Alpha-Cypermethrin Resistance in Musca domestica: Resistance Instability, Realized Heritability, Risk Assessment, and Insecticide Cross-Resistance
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Collection and Rearing of M. domestica
2.3. Selection of M. domestica with Alpha-Cypermethrin
2.4. Bioassay of Adults
2.5. Bioassay of Larvae
2.6. Alpha-Cypermethrin Resistance Stability in M. domestica
2.7. h2 Values for Alpha-Cypermethrin Resistance
2.8. Bioassay Data Analyses
3. Results
3.1. Alpha-Cypermethrin Resistance Selection in Alpha-Sel
3.2. Stability of Alpha-Cypermethrin Resistance in M. domestica
3.3. h2 of Alpha-Cypermethrin Resistance in M. domestica
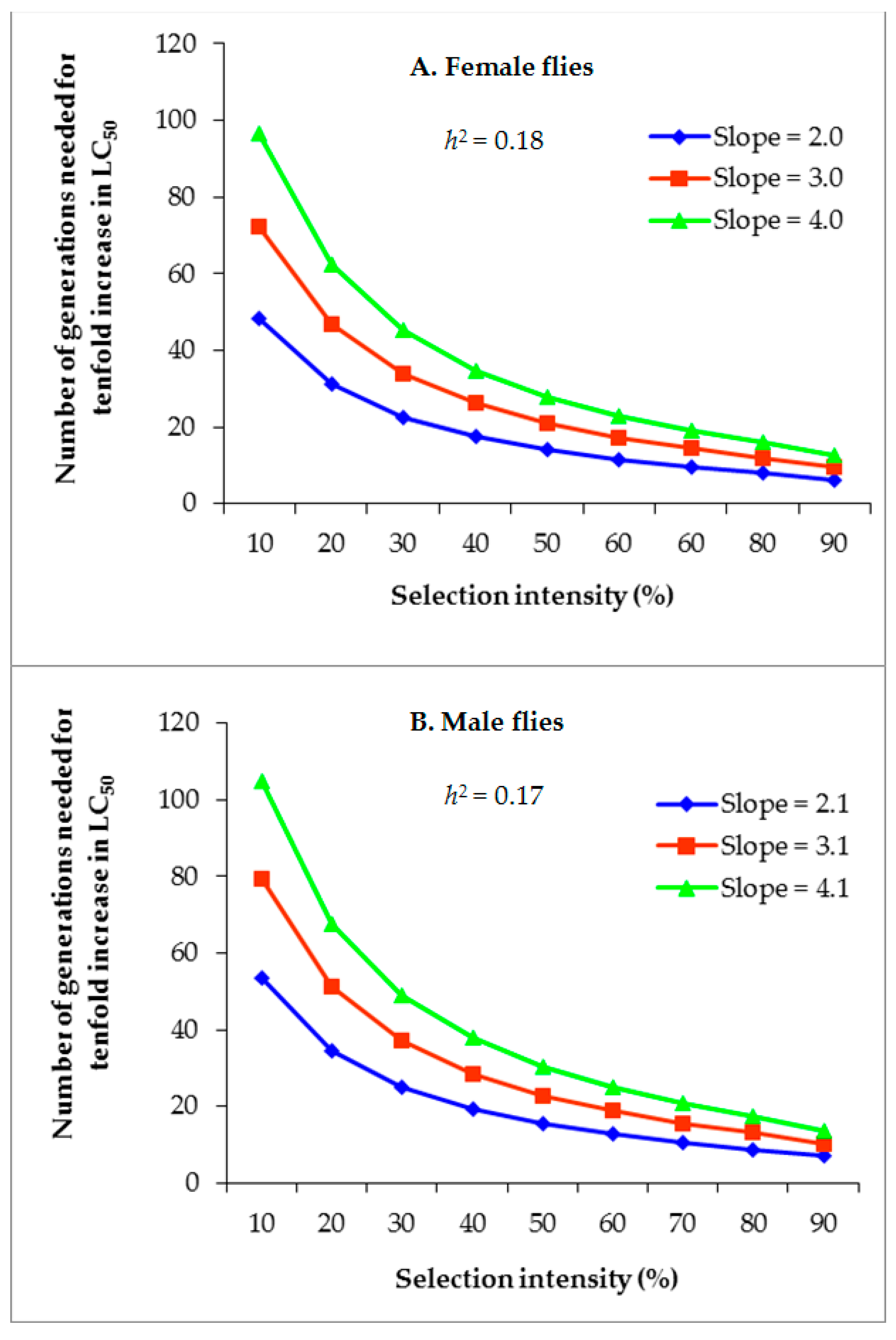
3.4. Projected Rate of Alpha-Cypermethrin Resistance Development
3.5. Cross-Resistance (CR) Patterns
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Narahashi, T.; Zhao, X.; Ikeda, T.; Nagata, K.; Yeh, J. Differential actions of insecticides on target sites: Basis for selective toxicity. Hum. Exp. Toxicol. 2007, 26, 361–366. [Google Scholar] [CrossRef]
- Abbas, N.; Khan, H.A.A.; Shad, S.A. Resistance of the house fly Musca domestica (Diptera: Muscidae) to lambda-cyhalothrin: Mode of inheritance, realized heritability, and cross-resistance to other insecticides. Ecotoxicology 2014, 23, 791–801. [Google Scholar] [CrossRef]
- Khan, H.A.A.; Akram, W.; Fatima, A. Resistance to pyrethroid insecticides in house flies, Musca domestica L., (Diptera: Muscidae) collected from urban areas in Punjab, Pakistan. Parasitol. Res. 2017, 116, 3381–3385. [Google Scholar] [CrossRef]
- Abbas, N.; Shad, S.A.; Ismail, M. Resistance to conventional and new insecticides in house flies (Diptera: Muscidae) from poultry facilities in Punjab, Pakistan. J. Econ. Entomol. 2015, 108, 826–833. [Google Scholar] [CrossRef] [PubMed]
- Hafez, A.M. First evaluation of field evolved resistance to commonly used insecticides in house fly populations from Saudi Arabian dairy farms. Insects 2021, 12, 1120. [Google Scholar] [CrossRef] [PubMed]
- Abbas, N.; Shad, S.A. Assessment of resistance risk to lambda-cyhalothrin and cross-resistance to four other insecticides in the house fly, Musca domestica L. (Diptera: Muscidae). Parasitol. Res. 2015, 114, 2629–2637. [Google Scholar] [CrossRef] [PubMed]
- Bass, C.; Jones, C. Editorial overview: Pests and resistance: Resistance to pesticides in arthropod crop pests and disease vectors: Mechanisms, models and tools. Curr. Opin. Insect Sci. 2018, 27, 4–7. [Google Scholar] [CrossRef] [PubMed]
- Khan, H.A.A. Characterization of permethrin resistance in a Musca domestica strain: Resistance development, cross-resistance potential and realized heritability. Pest Manag. Sci. 2019, 75, 2969–2974. [Google Scholar] [CrossRef] [PubMed]
- Abbas, N.; Ijaz, M.; Shad, S.A.; Binyameen, M. Assessment of resistance risk to fipronil and cross resistance to other insecticides in the Musca domestica L. (Diptera: Muscidae). Vet. Parasitol. 2016, 223, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Abbas, N.; Abubakar, M.; Hassan, M.W.; Shad, S.A.; Hafez, A.M. Risk assessment of flonicamid resistance in Musca domestica (Diptera: Muscidae): Resistance monitoring, inheritance, and cross-resistance potential. J. Med. Entomol. 2021, 58, 1779–1787. [Google Scholar] [CrossRef]
