Not All Field Margins Are Equally Useful: Effects of the Vegetation Structure of Margins on Cereal Aphids and Their Natural Enemies
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods
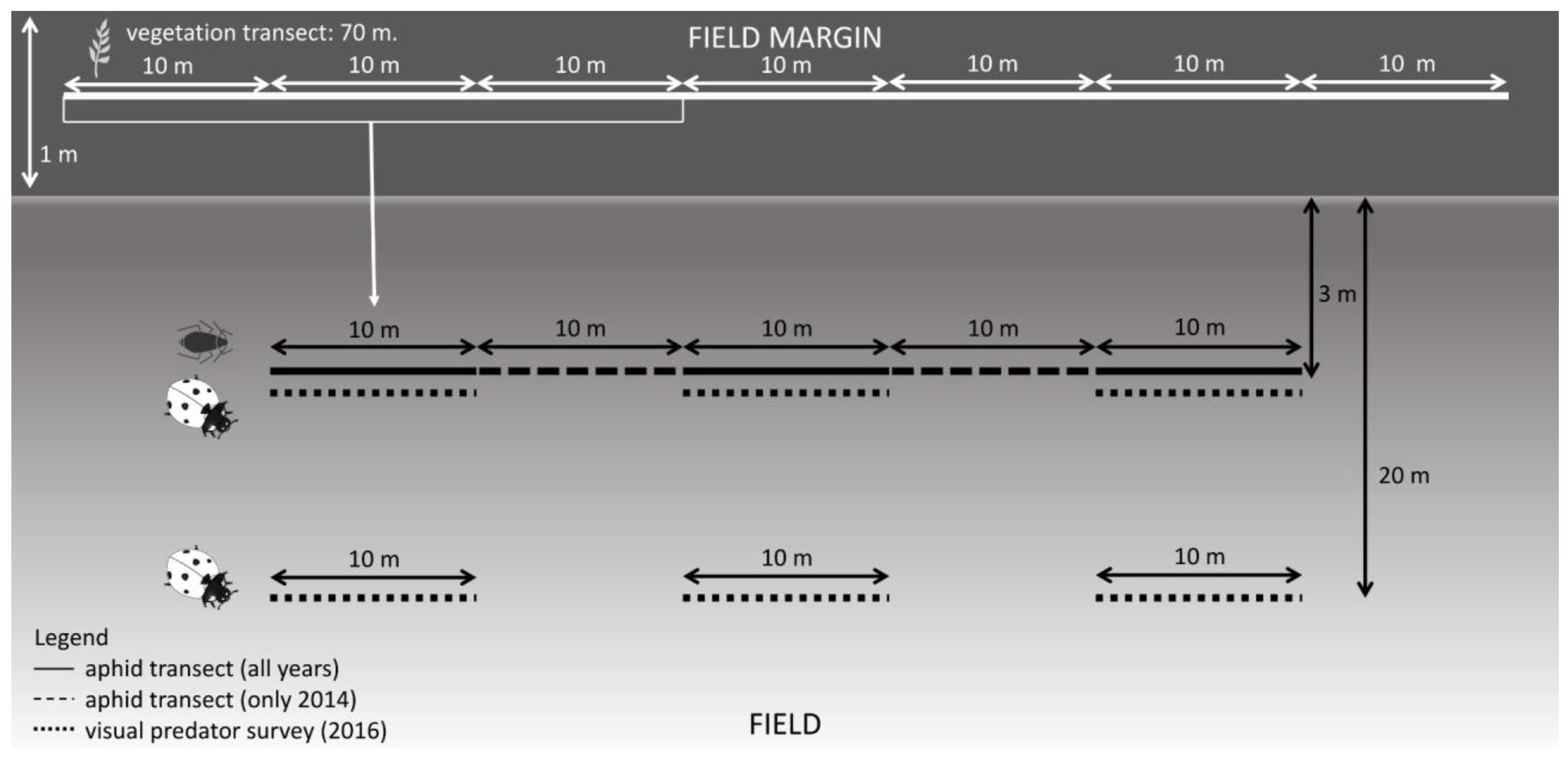
2.1. Sampling Site and Field Selection
2.2. Aphid Sampling
2.3. Predator Survey
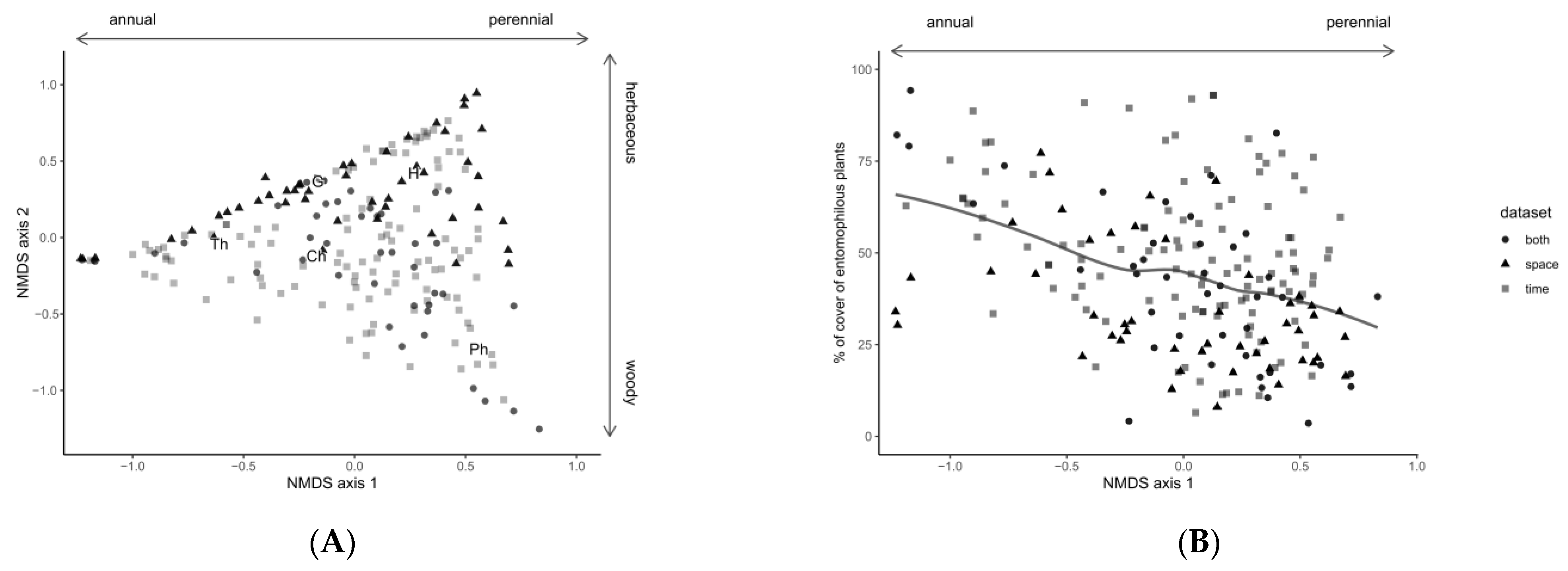
2.4. Vegetation Survey and Characterization
2.5. Vegetation and Data Analysis
2.6. Insect Data Analyses
| Dataset | Response | Fixed Effects | Random Effects | Distribution | ||
|---|---|---|---|---|---|---|
| ‘time’ dataset | Aphid abundance | vegetation NMDS axis 1 + axis 12 + axis 2 + axis 22 | + crop | + year | + (intercept|field) | Negative binomial |
| ‘space’ dataset | + area | |||||
| ‘time’ dataset | Parasitism | + log(aphid abundance) | + year | Binomial | ||
| ‘space’ dataset | + area | |||||
| 2016 visual transects | Ladybug abundance | + log(aphid abundance) + distance to margin | negative binomial | |||
| Hoverfly abundance | Poisson | |||||
3. Results
3.1. Field Margin Vegetation
3.2. Insects
| Variable | Aphid Abundances (‘Time’ Dataset) | Aphid Abundances (‘Space’ Dataset) | Parasitism (‘Time’ Dataset) | Parasitism (‘Space’ Dataset) | ||||
|---|---|---|---|---|---|---|---|---|
| axis 1 (linear) | 0.477 ± 0.252 | * | 0.854 ± 0.231 | *** | −0.334 ± 0.146 | * | −0.306 ± 0.254 | |
| axis 1 (quadratic) | 0.494 ± 0.312 | 0.335 ± 0.287 | −0.052 ± 0.175 | −0.566 ± 0.315 | m | |||
| axis 2 (linear) | −0.450 ± 0.235 | * | −0.535 ± 0.199 | ** | −0.303 ± 0.125 | * | −0.269 ± 0.167 | |
| axis 2 (quadratic) | −0.239 ± 0.371 | −0.475 ± 0.319 | 0.369 ± 0.237 | 0.233 ± 0.377 | ||||
| log(aphid abundance) | 0.027 ± 0.045 | −0.201 ± 0.107 | m | |||||
| barley vs. wheat | −1.173 ± 0.274 | *** | −1.232 ± 0.267 | *** | ||||
| year | ** | ns | ||||||
| area | ns | * | ||||||
