1. Introduction
Among the several species of
Frangula (Rosales: Rhamnaceae) in North America, glossy buckthorn (
Frangula alnus) is an invasive European shrub that has been spreading throughout the northeastern portion of the United States and Southeastern Canada [
1]. It is an aggressive shrub as it forms dense monospecific patches in disturbed areas, along roads, and throughout fields and clearings [
2]. In its native European range,
F. alnus was utilized as a source of superior charcoal for gunpowder production, as a laxative, and as a sap-green dye [
3]. In the late 1800s, it was introduced to Canada as an ornamental and was first collected outside of cultivation in London, Canada, in 1898. By 1970, it had spread to areas up to 150 km away from its original introduction [
4]. Currently,
F. alnus has been reported in 29 US states [
5].
As is typical of invasive species,
F. alnus has several factors contributing to its success in North America. It appears to lack the natural herbivores that it experienced in Europe, potentially not being exposed to the necessary pest pressure for control in North America.
Zygina suavis (Hemiptera: Cicadellidae) and
Gonopteryx rhamni (Lepidoptera: Pieridae) are both herbivorous insects known to be directly associated with
F. alnus in Europe [
6,
7]. These insects can limit the growth and reproduction of
F. alnus in its native habitats, but these inhibitory and specialist species are not known to exist in North America, highlighting the importance of identifying inhibitory species already present within North America. Light feeding damage due to deer browsing was also reported, but the damage did not affect invasion [
8]. In addition to occupying a natural enemy-free landscape, the ability of
F. alnus to flourish in a variety of soil conditions [
3] increases its suitable range and could, in turn, increase connectivity between potential open habitats. This sun-loving plant also puts out new leaves before other deciduous trees and retains its leaves longer into the fall than other deciduous trees [
9]. This results in a longer growing season, allowing the invasive shrub to monopolize soil nutrients and sunlight, crowding out native undergrowth with its vigorous growth.
In Europe, multiple projects have previously compiled lists of European insects associated with
F. alnus. A study in Southern Spain [
10] reported 47 insect species utilizing the flowers and suspected that 21 of them were likely to pollinate the flowers. Dipterans were the dominant insect group in terms of both the number of species interacting with the flowers and the number of individuals. To identify candidate natural enemies that could be introduced to North America as a weed control solution, Gassmann et al. [
6] collected 1000 insect samples from 99 sites containing
F. alnus and common buckthorn,
Rhamnus cathartica (Rosales: Rhamnaceae), in Europe. They discovered eight insect species associated with
F. alnus, with
Z. suavis being exclusive to
F. alnus. Brändle and Brandl [
11] found that 91 phytophagous insect species were associated with the combined genera
Frangula and
Rhamnus in Germany, 29 of which were specialists. Simandl [
12] specifically investigated wood-boring beetles associated with
F. alnus in the South Bohemian region and found a total of 13 associated species. Moreover, the specialist
G. rhamni is known to utilize
F. alnus in Europe [
6]. There are few mentions in the literature of individual insect species found to be associated with
F. alnus in North America, such as the soybean aphid,
Aphis glycines (Hemiptera: Aphididae), which utilizes
F. alnus for overwintering [
13]. Although these earlier studies have profiled insects associated with
F. alnus in Europe, a similar comprehensive compilation has not been conducted in North America.
Understanding how an invasive species interacts with ecosystems in its invaded range can lead to improvements in the management of the species. Differences in soil conditions, climate, pollinators, and herbivores in the invaded range compared to the native range could play a key role in the success or failure of an invasive plant. While the herbivorous arthropods that coevolved with
F. alnus in Europe do not exist in North America, it is still possible that North American insects could accept
F. alnus as a host plant. Insect-mediated pollination and the resulting fruit set are decisive factors in the successful spread of an invasive plant species. Thus, surveying insect communities on
F. alnus will help to identify key insect species that could be associated with
F. alnus, including those that utilize this shrub as a pest reservoir. Another important aspect of understanding the interaction of an invasive plant species with its biotic environment, including its interactions with insects, is detailed knowledge of the specialized plant metabolites that it produces.
