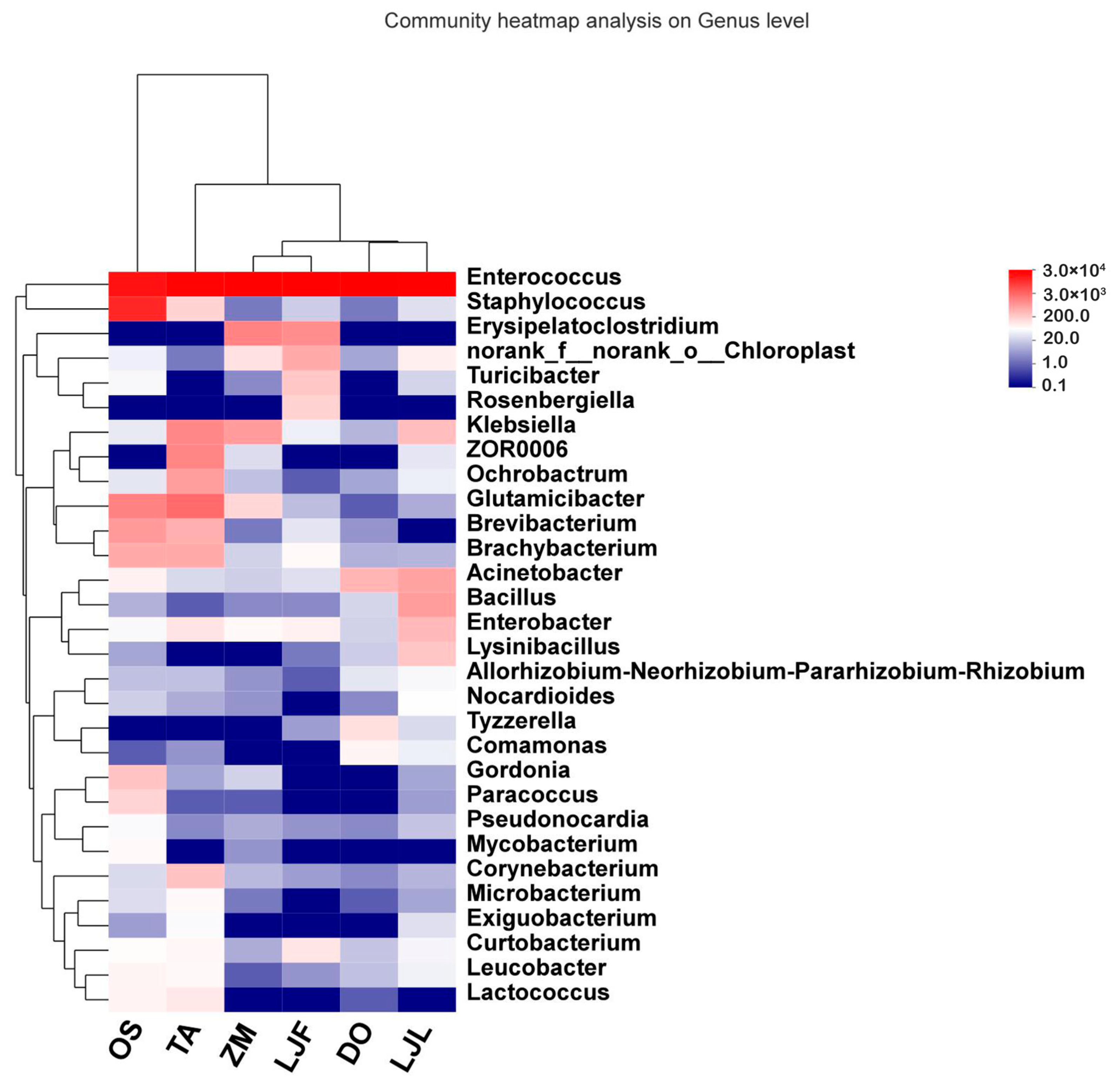

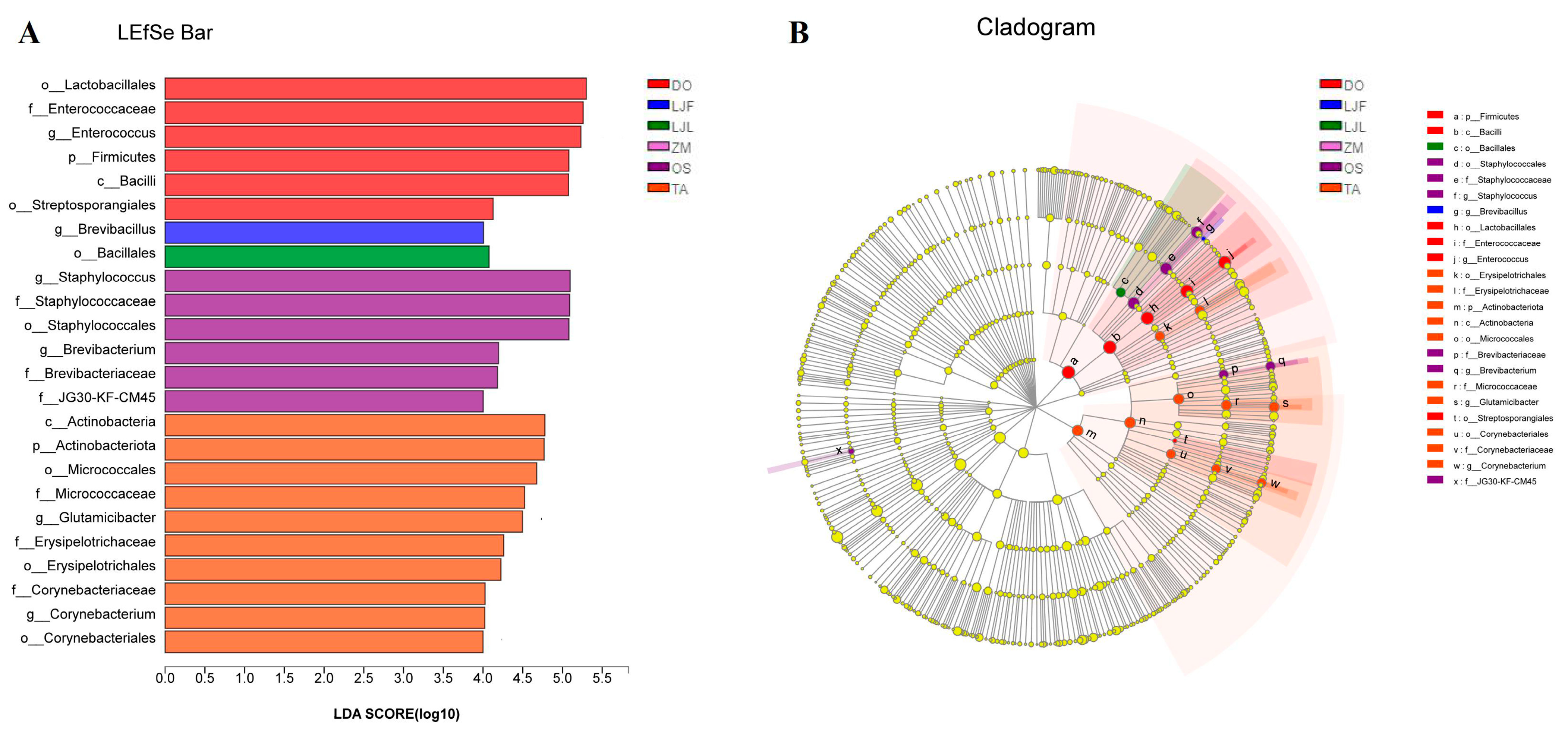
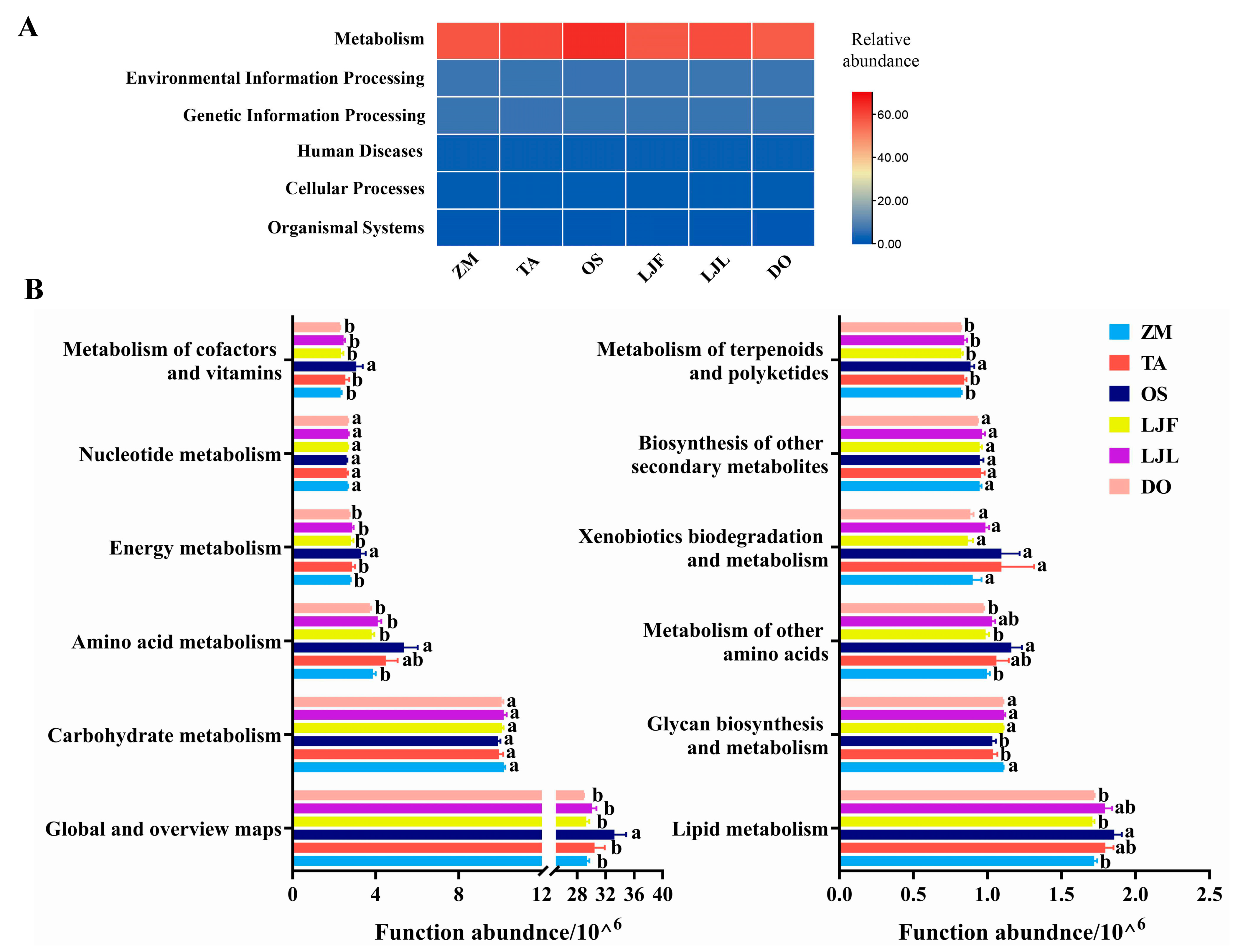
| Taxonomy | Group | ZM | TA | OS | LJF | LJL | DO | F-Value | p-Value |
|---|---|---|---|---|---|---|---|---|---|
| Phylum | Firmicutes | 98.39 ± 1.21 a | 80.80 ± 12.71 b | 83.93 ± 2.47 ab | 96.68 ± 4.68 a | 94.04 ± 0.90 ab | 98.97 ± 0.44 a | 5.731 | 0.006 |
| Actinobacteriota | 0.37 ± 0.20 b | 13.33 ± 3.89 a | 11.90 ± 2.26 a | 0.12 ± 0.06 b | 0.51 ± 0.16 b | 0.25 ± 0.08 b | 35.936 | <0.001 | |
| Proteobacteria | 0.79 ± 0.10 b | 0.40 ± 0.15 b | 0.75 ± 0.38 b | 0.47 ± 0.27 b | 5.17 ± 0.87 a | 0.69 ± 0.47 b | 51.616 | <0.001 | |
