Efficacy of In Vitro Lithium Chloride Treatments on Dermacentor reticulatus
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling
2.2. Immersion Contact Test
2.3. Statistical Analysis
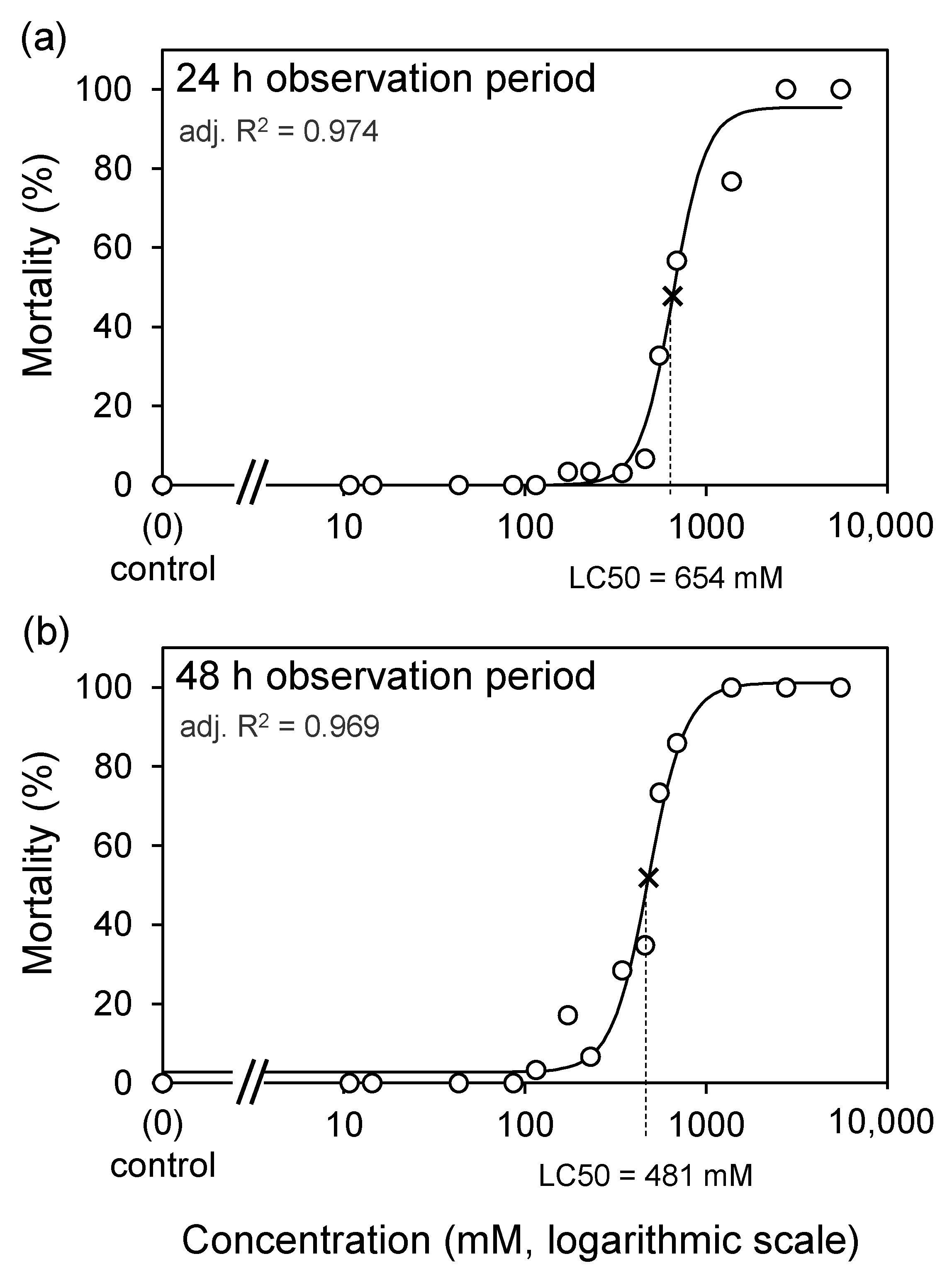
3. Results
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brown, M.; Hebert, A.A. Insect Repellents: An Overview. J. Am. Acad. Dermatol. 1997, 36, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Taylor, M.A. Recent Developments in Ectoparasiticides. Vet. J. 2001, 161, 253–268. [Google Scholar] [CrossRef] [PubMed]
- Hendricks, A.; Perrins, N. Recent Advance in Tick Control. In Practice 2007, 29, 284–287. [Google Scholar] [CrossRef]
- Quadros, D.G.; Johnson, T.L.; Whitney, T.R.; Oliver, J.D.; Oliva Chávez, A.S. Plant-Derived Natural Compounds for Tick Pest Control in Livestock and Wildlife: Pragmatism or Utopia? Insects 2020, 11, 490. [Google Scholar] [CrossRef] [PubMed]
- Wu-Chuang, A.; Obregon, D.; Mateos-Hernández, L.; Cabezas-Cruz, A. Anti-Tick Microbiota Vaccines: How Can This Actually Work? Biologia 2022, 77, 1555–1562. [Google Scholar] [CrossRef]
- Gu, Y.; Zhong, K.; Cao, R.; Yang, Z. Aqueous Lithium Chloride Solution as a Non-Toxic Bactericidal and Fungicidal Disinfectant for Air-Conditioning Systems: Efficacy and Mechanism. Environ. Res. 2022, 212, 113112. [Google Scholar] [CrossRef]
- Ziegelmann, B.; Abele, E.; Hannus, S.; Beitzinger, M.; Berg, S.; Rosenkranz, P. Lithium Chloride Effectively Kills the Honey Bee Parasite Varroa Destructor by a Systemic Mode of Action. Sci. Rep. 2018, 8, 683. [Google Scholar] [CrossRef]