F. alnus is well known for the formation of anthraquinones, including the abundant emodin [
14], which have antifeeding, laxative, antimicrobial, and allelopathic activity [
15]. In contrast, very little is yet known about the volatile organic compounds (VOCs) that are emitted from leaves and flowers of
F. alnus, although they are generally known to be involved in the interaction with herbivores and the attraction of pollinators [
16]. The interaction of plants with insect herbivores is influenced by VOCs, a group of specialized metabolites that are emitted from vegetative plant tissues into the surrounding atmosphere. While herbivores utilize some of these VOCs to localize suitable host plants, VOC emission is also an essential part of the plant defense against herbivores, either directly by acting as a repellent or indirectly by attracting predators and parasitoids [
17]. Moreover, the emission of defense-related plant VOCs is often induced by herbivory through plant tissue being wounded or exposed to herbivore-derived molecular patterns such as oral saliva [
18].
The goal of this study was to determine the associations between North American insects and F. alnus to provide a foundation for potential management strategies. This research specifically aimed to determine (1) the dominant insect orders and species found on F. alnus, (2) the changes in their species richness and diversity throughout the growing season, (3) insects that utilize the fruits of F. alnus, and (4) volatile organic compounds emitted from flowers and leaves of F. alnus that contribute to the interaction with insects.
2. Materials and Methods
2.1. Study Site
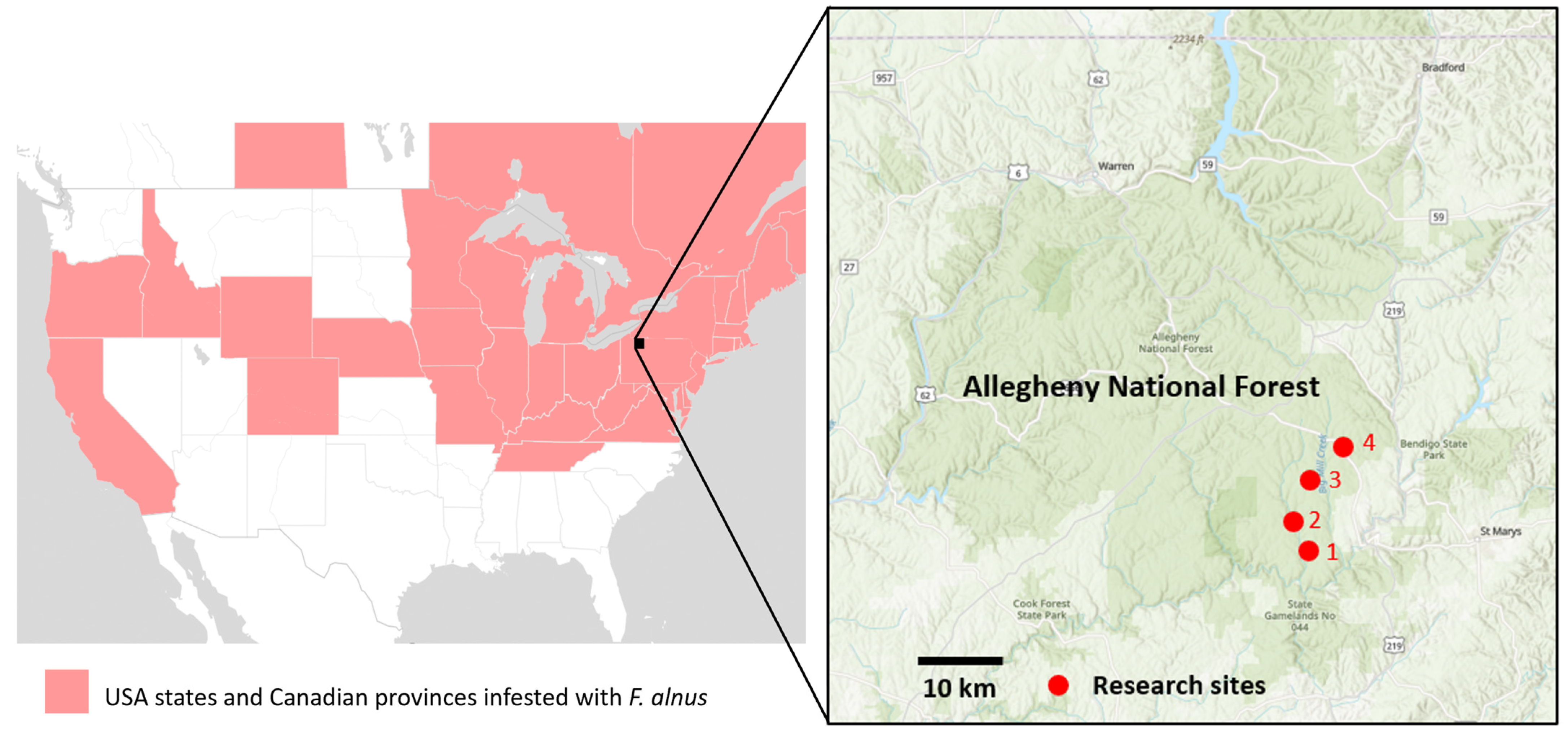
This research was conducted in the Allegheny National Forest near the borough of Ridgway, PA, USA. Four 2.5-ha sites (sites 1–4) infested with
F. alnus were selected, and, within each site, four 1-m
2 plots of
F. alnus (i.e., a total of 16 plots) were established. The geocoordinates of the four sites were 41.43704, −78.79137 for Site 1; 41.45674, −78.80247 for Site 2; 41.47875, −78.77288 for Site 3; and 41.485216, −78.741848 for Site 4 (
Figure 1). Sites 1 and 2 are primarily deciduous forests with intersecting unpaved forest roads. Site 3 is more varied, with a powerline, deciduous trees, grass fields, and a stream. Site 4 consists of mesic deciduous forests, paved and unpaved roads, wet areas, and a patch of conifers (
Figure 1). Our plot placements and the selection of individual
F. alnus for sampling were strategically determined to target areas where
F. alnus populations existed in isolation from other plant species, ensuring a focused investigation into the ecological interactions associated with this invasive shrub.