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- García-Feced, C.; Weissteiner, C.J.; Baraldi, A.; Paracchini, M.L.; Maes, J.; Zulian, G.; Kempen, M.; Elbersen, B.S.; Pérez-Soba, M. Semi-natural vegetation in agricultural land: European map and links to ecosystem service supply. Agron. Sustain. Dev. 2014, 35, 273–283. [Google Scholar] [CrossRef]
- Holland, J.M.; Douma, J.C.; Crowley, L.; James, L.; Kor, L.; Stevenson, D.R.; Smith, B.M. Semi-natural habitats support biological control, pollination and soil conservation in Europe. A review. Agron. Sustain. Dev. 2017, 37, 31. [Google Scholar] [CrossRef]
- Rusch, A.; Chaplin-Kramer, R.; Gardiner, M.M.; Hawro, V.; Holland, J.; Landis, D.; Thies, C.; Tscharntke, T.; Weisser, W.W.; Winqvist, C.; et al. Agricultural landscape simplification reduces natural pest control: A quantitative synthesis. Agric. Ecosyst. Environ. 2016, 221, 198–204. [Google Scholar] [CrossRef]
- Pollier, A.; Guillomo, L.; Tricault, Y.; Plantegenest, M.; Bischoff, A. Effects of spontaneous field margin vegetation on the regulation of herbivores in two winter crops. Basic Appl. Ecol. 2018, 27, 71–82. [Google Scholar] [CrossRef]
- Alignier, A.; Raymond, L.; Deconchat, M.; Menozzi, P.; Monteil, C.; Sarthou, J.-P.; Vialatte, A.; Ouin, A. The effect of semi-natural habitats on aphids and their natural enemies across spatial and temporal scales. Biol. Control 2014, 77, 76–82. [Google Scholar] [CrossRef]
- Botzas-Coluni, J.; Crockett, E.T.; Rieb, J.T.; Bennett, E.M. Farmland heterogeneity is associated with gains in some ecosystem services but also potential trade-offs. Agric. Ecosyst. Environ. 2021, 322, 107661. [Google Scholar] [CrossRef]
- Holland, J.M.; Bianchi, F.J.; Entling, M.H.; Moonen, A.-C.; Smith, B.M.; Jeanneret, P. Structure, function and management of semi-natural habitats for conservation biological control: A review of European studies. Pest Manag. Sci. 2016, 72, 1638–1651. [Google Scholar] [CrossRef]
- Balzan, M.V.; Moonen, A.-C. Field margin vegetation enhances biological control and crop damage suppression from multiple pests in organic tomato fields. Entomol. Exp. Appl. 2013, 150, 45–65. [Google Scholar] [CrossRef]