- Kolics, É.; Mátyás, K.; Taller, J.; Specziár, A.; Kolics, B. Contact Effect Contribution to the High Efficiency of Lithium Chloride against the Mite Parasite of the Honey Bee. Insects 2020, 11, 333. [Google Scholar]
- Kolics, B.; Kolics, É.; Mátyás, K.; Taller, J.; Specziár, A. Comparison of Alternative Application Methods for Anti-Varroa Lithium Chloride Treatments. Insects 2022, 13, 633. [Google Scholar]
- Kolics, B.; Kolics, É.; Solti, I.; Bacsi, Z.; Taller, J.; Specziár, A.; Mátyás, K. Lithium Chloride Shows Effectiveness against the Poultry Red Mite (Dermanyssus gallinae). Insects 2022, 13, 1005. [Google Scholar] [CrossRef]
- Földvári, G.; Široký, P.; Szekeres, S.; Majoros, G.; Sprong, H. Dermacentor reticulatus: A Vector on the Rise. Parasites Vectors 2016, 9, 314. [Google Scholar] [CrossRef]
- Földvári, G.; Farkas, R. Ixodid Tick Species Attaching to Dogs in Hungary. Vet. Parasitol. 2005, 129, 125–131. [Google Scholar] [CrossRef]
- Šimo, L.; Kocáková, P.; Sláviková, M.; Kubeš, M.; Hajnická, V.; Vančová, I.; Slovák, M. Dermacentor reticulatus (Acari, Ixodidae) Female Feeding in Laboratory. Biol. Bratisl. 2004, 59, 655–660. [Google Scholar]
- Nosek, J. The Ecology and Public Health Importance of Dermacentor marginatus and D. reticulatus Ticks in Central Europe. Folia Parasitol. 1972, 19, 93–102. [Google Scholar]
- Soto, P.F.; Sánchez, R.P.; Grandes, A.E.; Sanz, R. Rickettsia Slovaca in Dermacentor Ticks Found on Humans in Spain. Eur. J. Clin. Microbiol. Infect. Dis. 2006, 25, 129–131. [Google Scholar] [CrossRef]
- Gorenflot, A.; Moubri, K.; Precigout, E.; Carcy, B.; Schetters, T.P.M. Human Babesiosis. Ann. Trop. Med. Parasitol. 1998, 92, 489–501. [Google Scholar] [CrossRef]