2.2. Arthropod Sampling
Arthropods were collected from F. alnus on 16 plots using an aerial insect net. The insect net, with a diameter of 30 cm, was swept back and forth 10 times in a zig-zag pattern deeply and harshly into the branches of F. alnus to cover an area of 1 m in width by 2.5 m in height for each sample. All collections took place between noon and 4 p.m. on sunny or partly cloudy days, with wind speeds below 25 kph. This was replicated on five dates (4 August 2021; 6 September 2021; 19 May 2022; 30 June 2022; and 22 July 2022), resulting in a total of 80 samples (i.e., 16 plots on five sample dates). In addition to insect net sampling, we directly observed and recorded any insects feeding and visiting flowers and fruits.
The collected insects were brought to the lab and sorted to order and then to morphospecies by using a Dino-Lite Digital Microscope (Dunwell Tech., Inc. dba Dino-Lite Microscope, Torrance, CA, USA). The most common morphospecies were identified to species.
2.3. Fruit Sampling
Ripe F. alnus fruits were collected directly from the live branches of F. alnus at each of the 16 sites on three collection dates: 6 September 2021; 22 July 2022; and 10 August 2022. An average of 14 fruits were randomly selected and collected per plot on each date. The collected fruits were then placed in Solo P550N 5.5-oz plastic cups (Solo Cup Company, Lake Forest, IL, USA) with mesh placed over the top to prevent the escape of emerging insects. We collected an additional 434 F. alnus fruits at four different ripening stages based on color (i.e., green, greenish red, red, and black) in August and September 2023 to determine which fruit ripening stage that the insects used to lay eggs, with black representing fully ripened fruit. These cups were kept in the laboratory at ~21 °C, and the insect emergence from them was recorded. Each insect that emerged from these fruits was identified to the species level and sexed.
2.4. Collection and Analysis of Volatile Organic Compounds
Branches from
F. alnus were collected in the Allegheny National Forest and placed into a water-filled container for transport. Volatiles emitted from
F. alnus leaves were collected using a closed-loop stripping method as described by Wang et al. [
19,
20]. Five leaves were cut from freshly harvested branches for each volatile collection and supplemented with 10% (
w/
v) sucrose solution. In addition, leaves were also wounded using an array of needles as described by Gutensohn et al. [
21]. Headspace collections from detached unwounded and wounded leaves were performed for 24 h using Porapak-Q traps (Volatile Collection Trap LLC, Gainesville, FL, USA), and collected volatile compounds were subsequently eluted with dichloromethane. Moreover, 3.33 µg of naphthalene was added as an internal standard.
Samples from headspace collections were analyzed by combined gas chromatography/mass spectrometry (GC/MS) using a TRACE 1310 gas chromatograph system linked to a TSQ 8000 triple quadrupole mass spectrometer (Thermo Fisher Scientific, Waltham, MA, USA), as described by Wang et al. [
19,
20]. Individual compounds were identified using the Xcalibur 2.2 SP1.48 software (Thermo Fisher Scientific) by comparing their mass spectra with those deposited in the NIST/EPA/NIH Mass Spectral Library (NIST11) (National Institute of Standards and Technology NIST, Scientific Instrument Services, Inc., Ringoes, NJ, USA;
https://chemdata.nist.gov/mass-spc/ms-search/ accessed on 10 November 2023).