- Abbas, N.; Khan, H.A.A.; Shad, S.A. Cross-resistance, genetics, and realized heritability of resistance to fipronil in the house fly, Musca domestica (Diptera: Muscidae): A potential vector for disease transmission. Parasitol. Res. 2014, 113, 1343–1352. [Google Scholar] [CrossRef]
- Nielsen, A.A.; Skovgard, H.; Stockmarr, A.; Handberg, K.J.; Jorgensen, P.H. Persistence of low-pathogenic avian influenza H5N7 and H7N1 subtypes in house flies (Diptera: Muscidae). J. Med. Entomol. 2011, 48, 608–614. [Google Scholar] [CrossRef] [PubMed]
- Butler, J.F.; Garcia-Maruniak, A.; Meek, F.; Maruniak, J.E. Wild Florida house flies (Musca domestica) as carriers of pathogenic bacteria. Fla. Entomol. 2010, 93, 218–223. [Google Scholar] [CrossRef]
- Khan, H.A.A.; Shad, S.A.; Akram, W. Effect of livestock manures on the fitness of house fly, Musca domestica L. (Diptera: Muscidae). Parasitol. Res. 2012, 111, 1165–1171. [Google Scholar] [CrossRef] [PubMed]
- Nayduch, D.; Burrus, R.G. Flourishing in filth: House fly–microbe interactions across life history. Ann. Entomol. Soc. Am. 2017, 110, 6–18. [Google Scholar] [CrossRef]
- Abbas, N.; Hafez, A.M. Resistance to insect growth regulators and age-stage, two-sex life table in Musca domestica from different dairy facilities. PLoS ONE 2021, 16, e0248693. [Google Scholar] [CrossRef]
- Malik, A.; Singh, N.; Satya, S. House fly (Musca domestica): A review of control strategies for a challenging pest. J. Environ. Sci. Health B 2007, 42, 453–469. [Google Scholar] [CrossRef] [PubMed]
- Ngufor, C.; Agbevo, A.; Fagbohoun, J.; Fongnikin, A.; Rowland, M. Efficacy of Royal Guard, a new alpha-cypermethrin and pyriproxyfen treated mosquito net, against pyrethroid-resistant malaria vectors. Sci. Rep. 2020, 10, 1–15. [Google Scholar] [CrossRef]
- Pessoa, G.C.D.; Lopes, J.V.; Rocha, M.F.; Pinheiro, L.C.; Rosa, A.C.L.; Michalsky, É.M.; Dias, E.S. Baseline susceptibility to alpha-cypermethrin in Lutzomyia longipalpis (Lutz & Neiva, 1912) from Lapinha Cave (Brazil). Parasit. Vectors 2015, 8, 1–6. [Google Scholar]
- Kampouraki, A.; Stavrakaki, M.; Karataraki, A.; Katsikogiannis, G.; Pitika, E.; Varikou, K.; Vlachaki, A.; Chrysargyris, A.; Malandraki, E.; Sidiropoulos, N. Recent evolution and operational impact of insecticide resistance in olive fruit fly Bactrocera oleae populations from Greece. J. Pest Sci. 2018, 91, 1429–1439. [Google Scholar] [CrossRef]
- Mironidis, G.K.; Kapantaidaki, D.; Bentila, M.; Morou, E.; Savopoulou-Soultani, M.; Vontas, J. Resurgence of the cotton bollworm Helicoverpa armigera in northern Greece associated with insecticide resistance. Insect Sci. 2013, 20, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Margaritopoulos, J.T.; Skavdis, G.; Kalogiannis, N.; Nikou, D.; Morou, E.; Skouras, P.J.; Tsitsipis, J.A.; Vontas, J. Efficacy of the pyrethroid alpha-cypermethrin against Bactrocera oleae populations from Greece, and improved diagnostic for an iAChE mutation. Pest Manag. Sci. 2008, 64, 900–908. [Google Scholar] [CrossRef]
- Zhang, L.; Gao, X.; Liang, P. Beta-cypermethrin resistance associated with high carboxylesterase activities in a strain of house fly, Musca domestica (Diptera: Muscidae). Pestic. Biochem. Physiol. 2007, 89, 65–72. [Google Scholar] [CrossRef]
- Lorn, S.; Klakankhai, W.; Nusen, P.; Sumarnrote, A.; Tainchum, K. Pyrethroid susceptibility in Stomoxys calcitrans and Stomoxys indicus (Diptera: Muscidae) collected from cattle farms in Southern Thailand. Insects 2022, 13, 711. [Google Scholar] [CrossRef]