- Zahler, M.; Schein, E.; Rinder, H.; Gothe, R. Characteristic Genotypes Discriminate between Babesia Canis Isolates of Differing Vector Specificity and Pathogenicity to Dogs. Parasitol. Res. 1998, 84, 544–548. [Google Scholar] [CrossRef]
- Kalman, D.; Sreter, T.; Szell, Z.; Egyed, L. Babesia Microti Infection of Anthropophilic Ticks (Ixodes Ricinus) in Hungary. Ann. Trop. Med. Parasitol. 2003, 97, 317–319. [Google Scholar] [CrossRef]
- Meer-Scherrer, L.; Adelson, M.; Mordechai, E.; Lottaz, B.; Tilton, R. Babesia Microti Infection in Europe. Curr. Microbiol. 2004, 48, 435–437. [Google Scholar] [CrossRef]
- Hartelt, K.; Oehme, R.; Frank, H.; Brockmann, S.O.; Hassler, D.; Kimmig, P. Pathogens and Symbionts in Ticks: Prevalence of Anaplasma Phagocytophilum (Ehrlichia Sp.), Wolbachia Sp., Rickettsia Sp., and Babesia Sp. In Southern Germany. Int. J. Med. Microbiol. Suppl. 2004, 293, 86–92. [Google Scholar] [CrossRef]
- Rothschild, C.M. Equine Piroplasmosis. J. Equine Vet. Sci. 2013, 33, 497–508. [Google Scholar] [CrossRef]
- Halos, L.; Lebert, I.; Abrial, D.; Danlois, F.; Garzik, K.; Rodes, D.; Schillmeier, M.; Ducrot, C.; Guillot, J. Questionnaire-Based Survey on the Distribution and Incidence of Canine Babesiosis in Countries of Western Europe. Parasite 2014, 21, 13. [Google Scholar] [CrossRef] [PubMed]
- Halos, L.; Lebert, I.; Chao, I.; Vourc’h, G.; Ducrot, C.; Abrial, D.; Ravier, J.F.; Guillot, J. Questionnaire-Based Survey on Distribution and Clinical Incidence of Canine Babesiosis in France. BMC Vet. Res. 2013, 9, 41. [Google Scholar] [CrossRef] [PubMed]