For the analysis of floral volatile emissions, ten freshly sampled F. alnus flowers, either before or after anthesis, were placed in 2-mL glass vials and closed with an airtight screwcap. Floral volatiles were collected from the headspace via stir bar sorptive extraction (SBSE) by attaching a magnetic Twister® coated with polydimethylsiloxane (PDMS) (Gerstel, Mülheim, Germany) to the inside of the glass vial with an additional small magnet (D401-N52, K&J Magnetics, Pipersville, PA, USA). After 24 h, the Twisters were removed from the glass vials, and collected volatiles were analyzed by GC/MS utilizing a coupled gas chromatograph (Agilent Technologies 7890B series)/mass spectrometer (Agilent Technologies 5977B Inert Plus MSD Turbo EI, Santa Clara, CA, USA) equipped with a thermal desorption unit (TDU) and a cooled injection system (CIS 4C) (Gerstel, Mülheim, Germany).
2.5. Data Analysis
Arthropods collected in this study were grouped into eight categories: Arachnida, Coleoptera, Diptera, Hemiptera, Hymenoptera, Lepidoptera, Psocodea, and Others (all other minor insect orders). To determine which arthropod orders and morphospecies were dominant, the numbers of occurrences of each order and morphospecies were summed up and then ranked. The five dominant morphospecies were identified to species.
The arthropod abundance and arthropod diversity of each order over the five collection dates were analyzed using repeated-measures ANOVA followed by Tukey–Kramer comparisons of least squares means in PROC GLIMMIX of SAS. To measure the arthropod abundance, the statistical distribution of data was examined by the Shapiro–Wilk W test for a lack of normality. We found a lack of normality in the dataset and the data fit the Poisson error distribution, and thus we used PROC GLIMMIX with Poisson distribution as a log link function (SAS®, Version 9.4, SAS Institute Inc., Cary, NC, USA), after a constant 1 was added to the raw data. The model included the effects of order and time (i.e., the number of weeks since the beginning as a categorical variable type), and their interaction, with time as a random effect (repeated) and individual plots as subjects. A slicing method of multiple comparisons with Tukey–Kramer adjustment was employed. It enabled, in the case of an existing interaction of two effects, multiple comparisons among time points within each order (e.g., Hemiptera in August 2021 vs. Hemiptera in September 2021) or comparisons among the orders at each of the five sampling collections (e.g., Arachnida vs. Diptera in May 2022). Slicing prevents confounding and nonlogical comparisons (e.g., Arachnida in August 2021 vs. Lepidoptera in May 2022).
Arthropod richness was calculated by summing the number of morphospecies for each of the 16 plots on each of the five dates by using R software 9, version 4.1.2 [
22]. The effect of the collection date (categorical) on richness was analyzed using a repeated-measures generalized linear mixed model with a negative binomial error distribution using the package glmmTMB in R [
23]. To account for repeated measurements at each site, the site and plot nested in the site were included as random intercepts. Violations of assumptions were tested using the package DHARMa. The Levene test was used to check for homoscedasticity. Collection dates were compared using a post hoc Tukey–Kramer multiple comparison analysis (package emmeans) [
24].
Arthropod diversity was measured using the inverse Simpson’s diversity index (
DInvSimpson). The diversity index for each of the 16 plots on each of the five dates (for a total of 80 calculations) was calculated using the following equation:
where
s represents the number of unique morphospecies present in the given plot and date,
i represents each morphospecies, and
Pi represents the proportion of abundance of morphospecies
i within the given plot and date.
Pi =
Ni/
N, where
N represents total collection abundance and
Ni represents the abundance within morphospecies
i. It may be noted that it is common practice to divide one by the Simpson’s diversity index to obtain an inverse index, to ensure that higher indices represent higher diversity [
25]. To meet the assumptions of normality of distribution for repeated-measures ANOVA, the inverse Simpson’s diversity index was transformed by taking the log.