- Lundin, O.; Ward, K.L.; Williams, N.M. Identifying native plants for coordinated habitat management of arthropod pollinators, herbivores and natural enemies. J. Appl. Ecol. 2018, 56, 665–676. [Google Scholar] [CrossRef]
- Gardarin, A.; Plantegenest, M.; Bischoff, A.; Valantin-Morison, M. Understanding plant–arthropod interactions in multitrophic communities to improve conservation biological control: Useful traits and metrics. J. Pest Sci. 2018, 91, 943–955. [Google Scholar] [CrossRef]
- M’Gonigle, L.K.; Williams, N.M.; Lonsdorf, E.; Kremen, C. A Tool for Selecting Plants when Restoring Habitat for Pollinators. Conserv. Lett. 2016, 10, 105–111. [Google Scholar] [CrossRef]
- Irvin, N.A.; Pierce, C.; Hoddle, M.S. Evaluating the potential of flowering plants for enhancing predatory hoverflies (Syrphidae) for biological control of Diaphorina citri (Liviidae) in California. Biol. Control 2021, 157, 104574. [Google Scholar] [CrossRef]
- Bruelheide, H.; Dengler, J.; Purschke, O.; Lenoir, J.; Jiménez-Alfaro, B.; Hennekens, S.M.; Botta-Dukát, Z.; Chytrý, M.; Field, R.; Jansen, F.; et al. Global trait–environment relationships of plant communities. Nat. Ecol. Evol. 2018, 2, 1906–1917. [Google Scholar] [CrossRef]
- Pérez-Harguindeguy, N.; Díaz, S.; Garnier, E.; Lavorel, S.; Poorter, H.; Jaureguiberry, P.; Bret-Harte, M.S.; Cornwell, W.K.; Craine, J.M.; Gurvich, D.E.; et al. New handbook for standardised measurement of plant functional traits worldwide. Aust. J. Bot. 2013, 61, 167–234. [Google Scholar] [CrossRef]
- Blaix, C.; Moonen, A.-C. Structural field margin characteristics affect the functional traits of herbaceous vegetation. PLoS ONE 2020, 15, e0238916. [Google Scholar] [CrossRef]
- Pallavicini, Y.; Bastida, F.; Hernández-Plaza, E.; Petit, S.; Izquierdo, J.; Gonzalez-Andujar, J.L. Local Factors Rather than the Landscape Context Explain Species Richness and Functional Trait Diversity and Responses of Plant Assemblages of Mediterranean Cereal Field Margins. Plants 2020, 9, 778. [Google Scholar] [CrossRef]