- Falconer, D.S. Introduction to Quantitative Genetics, 3rd ed.; Longman: London, UK, 1989. [Google Scholar]
- Firkoi, M.J.; Hayes, J.L. Quantitative genetic tools for insecticide resistance risk assessment: Estimating the heritability of resistance. J. Econ. Entomol. 1990, 83, 647–654. [Google Scholar] [CrossRef] [PubMed]
- Haddi, K.; Valbon, W.R.; Viteri Jumbo, L.O.; de Oliveira, L.O.; Guedes, R.N.; Oliveira, E.E. Diversity and convergence of mechanisms involved in pyrethroid resistance in the stored grain weevils, Sitophilus spp. Sci. Rep. 2018, 8, 1–15. [Google Scholar] [CrossRef]
- Ahmad, M.; Sayyed, A.H.; Saleem, M.A.; Ahmad, M. Evidence for field evolved resistance to newer insecticides in Spodoptera litura (Lepidoptera: Noctuidae) from Pakistan. Crop Prot. 2008, 27, 1367–1372. [Google Scholar] [CrossRef]
- Abbas, N.; Khan, H.; Shad, S.A. Cross-resistance, stability, and fitness cost of resistance to imidacloprid in Musca domestica L., (Diptera: Muscidae). Parasitol. Res. 2015, 114, 247–255. [Google Scholar] [CrossRef]
- Hafez, A.M. Risk assessment of resistance to diflubenzuron in Musca domestica: Realized heritability and cross-resistance to fourteen insecticides from different classes. PLoS ONE 2022, 17, e0268261. [Google Scholar] [CrossRef]
- Shah, R.M.; Shad, S.A. Inheritance, stability, cross-resistance, and life history parameters of a clothianidin-selected strain of house fly, Musca domestica Linnaeus. Environ. Pollut. 2021, 278, 116880. [Google Scholar] [CrossRef]
- Tabashnik, B.E.; Groeters, F.R.; Finson, N.; Johnson, M.W. Instability of resistance to Bacillus thuringiensis. Biocontrol Sci. Technol. 1994, 4, 419–426. [Google Scholar] [CrossRef]
- Tabashnik, B.E. Resistance risk assessment: Realized heritability of resistance to Bacillus thuringiensis in diamondback moth (Lepidoptera: Plutellidae), tobacco budworm (Lepidoptera: Noctuidae), and Colorado potato beetle (Coleoptera: Chrysomelidae). J. Econ. Entomol. 1992, 85, 1551–1559. [Google Scholar] [CrossRef]
- Tabashnik, B.E.; McGaughey, W.H. Resistance risk assessment for single and multiple insecticides: Responses of Indianmeal moth (Lepidoptera: Pyralidae) to Bacillus thuringiensis. J. Econ. Entomol. 1994, 87, 834–841. [Google Scholar] [CrossRef]
- Finney, D. A Statistical Treatment of the Sigmoid Response Curve. In Probit Analysis, 3rd ed.; Cambridge University Press: London, UK, 1971; p. 333. [Google Scholar]
- LeOra, S. Poloplus, a User’s Guide to Probit or Logit Analysis; LeOra Software: Berkeley, CA, USA, 2003. [Google Scholar]
- Abbott, W. A method of computing the effectiveness of an insecticide. J. Econ. Entomol. 1925, 18, 265–267. [Google Scholar] [CrossRef]
- Torres-Vila, L.M.; Rodríguez-Molina, M.C.; Lacasa-Plasencia, A.; Bielza-Lino, P.; Rodríguez-del-Rincón, Á. Pyrethroid resistance of Helicoverpa armigera in Spain: Current status and agroecological perspective. Agric. Ecosyst. Environ. 2002, 93, 55–66. [Google Scholar] [CrossRef]
- Ullah, I.; Wazir, S.; Abbas, N.; Naeem, M.; Abdullah, K.; Mahmood, Z.; Rashid, M.-u.; Hafez, A.M. Monitoring of field-evolved resistance to flonicamid, neonicotinoid, and conventional insecticides in the Oxycarenus hyalinipennis costa. Environ. Monit. Assess. 2021, 193, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Khan, H.A.A.; Akram, W.; Haider, M.S. Genetics and mechanism of resistance to deltamethrin in the house fly, Musca domestica L., from Pakistan. Ecotoxicology 2015, 1–8. [Google Scholar] [CrossRef]