4. Discussion
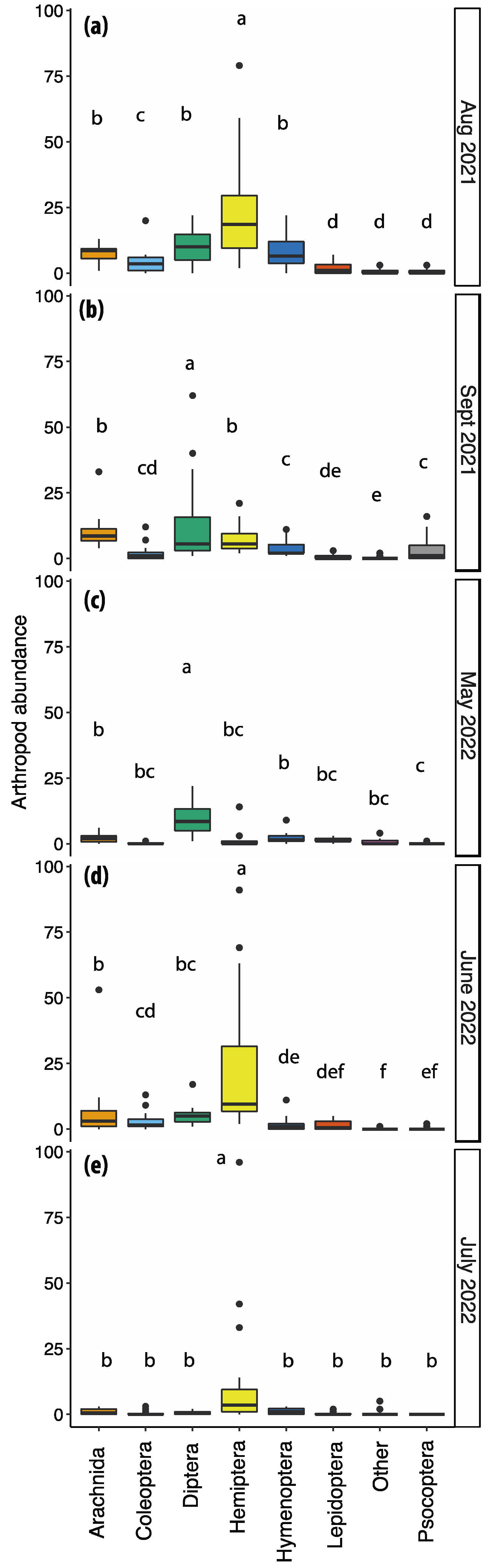
This study represents the first comprehensive investigation into the arthropod community associated with F. alnus conducted in North America. In total, 2845 specimens of insects and arachnids were systematically sampled, collectively constituting 563 distinct morphospecies. The abundance of arthropod orders was intricately linked to the phenological characteristics and seasonal variations observed during the survey. Notably, there were significant disparities in proportional representation among arthropod orders, being statistically different across the five designated collection dates, except for the other orders category.
Gassmann et al. [
6] previously conducted a parallel inquiry into the insect fauna linked with
F. alnus and
R. cathartica in Europe, differing in their approach by targeting specialized insects with direct plant interactions, in contrast to our holistic collection strategy with limitations including the less intensive sampling of target groups such as stem-feeding or root-feeding insects. Their study revealed Lepidoptera as the predominant insect order, encompassing 22 distinct species across the two plant species, followed by Hemiptera (eight species), Diptera (four species), and Acarina (four species). However, it is worth noting that our findings diverge from this pattern, with Hemiptera emerging as the dominant order, specifically exemplified by 625 individuals of
P. carpinicola, which was the dominant species; remarkably, 16.2% of these Hemiptera individuals were in the immature stages. Currently, there is extremely limited literature regarding
P. carpinicola, and no detailed life history information is available;
P. carpinicola is not known to feed on
F. alnus, and our field observations did not reveal any conspicuous feeding damage attributable to this species. However, we observed
Carpinus betulus (Betulaceae), the main host of
P. carpinicola, in all study sites. This observation is consistent with that of another entomologist [
26], who often observed
P. carpinicola resting on beech trees, which are similarly a non-host species. Whether
P. carpinicola feeds on
F. alnus or not needs to be investigated further in the future.
Drosophila suzukii was the second most abundant species captured through net sweeping and was the sole species successfully reared from collected
F. alnus fruits. Intriguingly, fruits from which
D. suzukii emerged exhibited conspicuous signs of damage, with extensive fruit consumption and residual rotting, corroborating findings from Hauser et al. [
27]. This suggests that
F. alnus serves as a reproductive host of
D. suzukii within forests with no agricultural fields nearby. Consequently, this suggests a potential negative impact on the dissemination and recruitment of
F. alnus, particularly if the seeds prove non-viable when the fruits are infested by
D. suzukii. It is pertinent to acknowledge that
D. suzukii also exploits other forest flora, such as
Rubus allegheniensis,
Prunus serotina, and
Sambucus nigra, as documented by Lee et al. [
28]. Additionally, Grassi et al. [
29] reported
D. suzukii infesting
F. alnus fruits in Italy. These interactions between
D. suzukii and
F. alnus could potentially influence avian consumption patterns, as birds might prefer insect-infested fruits due to their heightened protein content, as elucidated by Manzur and Courtney [
30]. Our results further unveiled that adult
D. suzukii’s flight activity and oviposition on
F. alnus were exclusively tied to the full maturation of
F. alnus fruits, signifying a clear attraction towards and utilization of mature fruits for reproduction. These findings are consistent with those of Kenis et al. [