- Thies, C.; Roschewitz, I.; Tscharntke, T. The landscape context of cereal aphid–parasitoid interactions. Proc. R. Soc. B Boil. Sci. 2005, 272, 203–210. [Google Scholar] [CrossRef]
- Tscharntke, T.; Brandl, R. Plant-Insect Interactions in Fragmented Landscapes. Annu. Rev. Entomol. 2004, 49, 405–430. [Google Scholar] [CrossRef]
- Rijn, P.C.J.; Kooijman, J.; Wäckers, F.L. The Impact of Floral Resources on Syrphid Performance and Cabbage Aphid Biological Control. IOBC/Wprs Bull. 2006, 29, 149. [Google Scholar]
- Bianchi, F.J.; Wäckers, F.L. Effects of flower attractiveness and nectar availability in field margins on biological control by parasitoids. Biol. Control 2008, 46, 400–408. [Google Scholar] [CrossRef]
- Tatchell, G. An estimate of the potential economic losses to some crops due to aphids in Britain. Crop Prot. 1989, 8, 25–29. [Google Scholar] [CrossRef]
- Dedryver, C.-A.; Le Ralec, A.; Fabre, F. The conflicting relationships between aphids and men: A review of aphid damage and control strategies. C. R. Biol. 2010, 333, 539–553. [Google Scholar] [CrossRef] [PubMed]
- Bassa, M.; Boutin, C.; Chamorro, L.; Sans, F.X.; José-María, L.; Armengot, L.; Blanco-Moreno, J.M.; Bassa, M.; Sans, F.X. Effects of Agricultural Intensification on Plant Diversity in Mediterranean Dryland Cereal Fields. J. Appl. Ecol. 2010, 47, 832–840. [Google Scholar] [CrossRef]
- Bassa, M.; Chamorro, L.; José-María, L.; Blanco-Moreno, J.M.; Sans, F.X. Factors affecting plant species richness in field boundaries in the Mediterranean region. Biodivers. Conserv. 2012, 21, 1101–1114. [Google Scholar] [CrossRef]
- Ferrer-Suay, M.; Selfa, J.; Pujade-Villar, J.; Elfa, J. Keys to world Charipinae (Hymenoptera, Cynipoidea, Figitidae). Zookeys 2019, 822, 79–139. [Google Scholar] [CrossRef]
- Noyes, J.S. Universal Chalcidoidea Database. Available online: https://www.nhm.ac.uk/our-science/data/chalcidoids/database/index.dsml (accessed on 1 December 2022).