- Prasad, T.P.N.H.; Shetty, N.J. Autosomal inheritance of alphamethrin, a synthetic pyrethroid, resistance in Anopheles stephensi-Liston, a malaria mosquito. Bull. Entomol. Res. 2013, 103, 547. [Google Scholar] [CrossRef]
- Rodríguez-Hidalgo, R.; Pérez-Otáñez, X.; Garcés-Carrera, S.; Vanwambeke, S.O.; Madder, M.; Benítez-Ortiz, W. The current status of resistance to alpha-cypermethrin, ivermectin, and amitraz of the cattle tick (Rhipicephalus microplus) in Ecuador. PLoS ONE 2017, 12, e0174652. [Google Scholar] [CrossRef]
- Emre, Ö.; Çetin, H.; YANIKOĞLU, A. Investigation of resistance to synthetic pyrethroids in Blattella germanica L., 1767 (Blattodea: Ectobiidae) and Periplaneta americana L., 1758 (Blattodea: Blattidae) populations in Turkey. Turk. J. Entomol. 2021, 45, 361–370. [Google Scholar]
- Ijaz, M.; Shad, S.A. Realized heritability, cross-resistance and high risk of resistance development to spirotetramat in dusky cotton bug, Oxycarenus hyalinipennis Costa (Hemiptera: Lygaeidae), an emerging threat to BT cotton in Pakistan. Phytoparasitica 2021, 50, 453–463. [Google Scholar] [CrossRef]
- Mansoor, M.M.; Abbas, N.; Shad, S.A.; Pathan, A.K.; Razaq, M. Increased fitness and realized heritability in emamectin benzoate-resistant Chrysoperla carnea (Neuroptera: Chrysopidae). Ecotoxicology 2013, 22, 1232–1240. [Google Scholar] [CrossRef]
- Shah, R.M.; Abbas, N.; Shad, S.A. Assessment of resistance risk in Musca domestica L. (Diptera: Muscidae) to methoxyfenozide. Acta Trop. 2015, 149, 32–37. [Google Scholar] [CrossRef]
- Shah, R.M.; Abbas, N.; Shad, S.A.; Sial, A.A. Selection, resistance risk assessment, and reversion toward susceptibility of pyriproxyfen in Musca domestica L. Parasitol. Res. 2015, 114, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Abbas, N.; Ijaz, M.; Shad, S.A.; Khan, H. Stability of field-selected resistance to conventional and newer chemistry insecticides in the house fly, Musca domestica L. (Diptera: Muscidae). Neotrop. Entomol. 2015, 44, 402–409. [Google Scholar] [CrossRef] [PubMed]
- Abbas, N.; Shah, R.M.; Shad, S.A.; Azher, F. Dominant fitness costs of resistance to fipronil in Musca domestica Linnaeus (Diptera: Muscidae). Vet. Parasitol. 2016, 226, 78–82. [Google Scholar] [CrossRef] [PubMed]
- Khan, H.M.U.; Banazeer, A.; Afzal, M.B.S.; Shad, S.A. Evaluation of resistance stability and fitness costs in dimethoate-selected strain of Oxycarenus hyalinipennis Costa (Hemiptera: Lygaeidae). J. Asia-Pacif. Entomol. 2021, 24, 798–804. [Google Scholar] [CrossRef]
- Kliot, A.; Ghanim, M. Fitness costs associated with insecticide resistance, Pest Manag. Sci. 2012, 68, 1431–1437. [Google Scholar]
- Abbas, N.; Shah, R.M.; Shad, S.A.; Iqbal, N.; Razaq, M. Biological trait analysis and stability of lambda-cyhalothrin resistance in the house fly, Musca domestica L. (Diptera: Muscidae). Parasitol. Res. 2016, 115, 2073–2080. [Google Scholar] [CrossRef]
- IRAC. IRAC: Mode of Action Classification Scheme; Version 10.4; Documents-Moa-Classification; IRAC: USA, 2022; pp. 1–26. Available online: www.irac-online.org (accessed on 30 December 2022).
- Wei, X.; Pan, Y.; Xin, X.; Zheng, C.; Gao, X.; Xi, J.; Shang, Q. Cross-resistance pattern and basis of resistance in a thiamethoxam-resistant strain of Aphis gossypii Glover. Pestic. Biochem. Physiol. 2017, 138, 91–96. [Google Scholar] [CrossRef]