31], who documented that
D. suzukii uses
F. alnus in European countries. Moreover, Lee et al. [
28] in Oregon identified
D. suzukii infesting the fruits of
F. purshiana, another
Frangula species that is native to Western North America. If
F. alnus inhabits near orchards, it can be used potentially as a trap plant for the monitoring of
D. suzukii.
Our survey revealed that there are numerous predatory arthropods indirectly associated with
F. alnus, warranting particular attention because they are generalist carnivores [
32]. Their pronounced presence in our samples indicates an abundant arthropod prey associated with
F. alnus. The presence of such natural enemies can affect the overall diversity because they can inhabit and feed on prey insects on
F. alnus throughout the year. Specifically, we observed significantly elevated arachnid abundance in August 2021, September 2021, and June 2022, when arthropod abundance was higher.
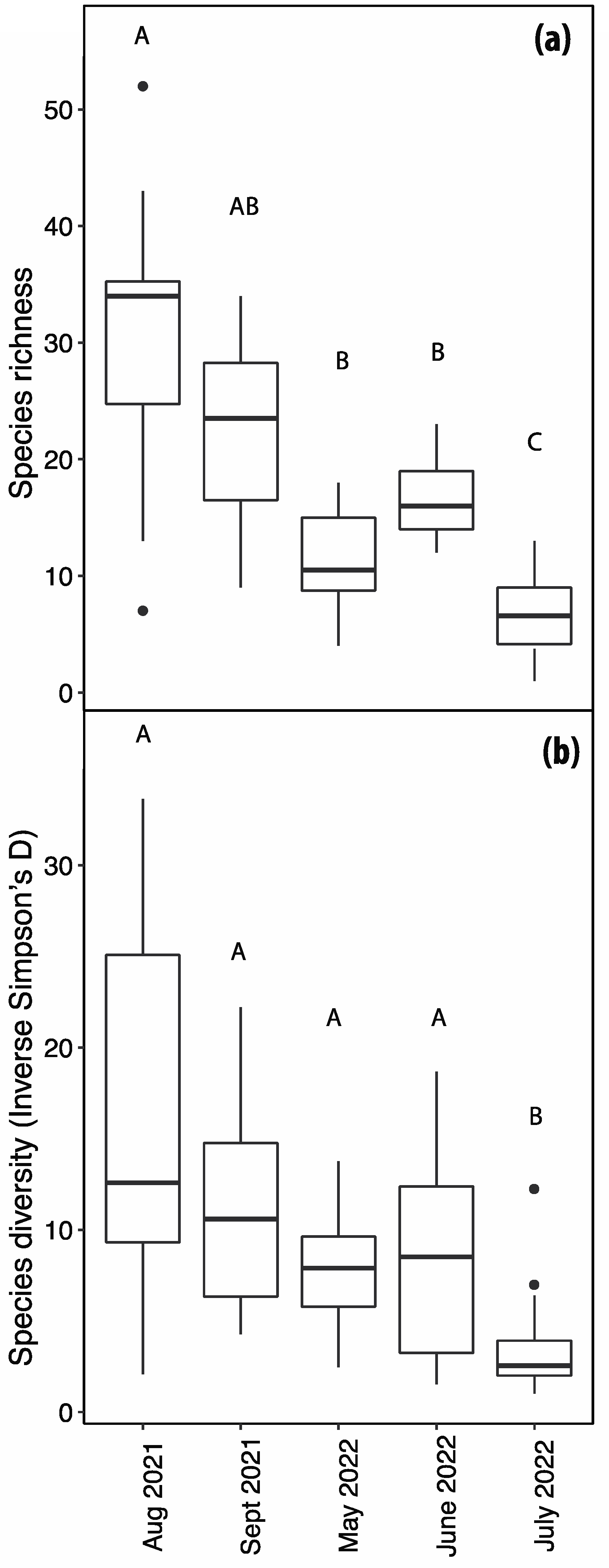
The arthropod community associated with F. alnus was highly dynamic, with substantial variations observed among different phenological stages (i.e., vegetative and reproductive stages). Both species richness and diversity exhibited a dependency on the sampling date, each dataset featuring at least one date with statistically distinct richness and diversity measures. Notably, the months of August and September 2021 exhibited a notable upsurge in arthropod abundance, coinciding with the peak foliage period when the plant provided substantial shade and refuge for insects traversing the adjacent roadways. These months were also characterized by heightened fruit availability. The patterns of richness and diversity were similarly influenced by the sampling date. July 2022 was marked by reduced insect and arachnid abundance and was not significantly abundant in any individual arthropod order. We attribute this phenomenon to the elevated temperatures experienced during sunny afternoons in July, which coincided with our insect collection efforts.