| Cyanobacteria | 0.54 ± 0.31 a | 0.02 ± 0.01 b | 0.14 ± 0.21 ab | 0.02 ± 0.02 b | 0.03 ± 0.05 b | 0.01 ± 0.01 b | 5.565 | 0.007 | |
| Bacteroidota | 0.04 ± 0.03 bc | 0.01 ± 0.01 c | 0.57 ± 0.13 a | 0.01 ± 0.01 bc | 0.19 ± 0.07 b | 0.07 ± 0.05 bc | 34.112 | <0.001 | |
| Family | Enterococcaceae | 92.35 ± 5.94 a | 82.25 ± 6.20 ab | 68.50 ± 9.75 b | 92.97 ± 5.94 a | 90.80 ± 0.54 a | 97.48 ± 2.07 a | 9.537 | 0.001 |
| Staphylococcaceae | 0.01 ± 0.00 b | 0.62 ± 0.44 b | 34.31 ± 11.58 a | 0.03 ± 0.02 b | 0.05 ± 0.01 b | 0.01 ± 0.00 b | 26.099 | <0.001 | |
| Micrococcaceae | 0.17 ± 0.16 b | 4.62 ± 0.74 a | 3.84 ± 2.01 a | 0.05 ± 0.02 b | 0.01 ± 0.00 b | 0.01 ± 0.00 b | 18.391 | <0.001 | |