- Flores, A.E.; Ponce, G.; Silva, B.G.; Gutierrez, S.M.; Bobadilla, C.; Lopez, B.; Mercado, R.; Black IV, W.C. Wide spread cross resistance to pyrethroids in Aedes aegypti (Diptera: Culicidae) from Veracruz state Mexico. J. Econ. Entomol. 2013, 106, 959–969. [Google Scholar] [CrossRef] [PubMed]


| IRAC † Chemical Class | Active Ingredient | Primary Site of Action | Trade Name | Formulation (%) | Manufacturer |
|---|---|---|---|---|---|
| 2A Organophosphate | Fenitrothion | Acetyl cholinesterase inhibitors | Fentox | 50% EC | Pioneers Chemicals Factory Co., Saudi Arabia |
| Chlorpyrifos | Chlorfet | 48% EC | Masani Chemicals, Jordan | ||
| Malathion | Delthion | 57% EC | Saudi Delta Company, Saudi Arabia | ||
| Diazinon | Diazinon | 60% EC | APCO, Saudi Arabia | ||
| Pirimiphos-methyl | Actikil | 50% EC | Astrachem, Saudi Arabia | ||
| 3A Pyrethroid | Alpha-cypermethrin | Sodium channel modulators | Alphaquest | 10% EC | Astrachem, Saudi Arabia |
| Cypermethrin | Montothrin | 10% EC | Montajat Agrochemicals, Saudi Arabia | ||
| Bifenthrin | Biflex | 8% SC | FMC, Belgium | ||
| Deltamethrin | K-Othrine | 25% SC | Bayer Crop Sciences, France | ||
| Cyfluthrin | Solfac | 5% EW | Bayer Crop Sciences, Germany | ||
| 7C Pyriproxyfen | Pyriproxyfen | Juvenile hormone mimics | Admiral | 10% EC | Sumitomo Chemicals, Japan |
| 15 Benzoylureas | Diflubenzuron | Inhibitors of chitin biosynthesis affecting CHS1 | Diflon | 25% WP | Saudi Delta Company, Saudi Arabia |
| Triflumuron | Starycide | 48% SC | Bayer Crop Sciences, Germany | ||
| 17 Cyromazine | Cyromazine | Molting disruptors, Diptera | Novasat | 75% WP | Astranova Chemicals, Saudi Arabia |
| 18 Diacylhydrazines | Methoxyfenozide | Ecdysone receptor agonists | Runner | 24% SC | Dow Agro Sciences, United Kingdom |
| Concentration (ppm) | Generation | Number of Males Exposed | Survival (%) | Number of Females Exposed | Survival (%) |
|---|---|---|---|---|---|
| 90 | G1 | 435 | 16.6 | 352 | 36.4 |
| 90 | G2 | 287 | 23.3 | 278 | 33.5 |
| 90 | G3 | 642 | 28.0 | 536 | 40.1 |
| 90 | G4 | 994 | 35.9 | 338 | 37.9 |
| 90 | G5 | 993 | 46.2 | 712 | 45.8 |
| 90 | G6 | 193 | 52.8 | 207 | 62.8 |
| 90 | G7 | 142 | 64.1 | 48 | 64.6 |
| 90 | G8 | 279 | 84.6 | 146 | 76.7 |
| 372 | G9 | 642 | 28.0 | 536 | 40.1 |
| 372 | G10 | 75 | 50.7 | 41 | 36.6 |
| 372 | G11 | 268 | 48.1 | 151 | 40.4 |
| 372 | G12 | 366 | 80.9 | 163 | 36.2 |
| 372 | G13 | 141 | 64.5 | 79 | 57.0 |
| 372 | G14 | 227 | 62.6 | 208 | 60.6 |
| 372 | G15 | 622 | 66.2 | 637 | 75.4 |
| 372 | G16 | 705 | 85.8 | 656 | 84.8 |
| 372 | G17 | 815 | 89.0 | 680 | 86.8 |
| 1000 | G18 | 573 | 62.5 | 663 | 71.2 |
| 1000 | G19 | 428 | 77.1 | 443 | 80.4 |
| 1000 | G20 | 546 | 71.4 | 465 | 80.6 |
| 1000 | G21 | 659 | 77.2 | 694 | 81.0 |
| 1000 | G22 | 656 | 80.9 | 707 | 80.6 |
| 1000 | G23 | 613 | 78.3 | 695 | 74.8 |
| Average | 59.8 | 60.2 |
| Strain (Generation) | Males | Females | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| LC50 (95% FL) † (ppm) | Slope ± SE | χ2 (df) | P | RR ‡ | LC50 (95% FL) † (ppm) | Slope ± SE | χ2 (df) | p | RR ‡ | |
| Alpha-Unsel (G24) | 4.4 (3.2–5.6) | 2.6 ± 0.4 | 0.6 (3) | 0.9 | 1.0 | 4.5 (3.1–5.9) | 2.0 ± 0.3 | 2.3 (3) | 0.5 | 1.0 |
| Field-Pop (G1) | 64.1 (51.5–79.8) | 2.7 ± 0.4 | 1.8 (3) | 0.6 | 14.6 | 90.1 (46.2–230.2) * | 2.4 ± 0.4 | 6.8 (3) | 0.1 | 20.0 |
| Alpha-Sel (G5) | 180.4 (136.3–232.2) | 2.5 ± 0.4 | 2.3 (3) | 0.5 | 41.0 | 208.8 (146.6–278.6) | 2.4 ± 0.4 | 1.2 (3) | 0.8 | 46.4 |