- Trjapitzin, V.A. Parasitic Hymenoptera of the Fam. Encyrtidae of Palaearctics. In Opredeliteli po Faune SSSR; Zoologicheskim Institutom Akademii Nauk SSR: Leningrad, Russia, 1989; pp. 1–489. [Google Scholar]
- de Graham, M.W. The Pteromalidae of north-western Europe (Hymenoptera: Chalcidoidea). Bull. Br. Mus. Nat. Hist. Zool. 1969, 16, 1–908. [Google Scholar] [CrossRef]
- Nieto Nafría, J.M.; Mier Durante, M.P. Hemiptera, Aphididae I; Consejo Superior de Investigaciones Científicas, Museo Nacional de Ciencias Naturales, Eds.; Museo Nacional de Ciencias Naturales: Madrid, Spain, 1998; ISBN 84-00-07774-1. [Google Scholar]
- Blackman, R.L.; Eastop, V.F. Aphids on the World’s Herbaceous Plants and Shrubs; Wiley: Hoboken, NJ, USA, 2006; ISBN 978-0-471-48973-3. [Google Scholar]
- Nieto-Nafría, J.M.; Mier-Durante, M.P.; García-Prieto, F.; Pérez-Hidalgo, N. Hemiptera, Aphididae III; Consejo Superior de Investigaciones Científicas, Museo Nacional de Ciencias Naturales, Eds.; Fauna Iberica; Museo Nacional de Ciencias Naturales: Madrid, Spain, 2005; Volume 28, p. 362. [Google Scholar]
- Favret, C. AphidNet—A Resource for Aphid Systematics and Taxonomy. Available online: http://aphidnet.org/ (accessed on 28 January 2022).
- Van Der Maabel, E. Transformation of cover-abundance values in phytosociology and its effects on community similarity. Plant Ecol. 1979, 39, 97–114. [Google Scholar] [CrossRef]
- Fitter, A.H.; Peat, H.J. The Ecological Flora Database. J. Ecol. 1994, 82, 415. [Google Scholar] [CrossRef]
- Julve, P. Baseflor. Index Botanique, Écologique et Chorologique de La Flore de France. Available online: http://philippe.julve.pagesperso-orange.fr/ (accessed on 1 October 2022).
- Klotz, S.; Kühn, I.; Durka, W. BIOLFLOR—Eine Datenbank mit Biologisch-Ökologischen Merkmalen zur Flora von Deutschland. Schriftenr. Vegetationskd. 2002, 38, 1–334. [Google Scholar]
- BiolFlor—A new plant-trait database as a tool for plant invasion ecology. Divers. Distrib. 2004, 10, 363–365. [CrossRef]
- de Bolòs, O.; Vigo, J.; Masalles, R.M.; Ninot, J.M. Flora Manual Dels Països Catalans, 3rd ed.; Pòrtic: Barcelona, Spain, 2005. [Google Scholar]
- Bocci, G. TR8: An R package for easily retrieving plant species traits. Methods Ecol. Evol. 2015, 6, 347–350. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing. 2018. Available online: https://www.r-project.org/ (accessed on 17 April 2019).
- Laliberté, E.; Legendre, P.; Shipley, B. FD: Measuring Functional Diversity from Multiple Traits, and Other Tools for Functional Ecology. R Package Version 1.0-12.1. 2014. Available online: https://cran.r-project.org/package=FD (accessed on 17 April 2019).
- Laliberté, E.; Legendre, P. A distance-based framework for measuring functional diversity from multiple traits. Ecology 2010, 91, 299–305. [Google Scholar] [CrossRef]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. vegan: Community Ecology Package. R Package Version 2.5.2. 2018. Available online: https://cran.r-project.org/package=vegan (accessed on 17 April 2019).
- Zuur, A.F.; Ieno, E.N.; Walker, N.; Saveliev, A.A.; Smith, G.M. Mixed Effects Models and Extensions in Ecology with R, 1st ed.; Springer: New York, NY, USA, 2009; pp. 209–259. ISBN 9780387874586. [Google Scholar]
- Pareja, M.; Brown, V.K.; Powell, W. Aggregation of parasitism risk in an aphid-parasitoid system: Effects of plant patch size and aphid density. Basic Appl. Ecol. 2008, 9, 701–708. [Google Scholar] [CrossRef]
- Fournier, D.A.; Skaug, H.J.; Ancheta, J.; Ianelli, J.; Magnusson, A.; Maunder, M.N.; Nielsen, A.; Sibert, J. AD Model Builder: Using automatic differentiation for statistical inference of highly parameterized complex nonlinear models. Optim. Methods Softw. 2012, 27, 233–249. [Google Scholar] [CrossRef]
- Skaug, H.; Fournier, D.; Bolker, B.; Magnusson, A.; Nielsen, A. GlmmADMB: Generalized Linear Mixed Models Using “AD Model Builder”. R Package Version 0.8.3.3. 2016. Available online: https://r-forge.r-project.org/projects/glmmadmb/ (accessed on 17 April 2019).