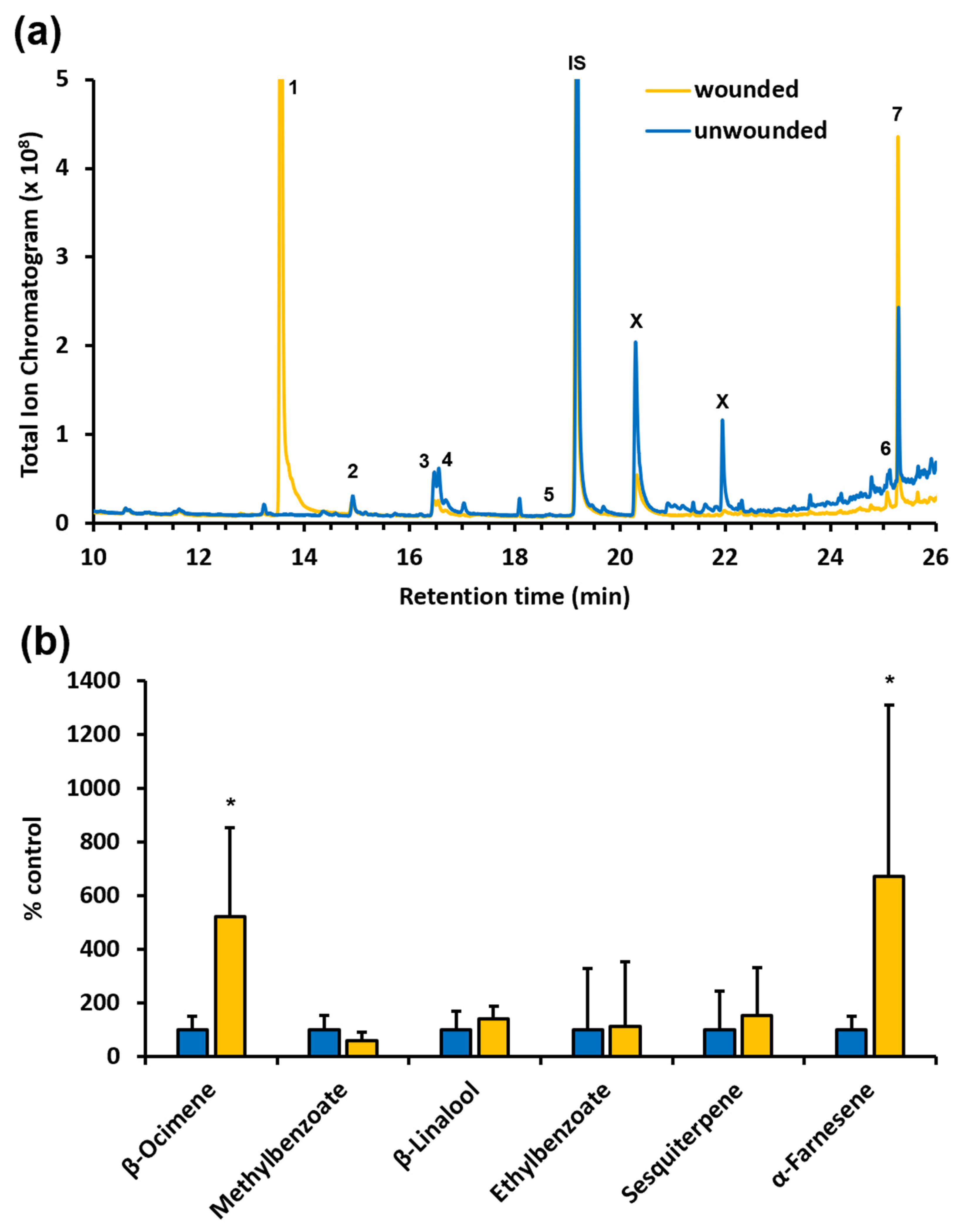
While plant-herbivore interactions frequently involve VOCs with attractive and/or repellent activity, our analysis of
F. alnus leaves revealed that these are not strongly scented and only emit comparatively small amounts of a few VOCs (
Figure 5a). This might be one reason why only a limited number of herbivores were found associated with
F. alnus in our study. It remains to be shown if this limited volatile emission is related to the relatively prominent cuticle, characteristic of
F. alnus leaves, as recent reports have demonstrated a significant effect of the cuticle thickness and composition on the emission of volatile compounds [
33,
34]. However, it is noteworthy that the wounding of
F. alnus leaves resulted in a significant increase in the emission of the terpenes β-ocimene and α-farnesene (
Figure 5b). Many previous studies have reported the induction of β-ocimene and α-farnesene upon herbivory in many plant species [
35,
36,
37]. Remarkably, α-farnesene is not only perceived in the antennal lobe of female
Helicoverpa assulta (Lepidoptera: Noctuidae) but also found to have an inhibitory effect on their oviposition [
35]. Moreover, several studies have shown that α-farnesene has an attractive effect on predators, such as anthocorid bugs (Hemiptera: Anthocoridae) [
38] and parasitoids including
Telenomus podisi (Hymenoptera: Platygastridae) and
Campoletis chlorideae (Hymenoptera: Ichneumonidae) [
35]. Further studies will be required to verify whether the observed α-farnesene emission of
F. alnus in its invasive range also contributes to the attraction of parasitoids or predators such as the arachnids found in our arthropod survey.
Insects are the only known pollinators of
F. alnus [
1], and since its flowers are self-incompatible and depend on cross-pollination, the interaction of
F. alnus with pollinator insects is essential for the reproduction of
F. alnus and the successful establishment and spread in its invasive range. It is important to note that the flowering period of
F. alnus is unusually long, which suggests that it is likely pollinated by many different insect species [
39]. While we only observed a relatively small number of potential pollinators that visited the
F. alnus flowers, the consistently high fruit set indicated that a sufficient number of pollinator insects must have been visiting the
F. alnus flowers. Of the four major
F. alnus flower-visiting insects,
B. impatiens,
A. pura, and
R. recta are well-known pollinators [
40,
41,
42]. In contrast,
V. maculifrons generally visit flowers to feed on nectar [