| Alpha-Sel (G6) | 201.2 (90.0–548.2) | 1.5 ± 0.3 | 4.2 (3) | 0.2 | 45.7 | 239.9 (175.5–346.5) | 1.6 ± 0.3 | 1.8 (3) | 0.6 | 53.3 |
| Alpha-Sel (G7) | 204.3 (155.0–267.2) | 2.3 ± 0.4 | 2.7 (3) | 0.4 | 46.4 | 283.5 (186.8–407.4) | 2.0 ± 0.4 | 2.8 (3) | 0.4 | 63.0 |
| Alpha-Sel (G8) | 316.3 (128.7–723.2) | 1.9 ± 0.3 | 5.8 (3) | 0.1 | 71.9 | 332.0 (254.3–420.8) | 2.3 ± 0.3 | 2.2 (3) | 0.5 | 73.8 |
| Alpha-Sel (G9) | 316.5 (148.3–478.2) | 1.4 ± 0.3 | 0.6 (3) | 0.9 | 71.9 | 393.0 (244.4–609.2) | 2.4 ± 0.3 | 3.5 (3) | 0.3 | 87.3 |
| Alpha-Sel (G10) | 319.3 (156.9–610.7) | 1.9 ± 0.3 | 4.3 (3) | 0.2 | 72.6 | 467.7 (259.2–1021.3) | 1.9 ± 0.3 | 4.4 (3) | 0.2 | 103.9 |
| Alpha-Sel (G11) | 352.4 (182.8–515.4) | 1.5 ± 0.3 | 0.8 (3) | 0.8 | 80.1 | 495.9 (199.5–1067.3) | 2.0 ± 0.4 | 3.9 (3) | 0.3 | 110.2 |
| Alpha-Sel (G12) | 367.3 (161.4–889.7) | 1.5 ± 0.3 | 3.9 (3) | 0.3 | 83.5 | 497.0 (315.1–686.0) | 1.6 ± 0.3 | 1.2 (3) | 0.8 | 110.4 |
| Alpha-Sel (G13) | 383.4 (277.5–504.9) | 2.4 ± 0.4 | 2.7 (3) | 0.4 | 87.1 | 500.0 (376.7–695.0) | 1.8 ± 0.3 | 0.6 (3) | 0.9 | 111.1 |
| Alpha-Sel (G14) | 410.3 (333.2–503.1) | 3.0 ± 0.4 | 1.5 (3) | 0.7 | 93.3 | 504.3 (375.7–714.1) | 1.8 ± 0.3 | 1.1 (3) | 0.8 | 112.1 |
| Alpha-Sel (G15) | 429.5 (117.7–666.3) | 2.9 ± 0.6 | 3.1 (3) | 0.4 | 97.6 | 527.7 (409.6–682.2) | 2.2 ± 0.3 | 0.4 (3) | 0.9 | 117.3 |
| Alpha-Sel (G16) | 440.8 (245.0–636.9) | 1.4 ± 0.3 | 1.1 (3) | 0.8 | 100.2 | 596.5 (389.5–823.8) | 1.6 ± 0.3 | 0.1 (3) | 0.9 | 132.6 |
| Alpha-Sel (G17) | 480.7 (345.4–628.6) | 2.6 ± 0.4 | 1.1 (3) | 0.8 | 109.3 | 649.1 (404.2–930.1) | 1.4 ± 0.3 | 0.3 (3) | 0.9 | 144.2 |
| Alpha-Sel (G18) | 486.6 (294.8–683.9) | 1.5 ± 0.3 | 0.3 (3) | 1.0 | 110.6 | 684.6 (545.7–877.0) | 2.5 ± 0.4 | 0.6 (3) | 0.9 | 152.1 |
| Alpha-Sel (G19) | 564.3 (382.1–855.0) | 3.8 ± 0.5 | 4.5 (3) | 0.2 | 128.3 | 740.5 (565.5–1019.1) | 2.0 ± 0.3 | 2.2 (3) | 0.5 | 164.6 |
| Alpha-Sel (G20) | 579.9 (357.6–821.2) | 1.6 ± 0.3 | 0.6 (3) | 0.9 | 131.8 | 967.5 (674.3–1379.5) | 1.5 ± 0.3 | 0.1 (3) | 0.9 | 215.0 |
| Alpha-Sel (G21) | 614.6 (218.2–1225.2) | 2.2 ± 0.4 | 4.1 (3) | 0.3 | 139.7 | 983.3 (692.5–1350.7) | 1.8 ± 0.3 | 0.6 (3) | 0.9 | 218.5 |
| Alpha-Sel (G22) | 688.4 (460.2–955.4) | 1.5 ± 0.3 | 0.7 (3) | 0.9 | 156.5 | 1122.6 (726.7–1642.6) | 1.7 ± 0.3 | 0.3 (3) | 0.9 | 249.5 |
| Alpha-Sel (G23) | 743.3 (343.1–1329.9) | 1.7 ± 0.3 | 3.6 (3) | 0.3 | 168.9 | 1137.5 (848.4–1560.4) | 1.8 ± 0.3 | 2.4 (3) | 0.5 | 252.8 |
| Alpha-Sel (G24) | 1113.9 (741.6–1640.6) | 1.6 ± 0.3 | 0.7 (3) | 0.9 | 253.2 | 2134.1 (1392.7–4225.5) | 2.4 ± 0.4 | 3.4 (3) | 0.3 | 474.2 |
| Strain (Generation) | Males | Females | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LC50 (95% FL) † (ppm) | Slope ± SE | χ2 (df) | P | RR ‡ | DR | LC50 (95% FL) † (ppm) | Slope ± SE | χ2 (df) | p | RR ‡ | DR | |
| Field-Pop (G1) | 64.1 (51.5–79.8) | 2.7 ± 0.4 | 1.8 (3) | 0.6 | 14.6 | 90.1 (46.2–230.2) | 2.4 ± 0.4 | 6.8 (3) | 0.1 | 20.0 | ||
| Alpha-Unsel (G5) | 20.5 (15.4–27.2) | 1.9 ± 0.3 | 2.0 (3) | 0.6 | 4.7 | −0.10 | 27.3 (20.7–36.9) | 1.9 ± 0.3 | 0.7 (3) | 0.9 | 6.1 | −0.10 |
| Alpha-Unsel (G6) | 17.5 (12.2–24.1) | 1.6 ± 0.3 | 2.4 (3) | 0.5 | 4.0 | −0.09 | 24.9 (17.1–35.5) | 1.7 ± 0.3 | 1.9 (3) | 0.6 | 5.5 | −0.09 |
| Alpha-Unsel (G7) | 16.6 (8.0–30.0) | 2.1 ± 0.3 | 4.8 (3) | 0.2 | 3.8 | −0.08 | 20.6 (15.3–27.6) | 1.8 ± 0.3 | 0.7 (3) | 0.9 | 4.6 | −0.09 |