- Sréter, T.; Sréter-Lancz, Z.; Széll, Z.; Kálmán, D. Anaplasma Phagocytophilum: An Emerging Tick-Borne Pathogen in Hungary and Central Eastern Europe. Ann. Trop. Med. Parasitol. 2004, 98, 401–405. [Google Scholar] [CrossRef] [PubMed]
- Raoult, D.; Roux, V. Rickettsioses as Paradigms of New or Emerging Infectious Diseases. Clin. Microbiol. Rev. 1997, 10, 694–719. [Google Scholar] [CrossRef]
- Randolph, S.E. The Shifting Landscape of Tick-Borne Zoonoses: Tick-Borne Encephalitis and Lyme Borreliosis in Europe. Philos. Trans. R. Soc. London. Ser. B: Biol. Sci. 2001, 356, 1045–1056. [Google Scholar] [CrossRef]
- Elllis, J.; Oyston, P.C.F.; Green, M. Tit-[18] Mattow, J., Jungblut, Pr, Schaible, Ue, Ball, Rw. Clin. Microbiol. Rev 2002, 15, 631–646. [Google Scholar]
- Ličková, M.; Havlíková, S.F.; Sláviková, M.; Slovák, M.; Drexler, J.F.; Klempa, B. Dermacentor reticulatus Is a Vector of Tick-Borne Encephalitis Virus. Ticks Tick-Borne Dis. 2020, 11, 101414. [Google Scholar] [CrossRef]
- Abbott, W.S. A Method of Computing the Effectiveness of an Insecticide. J. Econ. Entomol 1925, 18, 265–267. [Google Scholar] [CrossRef]
- Hill, A.V. The Possible Effects of the Aggregation of the Molecules of Haemoglobin on Its Dissociation Curves. J. Physiol. 1910, 40, 4–7. [Google Scholar]
- El-Samad, L.M.; El-Gendy, A.H.; Abdel-Moneim, A.M.; El-Ashram, S.; Augustyniak, M. Cuo Nps-Induced Damage to Testes and Deregulation of the Antioxidant System in Wild Terrestrial Organism Blaps sulcata (Coleoptera: Tenebrionidae). Environ. Nanotechnol. Monit. Manag. 2022, 18, 100751. [Google Scholar] [CrossRef]