43] and thus might not significantly contribute to the pollination of
F. alnus. Our analysis of floral volatiles revealed that four VOCs (i.e., phenylethanol, ethyl benzoate, ethyl salicylate, and α-farnesene) are emitted from open
F. alnus flowers at significantly higher rates, suggesting their involvement in the attraction of pollinators. These volatile compounds, particularly ethyl benzoate and ethyl salicylate, which are always found together, have previously been observed in the floral volatile profiles of other plant species, including strawberry (
Fragaria x ananassa), wild tobacco (
Nicotiana attenuata), lily (
Lilium spp.), orange jessamine (
Murraya paniculata), and
Viola tricolor [
44,
45,
46,
47,
48]. Remarkably,
Bombus terrestris (Hymenoptera: Apidae) and
Apis mellifera (Hymenoptera: Apidae) showed responses to phenylethanol, ethyl salicylate, and ethyl benzoate in an electroantennography (EAG) analysis [
44]. The very strong response of
B. terrestris towards ethyl benzoate reported in this recent study might explain our observation of
B. impatiens visiting
F. alnus flowers. An earlier EAG study also found a response of
Helicoverpa armigera (Lepidoptera: Noctuidae) towards ethyl benzoate [
49]. It should be considered that our survey of pollinator insects visiting
F. alnus was restricted to daylight hours, and thus we cannot exclude that night-active insects such as moths might also visit
F. alnus flowers and contribute to their pollination. In addition, some of the volatiles identified in this study can be used to develop attractants to increase the herbivory of
F. alnus, but this will also require additional studies under controlled conditions to identify those VOCs that are perceived by and act attractive towards different herbivores.
Future studies may seek to identify the insect pollinators and birds associated with F. alnus. It is also unknown which bird species in North America may be more likely to increase the germination and overall success of F. alnus seeds. In addition, research on feeding damage by the four major insect species found in this study would give further insight as to how the insects can affect F. alnus. Finally, as this study found that F. alnus can be an excellent reproductive host of D. suzukii, future studies should seek to identify how this interaction between these two invasives (1) affects the germination of F. alnus seeds, (2) decreases the desirability of the fruit for the birds that disperse the seeds, (3) increases connectivity between the suitable ranges of D. suzukii within forests, and (4) is affected by the VOCs associated with F. alnus fruits.