| Alpha-Unsel (G8) | 13.1 (9.2–17.2) | 2.7 ± 0.5 | 2.0 (3) | 0.6 | 3.0 | −0.09 | 17.3 (12.5–23.2) | 1.8 ± 0.3 | 1.1 (3) | 0.8 | 3.8 | −0.09 |
| Alpha-Unsel (G9) | 12.2 (8.4–16.2) | 1.9 ± 0.3 | 1.6 (3) | 0.7 | 2.8 | −0.08 | 14.0 (9.6–18.1) | 2.7 ± 0.6 | 2.9 (3) | 0.4 | 3.1 | −0.09 |
| Alpha-Unsel (G10) | 10.8 (6.6–17.8) | 2.6 ± 0.4 | 4.3 (3) | 0.2 | 2.5 | −0.08 | 12.1 (7.9–19.2) | 2.8 ± 0.4 | 4.0 (3) | 0.3 | 2.7 | −0.09 |
| Alpha-Unsel (G11) | 10.5 (4.9–18.2) | 2.0 ± 0.4 | 3.3 (3) | 0.4 | 2.4 | −0.07 | 11.2 (8.6–14.6) | 2.1 ± 0.3 | 1.4 (3) | 0.7 | 2.5 | −0.08 |
| Alpha-Unsel (G12) | 9.8 (7.8–12.4) | 2.4 ± 0.3 | 1.2 (3) | 0.8 | 2.2 | −0.07 | 10.6 (8.2–13.7) | 2.2 ± 0.3 | 2.7 (3) | 0.4 | 2.4 | −0.08 |
| Alpha-Unsel (G13) | 9.3 (7.0–11.8) | 2.8 ± 0.5 | 1.5 (3) | 0.7 | 2.1 | −0.06 | 10.5 (8.0–13.3) | 2.7 ± 0.4 | 0.7 (3) | 0.9 | 2.3 | −0.07 |
| Alpha-Unsel (G14) | 8.3 (4.0–14.4) | 1.9 ± 0.3 | 3.8 (3) | 0.3 | 1.9 | −0.06 | 10.2 (7.7–13.5) | 2.0 ± 0.3 | 2.9 (3) | 0.4 | 2.3 | −0.07 |
| Alpha-Unsel (G15) | 8.2 (3.4–15.9) | 1.9 ± 0.3 | 5.0 (3) | 0.2 | 1.9 | −0.06 | 9.9 (6.7–13.7) | 1.8 ± 0.3 | 0.2 (3) | 1.0 | 2.2 | −0.06 |
| Alpha-Unsel (G16) | 6.7 (2.1–13.8) | 1.4 ± 0.3 | 3.8 (3) | 0.3 | 1.5 | −0.06 | 9.1 (6.7–12.4) | 1.7 ± 0.3 | 0.5 (3) | 0.9 | 2.0 | −0.06 |
| Alpha-Unsel (G17) | 6.6 (4.9–8.4) | 2.3 ± 0.3 | 1.9 (3) | 0.6 | 1.5 | −0.06 | 8.0 (5.5–10.6) | 2.4 ± 0.4 | 2.4 (3) | 0.5 | 1.8 | −0.06 |
| Alpha-Unsel (G18) | 6.6 (4.5–9.0) | 1.6 ± 0.3 | 0.3 (3) | 1.0 | 1.5 | −0.05 | 7.8 (5.5–10.8) | 1.6 ± 0.3 | 0.2 (3) | 1.0 | 1.7 | −0.06 |
| Alpha-Unsel (G19) | 5.9 (4.0–8.0) | 1.7 ± 0.3 | 0.6 (3) | 0.9 | 1.3 | −0.05 | 7.6 (5.2–10.5) | 1.5 ± 0.3 | 0.3 (3) | 1.0 | 1.7 | −0.06 |
| Alpha-Unsel (G20) | 5.2 (3.7–6.8) | 2.0 ± 0.3 | 1.2 (3) | 0.8 | 1.2 | −0.05 | 6.6 (4.7–8.7) | 1.9 ± 0.3 | 1.3 (3) | 0.7 | 1.5 | −0.06 |
| Alpha-Unsel (G21) | 5.0 (3.4–6.5) | 2.6 ± 0.4 | 0.8 (3) | 0.9 | 1.1 | −0.05 | 6.4 (4.2–8.9) | 1.9 ± 0.3 | 0.2 (3) | 1.0 | 1.4 | −0.05 |
| Alpha-Unsel (G22) | 4.9 (3.4–6.6) | 1.9 ± 0.3 | 0.4 (3) | 0.9 | 1.1 | −0.05 | 6.2 (4.5–8.2) | 1.9 ± 0.3 | 0.2 (3) | 1.0 | 1.4 | −0.05 |
| Alpha-Unsel (G23) | 4.9 (3.2–6.7) | 2.2 ± 0.4 | 2.2 (3) | 0.5 | 1.1 | −0.05 | 5.4 (3.8–7.1) | 1.9 ± 0.3 | 1.1 (3) | 0.8 | 1.2 | −0.05 |
| Alpha-Unsel (G24) | 4.4 (3.2–5.6) | 2.6 ± 0.4 | 0.6 (3) | 0.9 | 1.0 | −0.05 | 4.5 (3.1–5.9) | 2.0 ± 0.3 | 2.3 (3) | 0.5 | 1.0 | −0.05 |
| Generation | Insecticide | Initial LC50 (log) (ppm) | Final LC50 (log) (ppm) | G 1 | R2 | p3 | i4 | Mean Slope | σp5 | S6 | h2 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Female flies | |||||||||||
| 12 (G1–G12) | Alpha-cypermethrin | 90.1 (2.0) | 497.0 (2.7) | 12 | 0.06 | 45.9 | 0.87 | 2.1 | 0.48 | 0.41 | 0.15 |
| 12 (G13–G24) | Alpha-cypermethrin | 500.0 (2.7) | 2134.1 (3.3) | 12 | 0.05 | 75.7 | 0.41 | 1.9 | 0.53 | 0.22 | 0.24 |
| 24 (G1–G24) | Alpha-cypermethrin | 90.1 (2.0) | 2134.1 (3.3) | 24 | 0.06 | 60.2 | 0.63 | 2.0 | 0.50 | 0.32 | 0.18 |
| Male flies | |||||||||||
| 12 (G1–G12) | Alpha-cypermethrin | 64.1 (1.81) | 367.3 (2.57) | 12 | 0.06 | 46.6 | 0.85 | 1.9 | 0.53 | 0.45 | 0.14 |
| 12 (G13–G24) | Alpha-cypermethrin | 383.4 (2.58) | 1113.9 (3.05) | 12 | 0.04 | 74.1 | 0.43 | 2.2 | 0.45 | 0.20 | 0.20 |
| 24 (G1–G24) | Alpha-cypermethrin | 64.1 (1.81) | 1113.9 (3.05) | 24 | 0.05 | 59.8 | 0.64 | 2.1 | 0.48 | 0.31 | 0.17 |
| Strain | Insecticide | Conc. (ppm) | LC50 (95% FL) † (ppm) | Slope ± SE | χ2 (df) | p | PR ‡ |
|---|---|---|---|---|---|---|---|