- Kolics, É.; Sajtos, Z.; Mátyás, K.; Szepesi, K.; Solti, I.; Németh, G.; Taller, J.; Baranyai, E.; Specziár, A.; Kolics, B. Changes in Lithium Levels in Bees and Their Products Following Anti-Varroa Treatment. Insects 2021, 12, 579. [Google Scholar] [CrossRef]
- Kolics, É.; Specziár, A.; Taller, J.; Mátyás, K.K.; Kolics, B. Lithium Chloride Outperformed Oxalic Acid Sublimation in a Preliminary Experiment for Varroa Mite Control in Pre-Wintering Honey Bee Colonies. Acta Vet. Hung. 2021, 68, 370–373. [Google Scholar] [CrossRef]
- Stanimirović, Z.; Glavinić, U.; Ristanić, M.; Aleksić, N.; Jovanović, N.; Vejnović, B.; Stevanović, J. Looking for the Causes of and Solutions to the Issue of Honey Bee Colony Losses. Acta Vet. 2019, 69, 1–31. [Google Scholar] [CrossRef]
- Prešern, J.; Kur, U.; Bubnič, J.; Šala, M. Lithium Contamination of Honeybee Products and Its Accumulation in Brood as a Consequence of Anti-Varroa Treatment. Food Chem. 2020, 330, 127334. [Google Scholar] [CrossRef]
- Aral, H.; Vecchio-Sadus, A. Toxicity of Lithium to Humans and the Environment—A Literature Review. Ecotoxicol. Environ. Saf. 2008, 70, 349–356. [Google Scholar] [CrossRef]
- Voica, C.; Roba, C.; Iordache, A.M. Lithium Levels in Food from the Romanian Market by Inductively Coupled Plasma–Mass Spectrometry (Icp-Ms): A Pilot Study. Anal. Lett. 2020, 54, 242–254. [Google Scholar] [CrossRef]
- Schrauzer, G.N. Lithium: Occurrence, Dietary Intakes, Nutritional Essentiality. J. Am. Coll. Nutr. 2002, 21, 14–21. [Google Scholar] [CrossRef]
- Mueller, R.; Betz, L.; Anke, M. Essentiality of the ultra trace element lithium to the nutrition of animals and man. In Proceedings of the 30 Scientific Symposium of Industrial Toxicology; Fargasova, A., Jambrich, M., Jambrich, M., Koprda, V., Koprda, V., Melnik, M., Eds.; Slovenska Spolocnost Priemyselnej Chemie: Bratislava, Slovakia, 2010; p. 376. [Google Scholar]