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using lme. J. Stat. Softw. 2015, 67, 48. [Google Scholar] [CrossRef]
- Bischoff, A.; Pollier, A.; Lamarre, E.; Salvadori, O.; Cortesero, A.-M.; Le Ralec, A.; Tricault, Y.; Jaloux, B. Effects of spontaneous field margin vegetation and surrounding landscape on Brassica oleracea crop herbivory. Agric. Ecosyst. Environ. 2016, 223, 135–143. [Google Scholar] [CrossRef]
- Ramsden, M.W.; Menéndez, R.; Leather, S.R.; Wäckers, F. Optimizing field margins for biocontrol services: The relative role of aphid abundance, annual floral resources, and overwinter habitat in enhancing aphid natural enemies. Agric. Ecosyst. Environ. 2015, 199, 94–104. [Google Scholar] [CrossRef]
- Haenke, S.; Scheid, B.; Schaefer, M.; Tscharntke, T.; Thies, C. Increasing syrphid fly diversity and density in sown flower strips within simple vs. complex landscapes. J. Appl. Ecol. 2009, 46, 1106–1114. [Google Scholar] [CrossRef]
- Tschumi, M.; Albrecht, M.; Collatz, J.; Dubsky, V.; Entling, M.H.; Najar-Rodriguez, A.J.; Jacot, K. Tailored flower strips promote natural enemy biodiversity and pest control in potato crops. J. Appl. Ecol. 2016, 53, 1169–1176. [Google Scholar] [CrossRef]
- Hatt, S.; Francis, F.; Xu, Q.; Wang, S.; Osawa, N. Perennial Flowering Strips for Conservation Biological Control of Insect Pests: From Picking and Mixing Flowers to Tailored Functional Diversity. In Integrative Biological Control: Ecostacking for Enhanced Ecosystem Services; Gao, Y., Hokkanen, H.M.T., Menzler-Hokkanen, I., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 57–71. ISBN 978-3-030-44838-7. [Google Scholar]
- Wäckers, F.L.; van Rijn, P.C.; Heimpel, G.E. Honeydew as a food source for natural enemies: Making the best of a bad meal? Biol. Control 2008, 45, 176–184. [Google Scholar] [CrossRef]
- Tena, A.; Wäckers, F.L.; Heimpel, G.; Urbaneja, A.; Pekas, A. Parasitoid nutritional ecology in a community context: The importance of honeydew and implications for biological control. Curr. Opin. Insect Sci. 2016, 14, 100–104. [Google Scholar] [CrossRef] [PubMed]
- Ricci, C.; Ponti, L.; Pires, A. Migratory flight and pre-diapause feeding of Coccinella septempunctata (Coleoptera) adults in agricultural and mountain ecosystems of Central Italy. Eur. J. Entomol. 2005, 102, 531–538. [Google Scholar] [CrossRef]
- Triltsch, H. Food Remains in the Guts of Coccinella septempunctata (Coleoptera: Coccinellidae) Adults and Larvae. Eur. J. Entomol. 1999, 96, 355–364. [Google Scholar]
- Schuldiner-Harpaz, T.; Coll, M. Effect of Diet History on Prey and Pollen Food Choice by Two Lady Beetle Species. J. Insect Behav. 2017, 30, 432–438. [Google Scholar] [CrossRef]
- Öberg, S.; Ekbom, B.; Bommarco, R. Influence of habitat type and surrounding landscape on spider diversity in Swedish agroecosystems. Agric. Ecosyst. Environ. 2007, 122, 211–219. [Google Scholar] [CrossRef]
- Pywell, R.; James, K.; Herbert, I.; Meek, W.; Carvell, C.; Bell, D.; Sparks, T. Determinants of overwintering habitat quality for beetles and spiders on arable farmland. Biol. Conserv. 2005, 123, 79–90. [Google Scholar] [CrossRef]