| Alpha-Unsel (G24) | Bifenthrin | 128–2048 | 254.8 (84.9–435.7) | 0.9 ± 0.3 | 0.03 (3) | 1.0 | 1.0 |
| Deltamethrin | 128–2048 | 146.5 (45.0–247.3) | 1.1 ± 0.3 | 1.0 (3) | 0.8 | 1.0 | |
| Cyfluthrin | 128–2048 | 139.3 (36.9–243.4) | 1.1 ± 0.3 | 0.5 (3) | 0.9 | 1.0 | |
| Cypermethrin | 128–2048 | 172.6 (60.0–283.8) | 1.1 ± 0.3 | 0.3 (3) | 0.9 | 1.0 | |
| Fenitrothion | 128–2048 | 140.1 (54.0–226.9) | 0.9 ± 0.2 | 0.3 (3) | 1.0 | 1.0 | |
| Malathion | 128–2048 | 213.8 (62.5–368.8) | 0.9 ± 0.3 | 0.2 (3) | 1.0 | 1.0 | |
| Diazinon | 2–32 | 3.0 (1.9–4.1) | 1.9 ± 0.3 | 1.9 (3) | 0.6 | 1.0 | |
| Pirimiphos-methyl | 128–2048 | 153.8 (68.4–236.0) | 1.4 ± 0.3 | 0.3 (3) | 1.0 | 1.0 | |
| Chlorpyrifos | 32–512 | 42.3 (18.6–65.3) | 1.3 ± 0.3 | 4.9 (3) | 0.2 | 1.0 | |
| Diflubenzuron | 0.25–4 | 0.7 (0.4–1.1) | 2.2 ± 0.3 | 3.6 (3) | 0.3 | 1.0 | |
| Triflumuron | 0.125–2 | 0.2 (0.1–0.3) | 1.7 ± 0.3 | 4.4 (3) | 0.2 | 1.0 | |
| Pyriproxyfen | 0.0078–0.125 | 0.01 (0.01–0.02) | 2.0 ± 0.4 | 2.0 (3) | 0.6 | 1.0 | |
| Cyromazine | 0.125–2 | 0.4 (0.3–0.5) | 2.3 ± 0.3 | 0.5 (3) | 0.9 | 1.0 | |
| Methoxyfenozide | 4–64 | 10.1 (7.6–12.9) | 2.2 ± 0.3 | 0.7 (3) | 0.9 | 1.0 | |
| Alpha-Sel (G24) | Bifenthrin | 625–10000 | 3941.5 (2012.4–12551.0) | 1.7 ± 0.3 | 4.6 (3) | 0.2 | 15.5 |
| Deltamethrin | 512–8192 | 4158.2 (3186.6–5879.9) | 2.1 ± 0.3 | 2.5 (3) | 0.5 | 28.4 | |
| Cyfluthrin | 562.5–9000 | 2336.6 (1559.3–3532.1) | 1.3 ± 0.3 | 0.9 (3) | 0.8 | 16.8 | |
| Cypermethrin | 562.5–9000 | 867.0 (671.4–1061.5) | 3.3 ± 0.6 | 2.7 (3) | 0.4 | 5.0 | |
| Fenitrothion | 128–2048 | 860.5 (622.8–1317.7) | 1.6 ± 0.3 | 0.5 (3) | 0.9 | 6.1 | |
| Chlorpyrifos | 128–2048 | 201.6 (42.8–362.3) | 1.5 ± 0.3 | 3.3 (3) | 0.4 | 4.8 | |
| Malathion | 128–2048 | 437.5 (293.7–625.7) | 1.4 ± 0.3 | 1.9 (3) | 0.6 | 2.1 | |
| Diazinon | 16–256 | 24.1 (5.2–43.5) | 0.9 ± 0.3 | 0.1 (3) | 1.0 | 8.1 | |
| Pirimiphos-methyl | 128–2048 | 332.1 (207.7–474.8) | 1.4 ± 0.3 | 1.1 (3) | 0.8 | 2.2 | |
| Triflumuron | 0.25–4 | 0.7 (0.4–1.0) | 1.2 ± 0.3 | 0.4 (3) | 0.9 | 3.3 | |
| Diflubenzuron | 0.25–4 | 0.9 (0.5–1.4) | 2.8 ± 0.4 | 4.2 (3) | 0.2 | 1.3 | |
| Cyromazine | 0.125–2 | 0.8 (0.5–1.7) | 2.2 ± 0.3 | 4.2 (3) | 0.2 | 1.9 | |
| Pyriproxyfen | 0.016–0.25 | 0.1 (0.03–0.1) | 1.6 ± 0.3 | 2.9 (3) | 0.4 | 5.0 | |
| Methoxyfenozide | 4–64 | 11.6 (5.8–19.7) | 2.2 ± 0.3 | 4.5 (3) | 0.2 | 1.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abbas, N.; Hafez, A.M. Alpha-Cypermethrin Resistance in Musca domestica: Resistance Instability, Realized Heritability, Risk Assessment, and Insecticide Cross-Resistance. Insects 2023, 14, 233. https://doi.org/10.3390/insects14030233
Abbas N, Hafez AM. Alpha-Cypermethrin Resistance in Musca domestica: Resistance Instability, Realized Heritability, Risk Assessment, and Insecticide Cross-Resistance. Insects. 2023; 14(3):233. https://doi.org/10.3390/insects14030233
Chicago/Turabian StyleAbbas, Naeem, and Abdulwahab M. Hafez. 2023. "Alpha-Cypermethrin Resistance in Musca domestica: Resistance Instability, Realized Heritability, Risk Assessment, and Insecticide Cross-Resistance" Insects 14, no. 3: 233. https://doi.org/10.3390/insects14030233
APA StyleAbbas, N., & Hafez, A. M. (2023). Alpha-Cypermethrin Resistance in Musca domestica: Resistance Instability, Realized Heritability, Risk Assessment, and Insecticide Cross-Resistance. Insects, 14(3), 233. https://doi.org/10.3390/insects14030233