- González-Weller, D.; Rubio, C.; Gutiérrez, A.J.; González, G.L.; Mesa, J.M.C.; Gironés, C.R.; Ojeda, A.B.; Hardisson, A. Dietary Intake of Barium, Bismuth, Chromium, Lithium, and Strontium in a Spanish Population (Canary Islands, Spain). Food Chem. Toxicol. 2013, 62, 856–868. [Google Scholar] [CrossRef]
- Licht, R.W. Lithium: Still a major option in the management of bipolar disorder. CNS Neurosci. Ther. 2012, 18, 219–226. [Google Scholar] [CrossRef]
- Dols, A.; Sienaert, P.; van Gerven, H.; Schouws, S.; Stevens, A.; Kupka, R.; Stek, M.L. The prevalence and management of side effects of lithium and anticonvulsants as mood stabilizers in bipolar disorder from a clinical perspective: A review. Int. Clin. Psychopharmacol. 2013, 28, 287–296. [Google Scholar] [CrossRef] [PubMed]
| Concentration LiCl (Test I & II) | Test I N | Test II N | Concentration NaCl (Test III) | Test III N |
|---|---|---|---|---|
| 5.520 M | 47 | 30 | 5.000 M | 30 |
| 2.760 M | 19 | 30 | 2.500 M | 30 |
| 1.380 M | 31 | 30 | 1.250 M | 30 |
| 0.690 M | 30 | 0.625 M | 30 | |
| 0.552 M | 31 | 0.316 M | 30 | |
| 0.460 M | 27 | |||
| 0.345 M | 30 | |||
| 0.230 M | 30 | |||
| 0.173 M | 29 | |||
| 0.115 M | 30 | |||
| 0.086 M | 59 | |||
| 0.043 M | 29 | |||
| 0.014 M | 30 | |||
| 0.011 M | 15 | |||
| 0.000 (control) | 50 | 37 | 30 | |
| Total | 147 | 463 | 180 |
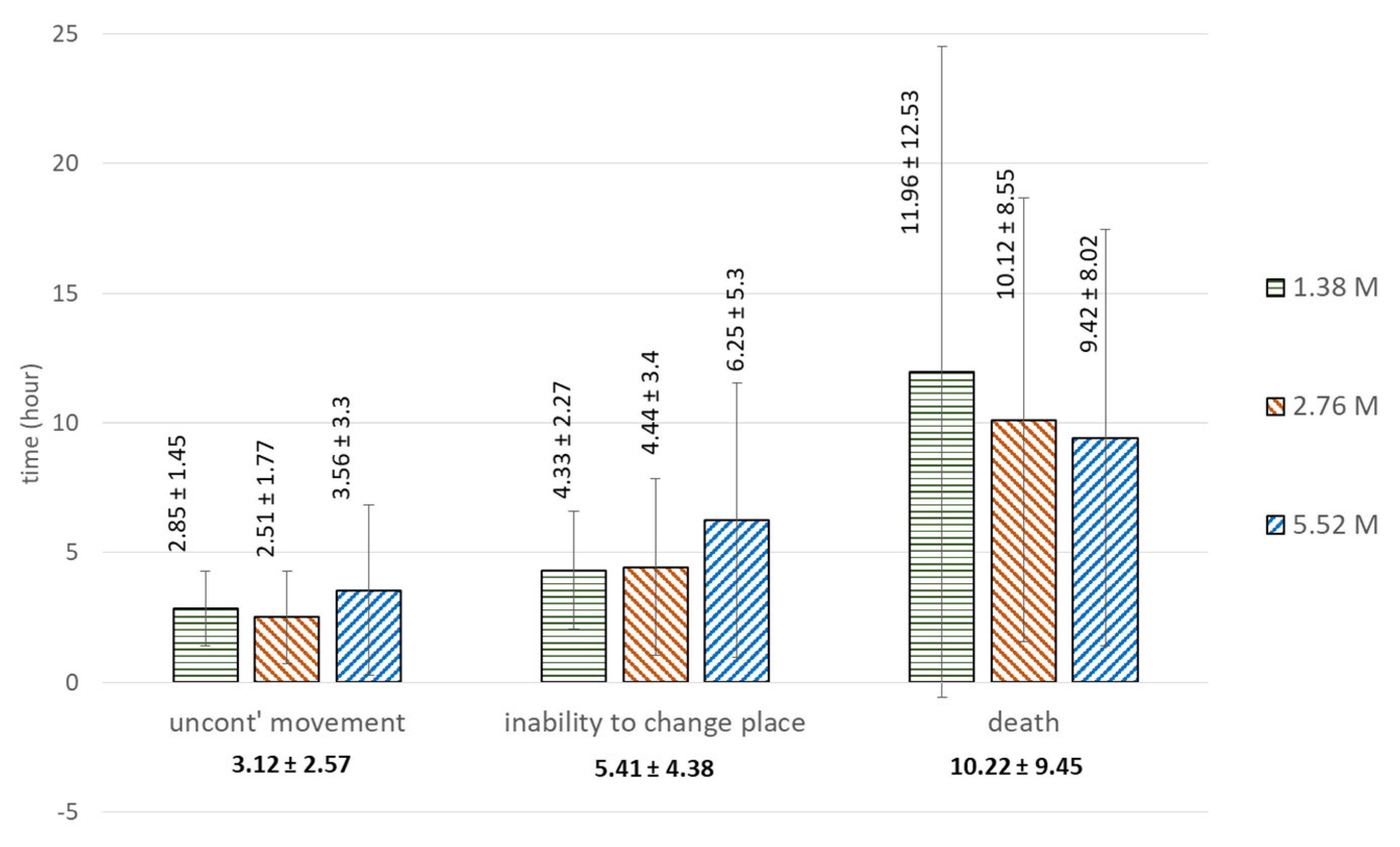
| Concentration | Onset of Uncontrolled Movement | Inability to Change Place | Death |
|---|---|---|---|