- Heath, S.K.; Soykan, C.U.; Velas, K.L.; Kelsey, R.; Kross, S.M. A bustle in the hedgerow: Woody field margins boost on farm avian diversity and abundance in an intensive agricultural landscape. Biol. Conserv. 2017, 212, 153–161. [Google Scholar] [CrossRef]
- Grass, I.; Lehmann, K.; Thies, C.; Tscharntke, T. Insectivorous birds disrupt biological control of cereal aphids. Ecology 2017, 98, 1583–1590. [Google Scholar] [CrossRef]
- Fusser, M.S.; Pfister, S.C.; Entling, M.H.; Schirmel, J. Effects of landscape composition on carabids and slugs in herbaceous and woody field margins. Agric. Ecosyst. Environ. 2016, 226, 79–87. [Google Scholar] [CrossRef]
- Pfiffner, L.; Luka, H. Overwintering of arthropods in soils of arable fields and adjacent semi-natural habitats. Agric. Ecosyst. Environ. 2000, 78, 215–222. [Google Scholar] [CrossRef]
- Caballero-López, B.; Bommarco, R.; Blanco-Moreno, J.; Sans, F.; Pujade-Villar, J.; Rundlöf, M.; Smith, H. Aphids and their natural enemies are differently affected by habitat features at local and landscape scales. Biol. Control 2012, 63, 222–229. [Google Scholar] [CrossRef]
- Snyder, W.E.; Ives, A.R. Generalist Predators Disrupt Biological Control by a Specialist Parasitoid. Ecology 2001, 82, 705–716. [Google Scholar] [CrossRef]

| Variable | Ladybug Abundance | Hoverfly Abundance | ||
|---|---|---|---|---|
| axis 1 (linear) | −3.514 ± 0.793 | *** | −1.429 ± 0.449 | ** |
| axis 1 (quadratic) | −2.472 ± 1.228 | * | 0.282 ± 0.228 | |
| axis 2 (linear) | 0.404 ± 0.339 | −1.626 ± 0.886 | ||
| axis 2 (quadratic) | 4.246 ± 1.006 | *** | 0.669 ± 0.595 | |
| 20 m vs. 3 m from margin | −0.560 ± 0.280 | * | −1.910 ± 0.268 | *** |
| log(aphid abundance) | 1.297 ± 0.359 | *** | 0.233 ± 0.206 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salat-Moltó, A.; Caballero-López, B.; Pérez-Hidalgo, N.; Michelena, J.M.; Ferrer Suay, M.; Guerrieri, E.; Blanco-Moreno, J.M. Not All Field Margins Are Equally Useful: Effects of the Vegetation Structure of Margins on Cereal Aphids and Their Natural Enemies. Insects 2023, 14, 156. https://doi.org/10.3390/insects14020156
Salat-Moltó A, Caballero-López B, Pérez-Hidalgo N, Michelena JM, Ferrer Suay M, Guerrieri E, Blanco-Moreno JM. Not All Field Margins Are Equally Useful: Effects of the Vegetation Structure of Margins on Cereal Aphids and Their Natural Enemies. Insects. 2023; 14(2):156. https://doi.org/10.3390/insects14020156
Chicago/Turabian StyleSalat-Moltó, Agnès, Berta Caballero-López, Nicolás Pérez-Hidalgo, José M. Michelena, Mar Ferrer Suay, Emilio Guerrieri, and José M. Blanco-Moreno. 2023. "Not All Field Margins Are Equally Useful: Effects of the Vegetation Structure of Margins on Cereal Aphids and Their Natural Enemies" Insects 14, no. 2: 156. https://doi.org/10.3390/insects14020156
APA StyleSalat-Moltó, A., Caballero-López, B., Pérez-Hidalgo, N., Michelena, J. M., Ferrer Suay, M., Guerrieri, E., & Blanco-Moreno, J. M. (2023). Not All Field Margins Are Equally Useful: Effects of the Vegetation Structure of Margins on Cereal Aphids and Their Natural Enemies. Insects, 14(2), 156. https://doi.org/10.3390/insects14020156