| Enterobacteriaceae | 0.53 ± 0.23 b | 0.22 ± 0.03 bc | 0.34 ± 0.08 bc | 0.35 ± 0.23 bc | 2.38 ± 0.09 a | 0.04 ± 0.04 c | 110.248 | <0.001 | |
| Erysipelatoclostridiaceae | 0.09 ± 0.01 b | 0.74 ± 0.11 a | 0.21 ± 0.06 b | 0.01 ± 0.00 b | 0.14 ± 0.23 b | 0.00 ± 0.00 b | 20.716 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, S.; Zhou, Y.; Wang, D.; Qin, Q.; Song, P.; He, Y. Effect of Different Host Plants on the Diversity of Gut Bacterial Communities of Spodoptera frugiperda (J. E. Smith, 1797). Insects 2023, 14, 264. https://doi.org/10.3390/insects14030264
Han S, Zhou Y, Wang D, Qin Q, Song P, He Y. Effect of Different Host Plants on the Diversity of Gut Bacterial Communities of Spodoptera frugiperda (J. E. Smith, 1797). Insects. 2023; 14(3):264. https://doi.org/10.3390/insects14030264
Chicago/Turabian StyleHan, Shipeng, Yayuan Zhou, Da Wang, Qiuju Qin, Peng Song, and Yunzhuan He. 2023. "Effect of Different Host Plants on the Diversity of Gut Bacterial Communities of Spodoptera frugiperda (J. E. Smith, 1797)" Insects 14, no. 3: 264. https://doi.org/10.3390/insects14030264
APA StyleHan, S., Zhou, Y., Wang, D., Qin, Q., Song, P., & He, Y. (2023). Effect of Different Host Plants on the Diversity of Gut Bacterial Communities of Spodoptera frugiperda (J. E. Smith, 1797). Insects, 14(3), 264. https://doi.org/10.3390/insects14030264