| 1.38 M | 2.85 ± 1.45 | 4.33 ± 2.27 | 11.96 ± 12.53 |
| 2.76 M | 2.51 ± 1.77 | 4.44 ± 3.4 | 10.12 ± 8.55 |
| 5.52 M | 3.56 ± 3.3 | 6.25 ± 5.3 | 9.42 ± 8.02 |
| ANOVA | F = 0.603, p = 0.549 | F = 0.266, p = 0.798 | |
| Welch Test | F = 0.910, p = 0.409 | ||
| Brown–Forsythe Test | F = 0.743, p = 0.479 | ||
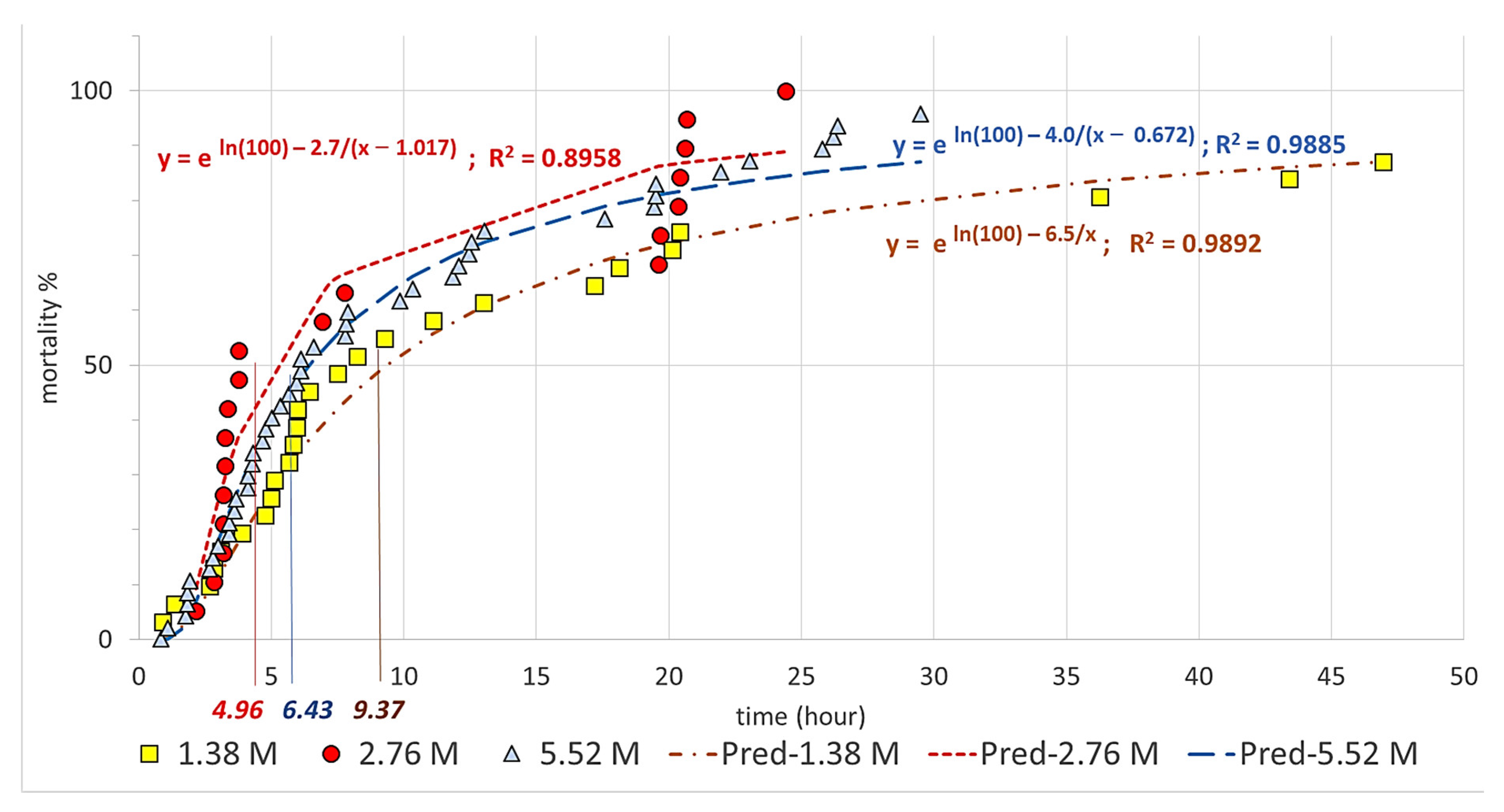
| Trend of mortality rates (y) by exposure times (x) | Estimated time to LT50 | ||
| 1.38 M | y = eln(100)−6.5/x; R2 = 0.989 | 9.37 | |
| 2.76 M | y = eln(100)−2.7/(x−1.017); R2 = 0.896 | 4.96 | |
| 5.52 M | y = eln(100)−4.0/(x−0.672); R2 = 0.989 | 6.43 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kolics, B.; Mátyás, K.; Solti, I.; Bacsi, Z.; Kovács, S.; Specziár, A.; Taller, J.; Kolics, É. Efficacy of In Vitro Lithium Chloride Treatments on Dermacentor reticulatus. Insects 2023, 14, 110. https://doi.org/10.3390/insects14020110
Kolics B, Mátyás K, Solti I, Bacsi Z, Kovács S, Specziár A, Taller J, Kolics É. Efficacy of In Vitro Lithium Chloride Treatments on Dermacentor reticulatus. Insects. 2023; 14(2):110. https://doi.org/10.3390/insects14020110
Chicago/Turabian StyleKolics, Balázs, Kinga Mátyás, Izabella Solti, Zsuzsanna Bacsi, Szilvia Kovács, András Specziár, János Taller, and Éva Kolics. 2023. "Efficacy of In Vitro Lithium Chloride Treatments on Dermacentor reticulatus" Insects 14, no. 2: 110. https://doi.org/10.3390/insects14020110
APA StyleKolics, B., Mátyás, K., Solti, I., Bacsi, Z., Kovács, S., Specziár, A., Taller, J., & Kolics, É. (2023). Efficacy of In Vitro Lithium Chloride Treatments on Dermacentor reticulatus. Insects, 14(2), 110. https://doi.org/10.3390/insects14020110





